Back to Journals » Clinical Ophthalmology » Volume 16
Performance of a Translucent Activator for LipiFlow Vectored Thermal Pulse (VTP) Treatment of Meibomian Gland Dysfunction
Authors Hu JG, Dang VT, Chang DH , Goldberg DF, McKinnon C , Makedonsky K, Laron M , Ji L
Received 18 December 2021
Accepted for publication 14 March 2022
Published 30 March 2022 Volume 2022:16 Pages 963—971
DOI https://doi.org/10.2147/OPTH.S354738
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Jerry G Hu,1 Vin T Dang,2 Daniel H Chang,2 Damien F Goldberg,3 Cheryl McKinnon,4 Katherine Makedonsky,4 Michal Laron,4 Leilei Ji5
1Texas Eye & Laser Center, P.A., Hurst, TX, USA; 2Empire Eye and Laser Center, Bakersfield, CA, USA; 3Wolstan & Goldberg Eye Associates, Torrance, CA, USA; 4Johnson & Johnson Surgical Vision, Inc., Milpitas, CA, USA; 5Johnson & Johnson Surgical Vision, Inc., Irvine, CA, USA
Correspondence: Cheryl McKinnon, Johnson & Johnson Surgical Vision, Inc., 510 Cottonwood Drive, Milpitas, CA, 95035, USA, Tel +1 408 273 4014, Email [email protected]
Purpose: Investigator feedback was used to assess the clinical use of the LipiFlow® System with the new translucent Activator Clear to successfully complete LipiFlow® treatments.
Patients and Methods: This was a prospective, open-label clinical investigation. A total of 88 eyes (44 subjects) were treated using the LipiFlow® System with the new Activator Clear. Subjects diagnosed with bilateral meibomian gland dysfunction (MGD) were enrolled in the study. Each investigator performed a complete LipiFlow® treatment with the translucent Activator on both eyes of each subject. Investigators completed a questionnaire assessing the clinical use of the Activator Clear on a 5-point scale (1 – very difficult or strongly disagree, 3 – neutral, 5 – very easy or strongly agree).
Results: The new translucent Activator provided successful LipiFlow® treatments in 100% of cases, with 95% confidence interval of (96%, 100%). Additionally, the investigators agreed or strongly agreed that the translucent components of the Activator Clear made it easy to access and position the activator with confidence on the subject’s eye.
Conclusion: The overall investigators’ impressions on usage and functionality of LipiFlow® System with the translucent Activator were very positive. The Activator Clear enables doctors with efficient and confident positioning around patient eyelids to ensure successful LipiFlow® treatment when used as indicated.
Keywords: LipiFlow® vectored thermal pulsation, Activator Clear
Introduction
Meibomian gland dysfunction (MGD) describes a chronic, diffuse abnormality of the meibomian glands, commonly characterized by terminal duct obstruction and/or qualitative/quantitative changes in the glandular secretion. It may result in alteration of the tear film, symptoms of eye irritation, clinically apparent inflammation, and ocular surface disease.1 The goal of MGD treatment is to improve meibomian gland function in order to restore a normal lipid layer of the tear film.2
The LipiFlow® Thermal Pulsation System is a commercially available medical device indicated for the application of localized heat and pressure therapy in adult patients with chronic cystic conditions of the eyelids, including meibomian gland dysfunction, also known as evaporative dry eye or lipid-deficiency dry eye. The LipiFlow® is used by a physician or technician in an in-office procedure to provide controlled heat to the inner eyelid surface and intermittent pressure to the outer eyelid to facilitate release of meibum from the cystic glands for 12 minutes. The device consists of a console, reusable cable, and a dome-shaped, single-use sterile device known as the Activator. The Activator cradles the upper and lower eyelids and applies regulated heat and pressure through an inflatable air bladder.3
There is a large body of evidence supported by existing well-controlled case studies,4–7 controlled8–16 and non-controlled clinical studies17–21 that demonstrate the efficacy, safety and clinical utility of the LipiFlow® system using VTP for the treatment of MGD.10,11,13–16,22–24 In addition, several studies have reviewed the different techniques for managing and treating MGD.22–28 This study evaluated the investigator’s experience in using the newly designed Activator model LFD-2100, ie, the Activator Clear with the unique property of being translucent.
The Activator (Disposable) consists of a combined eye cup and lid warmer with attached tubing and electrical wiring that connect to the Console with a connector. The connector plugs into the Console. The eye cup contacts the outer surface of the eyelids. The eye cup has a soft, flexible bladder that intermittently inflates with air to provide controlled massage (rolling motion) of the eyelids. The eye cup is composed of a plastic substrate that is over-molded with silicone in the patient contact area. The eye cup is latex free. The eye cup has attached air tubing that connects to the Console. The lid warmer contacts the inner eyelid surface. Within the lid warmer is a heater to provide controlled, outward directional (away from the eye) heat therapy to the eyelids. The lid warmer has a smooth surface and edges, where the circumference rests lightly on the conjunctiva of the eye. The lid warmer shape vaults above the eye surface to prevent corneal contact. The lid warmer has an integrated insulator to shield the eye from the heat and redundant temperature sensors to ensure precise control of the temperature. The lid warmer has attached electrical wiring that connects to the Console.
The current on-market Activator model has been modified to a newly designed model (LFD-2100) referred to as Activator Clear. The main clinical efficacy intended use of regulated heat and pressure delivery to the eyelid remained unchanged. The changes made included: integrate O-rings and updated air port geometry and non-patient contacting geometries to improve shut-offs during molding, and a reusable cable that reduces waste. There have been no changes to the two bladder designs between the original and the Activator Clear; no lid warmer or packaging changes were made. However, the main change is the translucent versus opaque bladder design between the two Activators. Activator Clear uses a similar material within the polycarbonate family to the previous iteration except that it is translucent as opposed to an opaque gray color. The change in polycarbonate material makes it less brittle and being translucent, makes it easier to handle, which helps with better Activator positioning than the previous iteration of Activator. The risk assessment supports that the use of the LipiFlow® with the Activator Clear in this study is non-significant risk because the system is designed to control the application of heat and pressure within a safe range and time. The use of the Activator Clear does not change the overall residual risk of the device or introduce new risks to the patient and/or user, as the design specifications of the Activator between the iterations remained unchanged, and that the main clinical efficacy intended use of regulated heat and pressure delivery to the eyelid remained unchanged.
The purpose of this study was to evaluate the clinical use of the LipiFlow® System with the Activator Clear to complete LipiFlow® treatments in patients. Additionally, the investigators rated the ease of use of the Activator Clear. Finally, the data from the treatment reports generated by the LipiFlow® console and the investigator questionnaire were used to assess the clinical utility of the Activator Clear.
Materials and Methods
Study Design and Enrollment Criteria
This prospective, open-label, multi-center clinical study was designed to evaluate the clinical use of the LipiFlow® System with the Activator Clear to successfully complete LipiFlow® treatments. Additionally, the five investigators rated the ease of use of the LipiFlow® system with the Activator Clear. Finally, the data from the treatment reports generated by the LipiFlow® console and from the investigator questionnaire was used to assess the clinical utility of the Activator Clear. The term investigator used in this study can refer to either ophthalmologists, optometrists, or technicians who are experienced in LipiFlow® treatment with Activator LFD-2000 (current version).
A total of 88 eyes (44 patients) were enrolled and treated at 3 sites in the USA. All patients were provided with written informed consent before enrollment in the trial and the conduct of any trial-related procedures. Institutional Review Board (IRB) approval was obtained from Salus IRB and was conducted in accordance with the Declaration of Helsinki. This study is registered at clinicaltrials.gov (identifier: NCT04500821).
The main inclusion criteria were at least 22 years of age at the time of consent, diagnosed with bilateral MGD prior to the study visit and documented as such in the medical, or had evidence of MGD in both eyes at the study visit. Exclusion criteria included prior eye surgery or trauma, medical conditions that have been identified as contraindications and precautions of the LipiFlow® System, and active ophthalmic disease or abnormality. Conditions that could result in reduced treatment effectiveness and require other medical management included moderate to severe allergic, vernal or giant papillary conjunctivitis; eyelid inflammation or infection; systemic disease conditions associated with dry eye; systemic medications known to cause dryness; and esthetic eyelid and eyelash procedures.
The entire exam and treatment were approximately 1–2 hours long. Pre-treatment assessments included slit lamp evaluation, non-dilated fundus exam, ocular surface staining, and optional meibomian gland assessment (at the investigator’s discretion). LipiFlow® treatment with the Activator Clear was performed on both eyes. The investigators completed a questionnaire regarding the clinical use of the Activator Clear following each bilateral procedure. The treatment reports automatically generated by the LipiFlow® Console after completion of each treatment were printed and collected. The reports presented confirmation of treatment completion and treatment information such as the changes in pressure and temperature during the treatment. A post-treatment slit lamp evaluation and ocular surface staining were performed.
Corneal and conjunctival staining was evaluated by slit-lamp examination based on the Report of the National Eye Institute/Industry Workshop on Clinical Trials in Dry Eye.29 On this scale, 0 = normal-no staining, 1 = mild-superficial stippling/micropunctate staining, 2 = moderate-macropunctate staining with some coalescent areas, and 3 = severe-numerous coalescent macropunctate areas and/or patches with a total corneal staining grade range from 0 to 15; and conjunctival staining grade range from 0 to 18.
Statistical Analysis
The confidence interval approach was used to estimate precision around the actual proportion of successful completion of LipiFlow® treatment with the Activator Clear. For example, assuming the proportion of successful completion of LipiFlow treatment is 95%, with n = 50 eyes, a two-sided 95% confidence interval will be (89%, 100%), i.e., a precision of 6.0%.
Results
Demographics and Baseline Characteristics
Patient characteristics and baseline measurements are found in Table 1. A total of 88 eyes from 44 patients (13 males, 31 females) had LipiFlow® treatment with the Activator Clear. The mean age was 67.0 years (SD 7.9; range 45 to 85). Most subjects were of Caucasian race (95.5%).
 |
Table 1 Demographic and Baseline Characteristics |
The successful completion of LipiFlow® treatment with the Activator Clear is summarized in Table 2 for all eyes tested. In 100% of cases (95% confidence interval of [96%, 100%]), the investigator successfully completed LipiFlow® treatment with the Activator Clear exceeding the target of 95% successfully treated. Additionally, feedback on the Activator Clear was very positive. The investigators agreed in 100% of cases and strongly agreed in at least 95.5% of cases that the translucent components of the Activator Clear made it (1) easy to confirm the right position, (2) efficient to position, (3) provide confidence in the position, and (4) easy to assess the position.
 |
Table 2 Questionnaire Items - LipiFlow Treatment with Activator CLEAR |
An investigator questionnaire was administered at each step during the LipiFlow® treatment procedure with Activator Clear. Steps consisted of disinfecting cables, connecting Activators to cables, passing self-test, inspecting and placing in patient’s eyes, stabilizing the Activators, running LipiFlow® Treatment with Activator, disconnecting Activators from patient’s eyes, and disconnecting Activators from the cables. The results for mean rating of the questionnaire by investigators on a 1 to 5 scale (where 1 refers to a negative rating, 3 being neutral, and 5 to a very positive rating) are reported in Table 3. In most cases, the investigator’s mean rating at each step in performing the procedures was high (5) and a lowest mean rating of 4.9, confirming a positive experience by the investigators at each step with the Activator Clear.
 |
Table 3 Summary of LipiFlow Treatment with Activator CLEAR Questionnaire Rating Scores |
A rating of 3 or lower occurred in 8 cases. A neutral rating (3) was given in six of these cases and was related to the rating of ease of fit in placing or removing the Activator in the subject’s eyes. Of these, four cases were related to difficulty inserting the device and securing the Activator into a proper and stable position (related to eye anatomy or patient squinting); two cases were related to difficulty removing the device. An additional two cases were rated a two (moderately difficult) for ease of completing the treatment. A system error was reported because there was no connection between the cable and the Activator. This was resolved with a new cable. Overall, there were two cases where the Activator did not pass the self-test (yes/no response) due to a cord connection error. Once the Activator was replaced, it passed the self-test, and treatment was completed.
The investigators assessed the corneal and conjunctival staining after the instillation of fluorescein dye in the eye pre-treatment and post-treatment (Tables 4 and 5). The mean sum of corneal staining scores across 5 corneal regions was low (less than 4.0 on a scale from 0 to 15). The mean sum of conjunctival staining scores across 6 conjunctival regions was also low (4.3 on a scale from 0 to 18). Both corneal and conjunctival staining scores showed a statistically significant mean increase of 0.9 from pre-treatment to immediately post-treatment. While the differences in staining scores were statistically significant for corneal staining (p < 0.0001) and conjunctival staining (p = 0.002), these differences (0.9, on a scale of 0–15 for corneal staining and 0–18 for conjunctival staining) are not considered clinically significant.
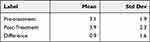 |
Table 4 Ocular Surface Staining – Cornea (N=88 Eyes); Staining Scale 0–15 |
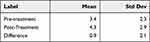 |
Table 5 Ocular Surface Staining – Conjunctiva (N=88 Eyes); Staining Scale 0–18 |
The most frequently reported associated conditions identified at the slit lamp were blepharitis (36.4%), and dry eye related findings such as superficial punctate keratitis (53.4%). Additionally, 6.8% of patients had conjunctival injection (mainly trace) which increased immediately post treatment to 20.5%. This increase in conjunctival injection most likely reflects transient irritation from the treatment and associate topical anesthetic drops. There were no device-related adverse events or safety-related concerns in the study.
Discussion
The goal of this study was to evaluate the investigators’ experience in using the LipiFlow® system with the Activator Clear to successfully complete LipiFlow® treatments. This was achieved in all study cases; the investigator successfully completed LipiFlow® treatment with the translucent Activator in 100% of cases.
Additionally, the investigators agreed in all cases and strongly agreed in at least 95.5% of cases that the translucent components of the Activator Clear made it easy to place the Activator efficiently, correctly, and with confidence on the patient’s eye.
For each step in the procedure on usage and functionality of the LipiFlow® System with the Activator Clear the overall investigators ratings were very favorable. Investigators rated as moderately easy or very easy during the LipiFlow® treatments with mean ratings ranging between 4.9 and 5.0.
Many clinical studies have demonstrated the safety, effectiveness, and clinical utility of the LipiFlow® system with the previous model of Activator over several years.9–11,13,14,18,20,21,30,31 To our knowledge, the present study is the first to gather feedback from investigators on the performance of the activator used in the treatment of MGD, which had both a positive impact and desirable outcomes. The translucent Activator provides benefits to both patients and clinicians, perhaps most significantly, this feature enhances clinician confidence in positioning and applying the device onto the lids. The clinicians successfully completed LipiFlow® treatment in 100% of cases providing evidence of the usability in positioning it and successfully performing the treatment on every attempt.
Corneal staining was significantly increased immediately after treatment but this increase was not deemed clinically significant. The increase in staining is likely due to the instrument’s contact with the eye and adnexa. Importantly, however, no epithelial abrasions were reported following the use of either device.9,13,14 An increase in staining scores immediately after treatment has been observed in similar procedures, such as the iLUX system,14 and this is not an unexpected finding.
No patient experienced any device-related adverse events. The most common slit-lamp findings were meibomian gland dysfunction, blepharitis, dry eye conditions/SPK, and conjunctival injection observed both pre- and post-treatment. The absence of device-related adverse events in this study further confirms the low-risk safety profile of the LipiFlow® System, as demonstrated in prior studies.9,13,30 In addition, slit lamp findings observed immediately after vector thermal pulsation (VTP) treatment required no medical treatment and were consistent with prior studies.9,13
The limitations of this study were related to its non-comparative open-label design. There was no control group using a previous version of the Activator; both subjects and investigators were not masked. Compared to the previous Activator model, no changes pertaining to device efficacy or the primary clinical intended use of regulated heat and pressure delivery to the eyelid were altered in the Activator Clear. Therefore, there is no reason to believe that the results would be any different if there was a control arm. The use of the newly designed Activator Clear does not change the overall residual risk of the device or introduce new risks to the patient and/or user compared to the previous Activator model. Use of the LipiFlow® System with the translucent Activator in subjects with the more complex conditions (ie, moderate to severe allergic, vernal or giant papillary conjunctivitis; eyelid inflammation or infection; systemic disease conditions associated with dry eye; systemic medications known to cause dryness; and esthetic eyelid and eyelash procedures) were excluded. Including complex conditions would provide additional data regarding the efficiency of the procedure, and possibly open the door for a future study.
As this was an open-label study, investigator bias may have affected their responses and the study outcomes. However, due to the nature of the study, masking was not possible. In order to mitigate the possibility of the investigator bias, the study was performed at 3 different sites with 5 investigators. The results are approximately evenly distributed across the sites. The clinical use of the LipiFlow® system with Activator Clear has demonstrated successful LipiFlow® treatment completion.
Conclusion
The newly designed translucent Activator Clear facilitates efficient and confident Activator placement and positioning around patient eyelids to ensure optimal vectored thermal pulsation treatment when used according to the indications for use.
Effectiveness results from this study are comparable to other heat treatments/eye massages. The absence of device-related adverse events in this study confirms the low-risk safety profile of the LipiFlow® System, as demonstrated in prior studies. In addition, slit lamp findings observed immediately after VTP treatment were transient and required no medical treatment.
Data Sharing Statement
Data are available upon reasonable request. The authors do not intend to share individual deidentified participant data. A summarized report with end point data tables based on statistical plan and analysis may be requested directly from the corresponding author for consideration. Access to anonymized data may be granted following review. Content with granted access will be available through email or other appropriate formats and for 3 months, upon review and consideration.
Acknowledgments
This study was funded by Johnson & Johnson, Inc. This study was presented in part as a poster at the American Academy of Optometry in November 2021 in Boston, USA. All authors contributed equally to the clinical trial.
Disclosure
Vin T Dang reports honoraria for consulting work from Johnson & Johnson Vision, outside the submitted work. Daniel H Chang reports personal fees, non-financial support for consultant, speaker, travel support, and grant support from Johnson & Johnson Vision, outside the submitted work. Cheryl McKinnon, Katherine Makedonsky, Michal Laron and Leilei Ji are employees of Johnson & Johnson, Inc. The other authors report no conflicts of interest in this work.
References
1. Nichols K, Foulks G, Bron A, et al. The international workshop on meibomian gland dysfunction: executive summary. Invest Ophthalmol Vis Sci. 2011;52:1922–1929. doi:10.1167/iovs.10-6997a
2. Qiao J, Yan X. Emerging treatment options for meibomian gland dysfunction. Clin Ophthalmol. 2013;7:1797–1803. doi:10.2147/OPTH.S33182
3. Johnson & Johnson Vision Care, Inc. and Johnson & Johnson Surgical Vision, Inc; 2021. TearScience® LipiFlow® Thermal Pulsation System. Available from: https://www.jnjvisionpro.com/products/eye-medical-devices/lipiflow-treatment.
4. Godin M, Stinnett S, Gupta P. Outcomes of thermal pulsation treatment for dry eye syndrome in patients with Sjogren disease. Cornea. 2018;37:1155–1158. doi:10.1097/ICO.0000000000001621
5. Kenrick C, Alloo S. The limitation of applying heat to the external lid surface: a case of recalcitrant meibomian gland dysfunction. Case Rep Ophthalmol. 2017;8:7–12. doi:10.1159/000455087
6. Korb D, Blackie C. Case report: a successful LipiFlow treatment of a single case of meibomian gland dysfunction and dropout. Eye Contact Lens. 2013;39:e1–3. doi:10.1097/ICL.0b013e31824ccbda
7. Schallhorn C, Schallhorn J, Hannan S, et al. Effectiveness of an eyelid thermal pulsation procedure to treat recalcitrant dry eye symptoms after laser vision correction. J Refract Surg. 2017;33:30–36. doi:10.3928/1081597X-20161006-05
8. Blackie C, Coleman C, Nichols K, et al. A single vectored thermal pulsation treatment for meibomian gland dysfunction increases mean comfortable contact lens wearing time by approximately 4 hours per day. Clin Ophthalmol. 2018;12:169–183. doi:10.2147/OPTH.S153297
9. Blackie CA, Coleman CA, Holland E. The sustained effect (12 months) of a single-dose vectored thermal pulsation procedure for meibomian gland dysfunction and evaporative dry eye. Clin Ophthalmol. 2016;10:1385–1396. doi:10.2147/OPTH.S109663
10. Finis D, Hayajneh J, König C, et al. Evaluation of an automated thermodynamic treatment (Lipiflow®) system for meibomian gland dysfunction: a prospective, randomized, observer-masked trial. Ocul Surf. 2014;12:146–154. doi:10.1016/j.jtos.2013.12.001
11. Hagen KB, Bedi R, Blackie CA, et al. Comparison of a single-dose vectored thermal pulsation procedure with a 3-month course of daily oral doxycycline for moderate-to-severe meibomian gland dysfunction. Clin Ophthalmol. 2018;12:161–168. doi:10.2147/OPTH.S150433
12. Kasetsuwan N, Suwajanakorn D, Tantipat C, et al. The efficacy between conventional lid hygiene and additional thermal pulsatile system in meibomian gland dysfunction patients treated with long-term anti-glaucoma medications in a randomized controlled trial. Clin Ophthalmol. 2020;14:2891–2902. doi:10.2147/OPTH.S259692
13. Lane SS, DuBiner HB, Epstein RJ, et al. A new system, the LipiFlow, for the treatment of meibomian gland dysfunction. Cornea. 2012;31:396–404. doi:10.1097/ICO.0b013e318239aaea
14. Tauber J, Owen J, Bloomenstein M, et al. Comparison of the iLUX and the lipiflow for the treatment of meibomian gland dysfunction and symptoms: a randomized clinical trial. Clin Ophthalmol. 2020;14:405–418. doi:10.2147/OPTH.S234008
15. Zhao Y, Veerappan A, Yeo S, et al. Clinical trial of thermal pulsation (LipiFlow) in meibomian gland dysfunction with pretreatment meibography. Eye Contact Lens. 2016;42:339–346. doi:10.1097/ICL.0000000000000228
16. Zhao Y, Xie J, Li J. Evaluation of monocular treatment for meibomian gland dysfunction with an automated thermodynamic system in elderly Chinese patients: a contralateral eye study. J Ophthalmol. 2016;27:1–8.
17. Chan A, Chuang J, Wong V. Evaluation of meibomian gland dysfunction among ophthalmic healthcare workers. Clin Ophthalmol. 2021;15:1201–1206. doi:10.2147/OPTH.S299338
18. Finis D, König C, Hayajneh J, et al. Six-month effects of a thermodynamic treatment for MGD and implications of meibomian gland atrophy. Cornea. 2014;33:1265–1270. doi:10.1097/ICO.0000000000000273
19. Greiner J. A single LipiFlow® Thermal Pulsation System treatment improves meibomian gland function and reduces dry eye symptoms for 9 months. Curr Eye Res. 2012;37:272–278. doi:10.3109/02713683.2011.631721
20. Greiner J. Long-term (3 year) effects of a single thermal pulsation system treatment on meibomian gland function and dry eye symptoms. Eye Contact Lens. 2016;42:99–107. doi:10.1097/ICL.0000000000000166
21. Kim M, Stinnett S, Gupta P. Effect of thermal pulsation treatment on tear film parameters in dry eye disease patients. Clin Ophthalmol. 2017;11:883–886. doi:10.2147/OPTH.S136203
22. O’Neil E, Henderson M, Massaro-Giordano M, et al. Advances in dry eye disease treatment. Curr Opin Ophthalmol. 2019;30:166–178. doi:10.1097/ICU.0000000000000569
23. Geerling G, Baudouin C, Aragona P, et al. Emerging strategies for the diagnosis and treatment of meibomian gland dysfunction: proceedings of the OCEAN group meeting. Ocul Surf. 2017;15:179–192.
24. Pang S, Chen Y, Tam K, et al. Efficacy of vectored thermal pulsation and warm compress treatments in meibomian gland dysfunction: a meta-analysis of randomized controlled trials. Cornea. 2019;38:690–697. doi:10.1097/ICO.0000000000001907
25. Arita R, Fukuoka S. Non-pharmaceutical treatment options for meibomian gland dysfunction. Clin Exp Optom. 2020;103:742–755. doi:10.1111/cxo.13035
26. Mandal P, Khan M, Shah S. Drugs - Do we need them? Applications of non-pharmaceutical therapy in anterior eye disease: a review. Cont Lens Anterior Eye. 2017;40:360–366. doi:10.1016/j.clae.2017.09.001
27. Sabeti S, Kheirkhah A, Yin J, et al. Management of meibomian gland dysfunction: a review. Surv Ophthalmol. 2020;65:205–217. doi:10.1016/j.survophthal.2019.08.007
28. Villani E, Marelli L, Dellavalle A, et al. Latest evidences on meibomian gland dysfunction diagnosis and management. Ocul Surf. 2020;18:871–892. doi:10.1016/j.jtos.2020.09.001
29. Lemp M. Report of the National Eye Institute/industry workshop on clinical trials in dry eyes. CLAO J. 1995;21:221–232.
30. Blackie C, Carlson A, Korb D. Treatment for meibomian gland dysfunction and dry eye symptoms with a single-dose vectored thermal pulsation: a review. Curr Opin Ophthalmol. 2015;26:306–313. doi:10.1097/ICU.0000000000000165
31. Hovanesian J, Bullimore M, Tauber J, et al. Comparison of the iLUX and the LipiFlow for the treatment of meibomian gland dysfunction and symptoms: a randomized clinical trial. Clin Ophthalmol. 2020;14:405–418.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
