Back to Journals » International Journal of Women's Health » Volume 13
Perinatal Outcomes and Influence of Amniotic Fluid Volume Following Previable, Preterm Prelabor Rupture of Membranes (pPPROM): A Historical Cohort Study
Authors Pylypjuk C , Majeau L
Received 17 February 2021
Accepted for publication 2 June 2021
Published 28 June 2021 Volume 2021:13 Pages 627—637
DOI https://doi.org/10.2147/IJWH.S303120
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Everett Magann
Christy Pylypjuk,1 Ladonna Majeau2
1Department of Obstetrics, Gynecology & Reproductive Sciences and Children’s Hospital Research Institute of Manitoba, University of Manitoba, Winnipeg, Manitoba, Canada; 2Department of Obstetrics, Gynecology & Reproductive Sciences, University of Manitoba, Winnipeg, Manitoba, Canada
Correspondence: Christy Pylypjuk
Department of Obstetrics, Gynecology & Reproductive Sciences, University of Manitoba, WN5002 HSC Women’s Hospital, 820 Sherbrook Street, Winnipeg, MB R3A 1R9, Canada
Tel +1 204 787-4821
Fax +1 204 787-2920
Email [email protected]
Purpose: To determine perinatal outcomes and influence of amniotic fluid volume in pregnancies complicated by previable, preterm prelabor rupture of membranes (pPPROM).
Patients and methods: This was a historical cohort study from two tertiary-level maternity hospitals (January 1, 2009 to December 31, 2015). All pregnancies complicated by pPPROM were identified using ICD coding of discharge abstracts. Hospital charts were reviewed to collect maternal demographics, pregnancy and delivery events, and immediate postnatal outcomes (including survival). Post-processing review of stored ultrasound images was performed to evaluate the relationship between amniotic fluid volume and outcomes.
Results: A total of 113 pregnancies were eligible and 99 were included in the final analysis (74 with “expectant management” and 25 opting for elective termination). The median gestational age at pPPROM was 20+6 weeks [IQR 19+4 to 21+5]. For those choosing expectant management, the median latency between pPPROM and delivery was 7 days, median gestational at delivery was 23+1 weeks, and neonatal survival to discharge was 27.5% overall. There was a trend towards higher rates of pregnancy termination at one hospital (31.7%) compared to the other (15.4%), but no difference between sites with respect to latency, mode of delivery, or survival amongst those managed expectantly. There was a relationship between survival and gestational age at pPPROM (p< 0.04), as well as initial amniotic fluid volume category: 52.6% of survivors had normal initial amniotic fluid volumes whereas the majority of previable losses had oligohydramnios and the majority of stillbirths had anhydramnios.
Conclusion: After expectant management, more than one in four newborns following pPPROM survived to hospital discharge. While gestational age at rupture was most strongly correlated with survival, normal initial amniotic fluid volumes were mostly seen in survivors whereas stillbirths more frequently had anhydramnios. These findings will help to improve counseling and care of patients with pPPROM and in guiding long-term follow-up studies.
Keywords: prelabor rupture of membranes, prematurity, previability, pregnancy complications, neonatal survival, amniotic fluid
Introduction
Spontaneous preterm prelabor rupture of membranes (PPROM) complicates about 3% of pregnancies worldwide and can be associated with multiple adverse outcomes for pregnant women and their babies, especially risks of ascending infection and related morbidity.1,2 PPROM that occurs at previability, or “pPPROM”, is a rarer complication of pregnancy, estimated to affect one to eight per 1000 births.3,4 When a low amniotic fluid environment occurs following pPPROM and coincides with the time of critical fetal lung development, there is a significant risk of life-limiting pulmonary hypoplasia or lifelong respiratory morbidity for those who survive.3–8 Owing to concerns about poor respiratory outcomes leading to neonatal death, many patients consider termination when pPPROM occurs.9,10 Regardless of these known complications, however, some patients choose expectant management following pPPROM and neonatal survival is possible, although outcomes reported in the literature vary considerably.7,8,11
While maternity care providers are acutely aware of the respiratory and infectious morbidity associated with pPPROM, less is known about other complications or the potential maternal risks directly resulting from management decisions (expectant versus termination).3–5 Many centers have developed clear management algorithms for PPROM after viability, but there remains considerable variation in practice patterns when membrane rupture occurs prior to viability; in addition, many of these medical interventions (including antenatal corticosteroids) have not been studied at previability or the extremes of prematurity.12–14 As there is no obvious way to risk-stratify pregnancies complicated by pPPROM at this time, and given that many of these cases will receive an obstetric ultrasound to evaluate fetal well-being, there remains a question about the potential role of amniotic fluid volume measurement in predicting outcome after previable membrane rupture.15,16 The goal of this study was to determine the perinatal outcomes and influence of amniotic fluid volume in pregnancies complicated by previable, preterm prelabor rupture of membranes (pPPROM). The results of this study could be used to refine patient counseling and develop management strategies to improve outcomes, as well as to direct much-needed future research.
Materials and Methods
This was a historical cohort study conducted at two tertiary-level maternity hospitals in Canada between January 1, 2009 and December 31, 2015. Both hospitals serve as the regional referral sites for a total population of 1.3 million inhabitants and a geographic region which includes urban, rural, and northern/remote communities. There are approximately 10,000 deliveries between the two sites per year. Research ethics approval was obtained from the University of Manitoba Health Research Ethics Board [HS23563 (H2020:012)]. Patient confidentiality was maintained in compliance with institutional standards and in accordance with the Declaration of Helsinki: because this project was retrospective in nature and did not require any direct patient contact, nor did it influence patient management or outcomes, informed consent forms were not required by our institution.
All pregnant patients diagnosed with PPROM prior to 24+0 weeks of gestation during the study period were eligible for inclusion. Potential cases of pPPROM were identified by the health records department using the International Classification of Disease, ninth revision (ICD-9) coding of PPROM from hospital discharge abstracts of in-patient medical records. Consistent with local maternity and neonatal resuscitation practices at that time, “previability” was defined as a gestational age of <24+0 weeks. (The study was bound by the year 2015 because neonatal resuscitation became offered in the following year for preterm births beginning at 23 weeks’ gestation.) From those cases coded as PPROM, the diagnosis of membrane rupture was confirmed during the chart review and required documented presence of pooling and ferning on speculum examination. In order to best isolate cases of spontaneous pPPROM, pregnancies with congenital anomalies, planned postnatal palliation, iatrogenic membrane rupture, rescue cerclage within 14 days, or prelabor rupture of membranes occurring at gestational ages after viability (and including those at term) were excluded. Cases were also excluded if the latency interval between membrane rupture and delivery was less than 24 hours, in keeping with the case definitions used in other published pPPROM literature, as it was difficult to ascertain with certainty from retrospective chart review whether or not these cases represented “true” spontaneous pPPROM versus membrane rupture that occurred during the course of preterm labor.3,4 Final analysis was also restricted to singleton pregnancies to avoid confounding by chorionicity.
Hand-searches of maternal and newborn hospital charts were performed by experienced research personnel, and information regarding maternal demographics, pregnancy and delivery information, and early postnatal outcomes (including neonatal survival) was abstracted using standardized data collection sheets. Post-processing review of stored ultrasound images and fetal assessment reports were also performed to determine the initial amniotic fluid volume following membrane rupture. Amniotic fluid volumes were primarily coded categorically using single deepest vertical pocket (“normal” = single deepest vertical pocket >2 cm; ‘oligohydramnios’ = measureable single deepest vertical pocket <2 cm; or ‘anhydramnios’ = no visible amniotic fluid pocket), and as a continuous variable using single deepest vertical pocket in centimeters. The primary outcome was neonatal survival to hospital discharge following expectant management of pPPROM. Secondary outcomes included: 1) characteristics of patients with pPPROM and between those opting for expectant management versus termination of pregnancy; 2) relationship between initial amniotic fluid volume and pregnancy outcomes; and 3) additional perinatal factors associated with neonatal survival versus perinatal loss.
Statistical analysis was performed using Stata v.14.2 (Stata Corp, College Station, TX, USA) software. Continuous variables were presented as means with standard deviations (SDs) if normally distributed or as medians with interquartile ranges (IQRs) if non-parametrically distributed. Dichotomous and categorical variables were described as proportions. Student’s t-, chi-squared, Kruskal–Wallis, and analysis of variance tests were used to compare outcomes between groups depending on data type and distribution. Regression analyses were performed to evaluate the relationship between amniotic fluid outcome and survival.
Results
A total of 161 potential cases were identified using ICD codes alone, although 48 were excluded upon chart review owing to a combination of factors related to coding errors, incomplete records, and/or membrane rupture occurring after 24 weeks of gestation (Figure 1). The remaining 113 pregnancies were then categorized according to management group (expectant management versus termination). Initially, 88 patients with pPPROM (77.9%) elected to proceed with expectant management while the other 25 (22.1%) opted for termination (Figure 1); however, 14 of those cases choosing expectant management were ultimately excluded because the latency between rupture of membranes and delivery was less than 24 hours: 15.9% (14/88) of women choosing expectant management delivered spontaneously within 24 hours of diagnosis of pPPROM and thus were excluded from the final analysis (Figure 1).
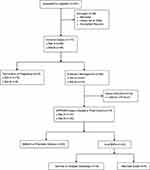 |
Figure 1 Flow diagram of study subjects. Abbreviations: GA, gestational age; ROM, rupture of membranes; pPPROM, previable, preterm prelabor rupture of membranes. |
The median gestational age at rupture of membranes for the cohort was 20+6 weeks [IQR 19+4 to 21+5], and those choosing termination tended to have membrane rupture at earlier gestational ages than those opting for expectant management (p=0.023) (Table 1). The baseline maternal age of women with pPPROM was 29.7 years (SD 6.0) and body mass index (BMI) was 30.1 kg/m2 (SD 6.5). Over half (55%) of patients with pPPROM were urban residents and the remainder of patients were from surrounding areas within the catchment (31.5% rural and 13.5% northern/remote). Most women in the cohort were multiparous (69.9%), with gravidity and parity equal to 4 [IQR 2 to 6] and 1 [IQR 0 to 3] respectively. Amongst those with prior pregnancies, 17.8% had a history of preterm birth (16.4% with one prior preterm delivery and 8.2% with two or more prior preterm deliveries) and 9.6% had a prior pregnancy complicated by stillbirth. Over half of the cohort (56%) had had a prior “abortion”; however, we were limited in our ability to differentiate spontaneous abortions from those that were elective or planned. A trend towards higher rates of pregnancy termination existed at one hospital site (31.7%) compared to the other (15.4%; p=0.070), but there were no obvious demographic differences noted between sites (Supplementary Table S1). When comparing cases within the different management subgroups (expectant vs termination), women choosing expectant management were significantly older (30.4 years vs 27 years; p=0.012) and more tended to reside in urban locations, although this finding was not statistically significant (58.7% vs 40%; p=0.155) (Table 1).
 |
Table 1 Maternal and Pregnancy Characteristics Associated with pPPROM Comparing Management Groups (Expectant versus Termination) |
Within the expectant management group, the median latency between rupture of membranes and delivery was 7 days [IQR 3 to 29] and the median gestational age at delivery was 23+1 weeks [IQR 21+0 to 24+4] (Table 1). Furthermore, 27% of expectantly managed pregnancies delivered over 24 weeks of gestation and 28.4% required induction of labor. The main indications for induction were stillbirth (33.8%), chorioamnionitis (8.1%), and antepartum hemorrhage or abruption (6.8%); however, the prevalence of these individual complications was even higher clinically (Table 2). The prevalence of histologically confirmed chorioamnionitis was significantly higher amongst those patients managed expectantly versus those undergoing termination of pregnancy (58.4% vs 8%; p<0.0001). In over half of patients with histological chorioamnionitis in the expectant management group, there was no clinical suspicion of infection prior to delivery and the only evidence of chorioamnionitis came from placental pathology after birth (Table 2). There were more cases of antepartum hemorrhage and abruption amongst those in the expectant management group (41.9% vs 16%; p=0.022), but no significant difference in prevalence of postpartum hemorrhage or need for blood transfusion. There was no obvious difference in the risk of placenta accreta or maternal sepsis between groups, although the absolute numbers were small (Table 2). There were no maternal deaths in this cohort. The risks of stillbirth and periviable delivery following expectant management were 33.8% and 29.7%, respectively (Table 2). About one-third of newborns in the expectantly managed group reached the neonatal intensive care unit (NICU) (32.4%), with overall neonatal survival to hospital discharge of 25.7%. Amongst those newborns admitted to the NICU, survival to hospital discharge reached 79.2% (Table 2).
 |
Table 2 Maternal Complications and Pregnancy Outcomes Following pPPROM Comparing Management Groups (Expectant versus Termination) |
Of all pregnancies that achieved over 24 hours of latency following pPPROM, only 37.8% received antenatal corticosteroids prior to delivery and 10.8% received tocolytics. There was considerable variation regarding timing of the initial corticosteroid doses: 8% received steroids prior to 23 weeks of gestation; 48% at 23 weeks, 40% at 24 weeks, and the remaining 4% at 25 weeks. Moreover, 39.3% of cases received appropriately timed steroids within 1–7 days of delivery, whereas 12% received only a partial dose and another 6.8% received multiple rescue doses prior to delivery because more than 2 weeks had elapsed since the initial course. Amongst live births admitted to the NICU, all 19 survivors received steroids prior to delivery; all rescue courses were also given within this subgroup and all but one of the neonatal deaths also received steroids.
There were almost no differences in maternal characteristics or perinatal risk factors differentiating neonatal survivors from perinatal losses; however, there were significantly more multiparous women represented amongst the pregnancies resulting in neonatal survival (89.5%) versus those with perinatal deaths (61.8%; p=0.026) (Table 3). While there was a trend towards a later gestational age at membrane rupture amongst survivors (21+5 weeks [IQR 20+1 to 23+3]) compared to perinatal losses (20+4 weeks [IQR 18+4 to 22+4]; p=0.043), much of the improved prognosis was attributable to the significantly prolonged latency amongst survivors (median 30 days vs 5 days for perinatal losses; p<0.0001); consequently, this delay in onset of labor was related to an even later gestational age at delivery for survivors (median gestational age 25+6 weeks vs 22+2 weeks; p<0.0001) and ultimately a significantly higher birth weight as well (p<0.0001) (Table 3). While over half of cases in the expectant management group were complicated by histologically confirmed chorioamnionitis, there was no difference in the frequency of chorioamnionitis between survivors (63.2%) and those ending with perinatal death (50.9%; p=0.357). The main cause of perinatal loss was stillbirth (33.8%). followed by extreme prematurity (29.7%) (Table 2). Amongst newborns resuscitated and admitted to the NICU, 48% had respiratory distress syndrome and 25% had bronchopulmonary dysplasia, 17% had early neonatal sepsis, 17% had limb contractures, and 4% had grade III or IV intraventricular hemorrhage: there were three neonatal deaths from respiratory failure and another resulting as a consequence of a severe intracranial bleed. Overall, 15 of 52 (21.1%) expectantly managed cases of pPPROM with planned postnatal resuscitation were delivered by Cesarean section, with approximately half surviving to hospital discharge (46.7%; 7/15); upon further analysis of mode of delivery by outcome group, Cesarean section was used to deliver 36.8% of neonates that survived and 15.4% of those that died from extreme prematurity or from other complications in the NICU (p=0.052).
 |
Table 3 Perinatal Characteristics Associated with Neonatal Survival versus Perinatal Loss Following Expectant Management of pPPROM |
Most cases of pPPROM were complicated by oligohydramnios (43.2%) or anhydramnios (39.2%), but 17.6% had normal initial amniotic fluid volumes. Survivors were significantly more likely to have normal amniotic fluid volumes at initial ultrasound (52.6%) compared to only 5.6% of those pregnancies ending with a perinatal loss (p<0.0001), whereas there was a higher prevalence of anhydramnios amongst perinatal losses (47.3% vs 15.8%; p=0.0254) (Table 3). While there is a significant association between initial amniotic fluid volume category at time of membrane rupture and neonatal survival, there was no obvious linear relationship when examining single deepest vertical pocket as a continuous variable (p=0.23). For newborns surviving to hospital discharge, three of 19 cases had complete anhydramnios immediately after pPPROM. For those cases resulting in neonatal death after admission to the NICU (n=5), four had oligohydramnios and one had normal amniotic fluid volume; there were no cases of complete anhydramnios in this subgroup (Figure 2). Cases of pPPROM resulting in a previable delivery or intrapartum stillbirth (n=31) were significantly more likely to have oligohydramnios (48.4%) or anhydramnios (45.2%) as opposed to normal amniotic fluid volume (6.5%; p=0.002). None of the pregnancies ending in prelabor stillbirth (n=12) had normal amniotic fluid volume at initial ultrasound; 33.3% had oligohydramnios while 66.7% had complete anhydramnios (Figure 2). Most cases of complete anhydramnios (87%) resulted in stillbirth or periviable loss prior to NICU admission, although notably there were three survivors (13%) in this category as well (Figure 2). Upon further stratification by median membrane rupture of 21 weeks’ gestation, there was no obvious evidence of confounding by gestational age on survival to discharge by amniotic fluid volume: the crude relative risk of neonatal death following anhydramnios compared to those cases with amniotic fluid was 1.33 (0.98–1.78), whereas the adjusted relative risks were 1.35 (95% CI 0.97–1.96) and 1.24 (95% CI 0.75–2.04) for rupture of membranes less than 21 weeks versus 21 weeks of gestation and greater.
Discussion
pPPROM is an uncommon but serious complication of pregnancy and is frequently associated with poor outcomes. However, with one-quarter of newborns surviving to hospital discharge following expectant management, survival in our cohort was much better than traditionally expected and even higher amongst those newborns receiving immediate resuscitation and reaching admission to the NICU. For patients opting for expectant management, the first 24 hours following membrane rupture remain critical, with almost 16% of patients delivering spontaneously within that time period. The strongest predictors of survival were the latency period and, consequently, the gestational age at delivery and related birth weight. However, less is known about the specific predictors of prolonged latency beyond gestational age at rupture of membranes alone. Given that normal amniotic fluid volume was significantly associated with survival (and, conversely, low fluid was associated with perinatal loss), this raises some questions about a potential relationship between amniotic fluid and latency versus some other protective mechanism. There was no obvious confounding by gestational age on risk of neonatal death by amniotic fluid volume category, although the subgroups are small and will need validation in larger, prospective studies. As this was a retrospective cohort study, it is impossible to determine the causal relationship between amniotic fluid volume and latency: is amniotic fluid directly related to latency duration or is it merely a marker of other underlying factors which confer a “protective” effect and correspond to improved outcomes including neonatal survival? While mechanical stretch of the cervix is known to cause hypothalamic stimulation, triggering oxytocin release from the posterior pituitary, which leads to uterine contractions during normal labor,17 it is unclear whether this is also the mechanism by which low amniotic fluid might contribute to a shorter latency and subsequently increased perinatal loss. Irrespective of the exact mechanism between amniotic fluid and labor onset, utilization of fetal ultrasound to measure amniotic fluid volume at the time of rupture may offer some insights into prognosis and pregnancy outcomes following pPPROM. Complete anhydramnios at the initial scan was most often seen amongst those pregnancies ending in stillbirth and may be an independent predictor of perinatal loss following pPPROM, as there were no cases of normal initial amniotic fluid volume in this group. Cases that ended in previable delivery were more likely to have an initial diagnosis of oligohydramnios, whereas more than half of pregnancies with a newborn that survived to hospital discharge had normal amniotic fluid at the initial scan. At a minimum, it should be noted that approximately one in five pregnancies complicated by pPPROM had normal amniotic fluid volumes on the initial scan; this finding highlights the fact that ultrasound should not be used for the diagnosis of membrane rupture, but, rather, may offer some information about prognosis following clinically diagnosed pPPROM.
While infectious morbidity and the potential for pulmonary hypoplasia/respiratory compromise are frequently identified as risks of pPPROM, other complications are less commonly recognized in the management and counseling of these patients and, in some instances, were even more prevalent in our cohort. Approximately one-third of expectantly managed cases ended in stillbirth and another third of cases ended in previable delivery, over 40% were complicated by antepartum hemorrhage/abruption, and 21% required delivery by Cesarean section. However, as this was a retrospective study, there was no way to account for potential confounding by indication of mode of delivery (ie, might Cesarean section have been reserved for only those cases perceived to have better outcomes and withheld from those with a presumed poorer prognosis?); thus, results pertaining to the influence of mode of delivery on survival should be interpreted with caution. Almost half of newborns admitted to NICU had respiratory distress syndrome, but only 25% were diagnosed with bronchopulmonary dysplasia. Limb contractures occurred in 17% of newborns delivered after expectantly managed pPPROM and other neonatal complications were rare. These findings serve as a reminder of the non-respiratory and non-infectious complications of pPPROM and may be used to guide more refined counseling of patients and families experiencing this complication of pregnancy. Over half of pPPROM cases had chorioamnionitis, although the prevalence of histological chorioamnionitis was significantly higher amongst those patients managed expectantly compared to those undergoing termination, thus suggesting that underlying infection is not the cause of all pPPROM but rather a consequence of ascending, later-onset infection. Despite the high index of suspicion regarding risk of infection in pregnancies complicated by pPPROM, only about half of the cases of histological chorioamnionitis were diagnosed as “clinical” chorioamnionitis based on bedside characteristics prior to delivery. While specific information about the individual clinical findings used to diagnose chorioamnionitis clinically was not available as part of this study, this discrepancy raises the suspicion that traditional diagnostic algorithms may need to be modified to improve antenatal detection of chorioamnionitis amongst patients delivering preterm and particularly those giving birth following pPPROM.18,19 This finding also highlights the important role of placental pathology in cases of pPPROM and for consideration by neonatologists in planning early postnatal management due to the risks of early neonatal sepsis. These results will help in alerting patients and healthcare providers to possible complications following pPPROM beyond respiratory morbidity and infection.
Despite a heightened risk of antepartum hemorrhage with expectant management compared to termination, there were no other differences in the risk of maternal medical complications between the different management groups. Specifically, there were no significant differences between postpartum hemorrhage risk or need for blood transfusion following pPPROM, regardless of management choice. Also, even though over half of expectantly managed cases of pPPROM were diagnosed with chorioamnionitis, the prevalence of maternal sepsis was low overall and without any significant difference between groups, including no cases of septic shock or maternal death in the cohort; however, the absolute numbers of complications remained small and warrant further study. There was a striking difference between hospitals regarding patients opting for termination as opposed to expectant management. As the only measurable patient-level difference between management groups and study sites was maternal age, one possible explanation for this observation might relate to differential access to onsite surgical termination services: with higher termination rates at the site with onsite surgical termination available, subtle differences in counseling practices and/or management styles or underlying patient preferences for treatment by their own physician may be potential reasons for this variation in management choice between sites. However, within the relatively small urban center with regional accessibility to these services (ie, patients from one hospital could be referred to an alternate site for surgical termination), it is unclear that this would be the sole explanation for this difference in management trends. There was no difference in outcomes amongst expectantly managed pregnancies between sites. The observed variation in administration and timing of antenatal corticosteroids, as well as usage of tocolytics, amongst expectantly managed cases underscores the considerable variability between healthcare providers in the management of pPPROM, and is consistent with other reported literature.13,14,20,21 While most cases of PPROM would be administered steroids at the time of presentation to hospital with membrane rupture, only one-third of patients with pPPROM in our study received steroids prior to delivery. Acknowledging the inherent challenges of optimal timing of antenatal corticosteroid administration within 1–7 days of preterm delivery, most cases of PPROM are associated with a risk of “stale” steroids (given more than 7 days prior to delivery) as opposed to non-receipt.21–23 This study raises awareness of the variation that exists regarding treatment of pregnancies complicated by pPPROM and the need for future interventional controlled trials to evaluate the optimal management of these pregnancies, including mode and timing of delivery; the potential role of amnioinfusion for improving outcomes specifically in the setting of pPPROM also needs to be explored.24,25
Our study is unique as one of the only studies about pPPROM incorporating initial amniotic fluid volume by single deepest vertical pocket into the risk stratification of pregnancy outcomes in this high-risk group; it is also one of the largest cohorts of outcomes following pPPROM in the published literature. Because single deepest vertical pocket measurements are readily available in most maternity wards with access to bedside ultrasound, use of this measurement could easily be incorporated as part of counseling of patients and their families. We have also brought heightened awareness to the potential perinatal risks of pPPROM beyond infectious and respiratory morbidity. As all high-risk pregnancies of ≤34 weeks’ gestation in the region are delivered at either of the two participating study hospitals, we are fairly confident that this cohort represented close to the entire complement of cases of pPPROM in our population which reached viability; however, we are not able to quantify the number of cases outside our catchment area that either underwent a termination prior to referral or were managed expectantly but never reached viability and delivered elsewhere. It should also be noted that the abstracter was blinded to the neonatal outcomes at the time of collection of the antenatal and obstetric variables, as well as initial amniotic fluid volumes, which should mitigate risks of observer bias. Another potential limitation was restriction of the study period to 2015 and earlier because it coincided with changes to local practice patterns which saw a shift in the definition of “viability” from 24 weeks of gestation down to 23 weeks that same year, and may have otherwise confounded study results. For this reason, studies are still needed to determine outcomes for births at extreme prematurity (around 22–23 weeks’ gestation) following pPPROM. This study was also not designed to evaluate the utility of the amniotic fluid index in this population or the influence of amniotic fluid reaccumulation on the impact of neonatal survival to discharge. Future studies are still needed to evaluate the influence of mode of delivery on survival and the long-term health and neurodevelopmental outcomes of survivors following pPPROM, particularly as modified by amniotic fluid volumes.
Conclusion
More than one in four pregnancies complicated by pPPROM and managed expectantly resulted in neonatal survival to hospital discharge. Gestational age at time of rupture and subsequent latency were most strongly correlated with outcome; however, there was also a non-linear association between initial amniotic fluid volume and survival, with more than half of survivors having normal amniotic fluid following membrane rupture and most cases of prelabor stillbirth having complete anhydramnios at the initial scan. These results can be used to better refine prognostication and counseling of similar patients opting for expectant management following this high-risk pregnancy complication.
Acknowledgments
Thank you to the staff in health records for supporting this project.
Disclosure
Dr Christy Pylypjuk reports receiving grants from Manitoba Medical Services Foundation and Children's Hospital Research Institute of Manitoba, and the Winnipeg Foundation Martha Donovan Women’s Leadership Award, as well as transportation and lodging to speak at the annual conference of the Society of Obstetricians and Gynecologists of Canada (no direct payments received), outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Rouzaire M, Corvaisier M, Roumeau V, et al. Predictors of short latency period exceeding 48 h after preterm premature rupture of membranes. J Clin Med. 2021;10(1):150. doi:10.3390/jcm10010150.
2. Dotters-Katz S. Antibiotics for prophylaxis in the setting of preterm prelabor rupture of membranes. Obstet Gynecol Clin North Am. 2020;47(4):595–603.
3. Van der Marel I, de Jonge R, Duvekot J, et al. Maternal and neonatal outcomes of preterm premature rupture of membranes before viability. Klin Padiatr. 2016;228(2):69–76.
4. Linehan LA, Walsh J, Morris A, et al. Neonatal and maternal outcomes following midtrimester preterm premature rupture of the membranes: a retrospective cohort study. BMC Pregnancy Childbirth. 2016;16:26. doi:10.1186/s12884-016-0813-3.
5. Margato MF, Martins GLP, Junior RP, Nomura ML. Previable preterm rupture of membranes: gestational and neonatal outcomes. Arch Gynecol Obstet. 2012;285(6):1529–1534.
6. Gafner M, Borovich A, Gimpel A, et al. Risk factors and maternal outcomes following preterm premature rupture of membrane in the second trimester of gestation. Arch Gynecol Obstet. 2020;301(5):1207–1212.
7. Sorano S, Fukuoka M, Kawakami K, Momohara Y. Prognosis of preterm premature rupture of membranes between 20 and 24 weeks of gestation: a retrospective cohort study. Eur J Obstet Gynecol Reprod Biol. 2019;5:1001002. doi:10.1016/j/eurox.2019.100102.
8. Sim WH, Araujo junior E, Da Silva Costa F, Sheehan PM. Maternal and neonatal outcomes following expectant management of preterm prelabour rupture of membranes before viability. J Perinat Med. 2017;45(1):29–44.
9. Yeast JD. Preterm premature rupture of membranes before viability. Clin Perinatol. 2001;28(4):849–860.
10. Nakamura E, Matsunga S, Ono Y. Risk factors for neonatal bronchopulmonary dysplasia in extremely preterm premature rupture of membranes: a retrospective study. BMC Pregnancy Childbirth. 2020;20(1):
11. Gibson KS, Brackney K. Periviable premature rupture of membranes. Obstet Gynecol Clin North Am. 2020;47(4):633–651.
12. Mercer BM, Miodovnik M, Thurnau GR, et al. Antibiotic therapy for reduction in infant morbidity after preterm premature rupture of the membranes: a randomized controlled trial. JAMA. 1997;278(12):989–995.
13. Waters TP, Mercer BM. The management of preterm premature rupture of the membranes near the limit of fetal viability. Am J Obstet Gynecol. 2009;201(3):230–240.
14. Mercer BM. Is there a role for tocolytic therapy during conservative management of preterm premature rupture of the membranes? Clin Obstet Gynecol. 2007;50(2):487–496.
15. Peaceman AM, Lai Y, Rouse DJ, et al. Length of latency with preterm premature rupture of membranes before 32 weeks’ gestation. J Perinatol. 2015;32(1):57–62.
16. Gunay T, Erdem G, Bilir RA, et al. The association of the amniotic fluid index (AFI) with perinatal fetal and maternal outcomes in pregnancies complicated by preterm premature rupture of membranes (PPROM). Ginekol Pol. 2020;91(8):465–472.
17. Gibb W, Challis JRG. Mechanisms of term and preterm birth. J Obstet Gynaecol Can. 2002;24(11):873–883.
18. Denoble AE, Wu J, Mitchell CJ, et al. Chorioamnionitis versus intraamniotic infection among preterm deliveries – is postpartum infectious morbidity different? Am J Obstet Gynecol. 2020;2(3):100176. doi:10.1016/j.ajogmf.2020.100176.
19. Sahni M, Franco-Fuenmayor ME, Shattuck K. Management of late preterm and term neonates exposed to maternal chorioamnionitis. BMC Pediatr. 2019;282:19. doi:10.1186/s12887-019-1650-0
20. Ramsey PS, Nuthalapaty FS, Lu G, et al. Contemporary management of preterm premature rupture of membranes (PPROM): a survey of maternal-fetal medicine providers. Am J Obstet Gynecol. 2004;191(4):497–502.
21. Battarbee A. Use of antenatal corticosteroids in preterm prelabor rupture of membranes. BMC Preg Childbirth. 2020;47(4):587–594.
22. Vermillion ST, Soper DE, Bland ML, Newman RB. Effectiveness of antenatal corticosteroid administration after preterm premature rupture of the membranes. Am J Obstet Gynecol. 2000;183(4):925–929.
23. Stoll BJ, Hansen NI, Bell EF, et al. Trends in care practices, morbidity, and mortality of extremely preterm neonates, 1993-2012. JAMA. 2015;314(10):1039–1051.
24. Miyazaki K, Furuhashi M, Yoshida K, Ishikawa K. Aggressive intervention of previable preterm premature rupture of membranes. Acta Obstet Gynecol Scand. 2012;91(8):923–929.
25. Kozinszky Z, Sikovanyecz J, Pasztor N. Severe midtrimeter oligohydramnios: treatment strategies. Curr Opin Obstet Gynecol. 2014;26(2):67–76.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

