Back to Journals » Clinical Interventions in Aging » Volume 13
Percutaneous kyphoplasty combined with zoledronic acid infusion in the treatment of osteoporotic thoracolumbar fractures in the elderly
Authors Shi C, Zhang M, Cheng AY, Huang ZF
Received 20 July 2017
Accepted for publication 22 February 2018
Published 4 May 2018 Volume 2018:13 Pages 853—861
DOI https://doi.org/10.2147/CIA.S146871
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Zhi-Ying Wu
Chen Shi,1,* Mi Zhang,2,* An-Yuan Cheng,1 Zi-Feng Huang1
1Department of Trauma Surgery, Wuhan No 1 Hospital, Wuhan, China; 2Department of Orthopedics, Wuhan No 5 Hospital, Wuhan, China
*These authors contributed equally to this work
Objective: We studied the efficacy of zoledronic acid (ZOL) infusion on radiographic and clinical outcomes after percutaneous kyphoplasty (PKP) for elderly patients with osteoporotic thoracolumbar fractures (osteoporotic vertebral compression fractures [OVCFs]).
Materials and methods: We retrospectively analyzed 95 elderly patients (age >65 years) with OVCF. All patients were followed up for 2 years. Thirty-two patients were treated with only once-yearly 5 mg ZOL infusion (ZOL group), 34 patients with only PKP (PKP group) and 29 patients received ZOL infusion 3 days after PKP (PKP+ZOL group).
Results: There were no significant differences in the patients’ age, gender, body mass index, lumbar spine bone mineral density T-scores, baseline of Visual Analog Scale scores and Oswestry Disability Index scores (P>0.05). The postoperative vertebral heights of patients with OVCF after PKP and PKP+ZOL were 23.70±3.03 and 24.30±3.13 mm, respectively, which were significantly higher than that of patients in ZOL group (P<0.05). The reduction in degrees of kyphotic deformity in the PKP and PKP+ZOL groups were corrected to 8.4° and 8.7°. The bone mineral density T-scores of patients with OVCF in the ZOL group and PKP+ZOL group were significantly higher than that in the PKP group (P<0.05). The Visual Analog Scale and the Oswestry Disability Index scores of the PKP+ZOL and PKP groups were significantly lower than those of the ZOL group (P<0.05). The incidence of recompression vertebral fracture (RVF) in the PKP group was 14.7%, but there was no patient with RVF in the PKP+ZOL group (P<0.05).
Conclusion: Once-yearly 5 mg ZOL infusion combined with PKP could provide beneficial effects in elderly osteoporotic patients with OVCF.
Keywords: zoledronic acid, percutaneous kyphoplasty, osteoporotic thoracolumbar fractures, elderly, clinical outcomes, osteoporosis
Introduction
Osteoporosis is a systemic disease which can lead to increased bone fragility and a propensity to fracture.1 According to statistics, there are ~144 million Chinese people over the age of 65 and the mean prevalence of osteoporosis among this population is estimated at 15.7%.2 The most severe complication of osteoporosis is osteoporotic fracture, and the osteoporotic compression fracture in the thoracic or lumbar spine (osteoporotic vertebral compression fractures [OVCFs]) is the most common type accounting for almost 50% of the osteoporotic fractures.3 However, many patients with OVCFs lack significant clinical symptoms, including back pain, height loss and kyphosis, and only 30%–40% patients with OVCFs seek medical assistance.4 Thus, a large number of patients with OVCFs suffer from serious clinical consequences, such as chronic disabling pain, spinal kyphosis, psychological disorders, decreased quality of life (QoL), loss of mobility and even death.5
Traditionally, the conservative therapy for OVCFs, including bed rest, pain medication, anti-osteoporosis drugs, physical therapy and bracing, can relieve pain during the natural healing process of fractures.6 However, it is not appropriate for elderly patients due to high risk of hypostatic pneumonia, decubitus ulcers and venous thromboembolism.7 Since the late 1980s, percutaneous vertebroplasty (PVP) and percutaneous kyphoplasty (PKP) have gradually become the most popular treatments of OVCFs for quickly relieving pain and achieving spinal stabilization.8 PKP, firstly reported by Garfin et al in 1998, was introduced to correct the spinal kyphotic deformity and restore the vertebral height via balloon expansion.9 PKP is the modification of PVP with more injected cement volume, short-term pain relief, improvement of kyphotic angle and lower incidence of cement leakage.10 However, Kim et al’s study has reported that the incidence of new fractures after PKP is about 12.5%–36.8%.11 The widely accepted mechanism for recompression is the load transfer mechanism after augmentation, indicating that PKP increases the stiffness of treated vertebral segments and it may change the biomechanical properties of adjacent vertebral body leading to increased stresses and strains in the levels of adjacent vertebra.12,13
Bisphosphonates have been used as the first-line agents for prevention and treatment of osteoporosis.14 Numerous clinical studies have proved that bisphosphonates could reduce the bone turnover rate, increase bone mineral density (BMD) and decrease the risk of osteoporotic fractures by inhibiting osteoclastic function and inducing osteoclast apoptosis.15,16 Zoledronic acid (ZOL) is a third-generation, nitrogen-containing bisphosphonate, and a once-yearly intravenous infusion of ZOL 5 mg can ensure bisphosphonate adherence and persistence over 12-month interval.17,18 Moreover, ZOL is the most potent inhibitor of farnesyl diphosphate synthase, resulting in strongly inhibiting bone resorption, increasing secondary mineralization for refilling of remodeling space and reducing the incidence of vertebral/non-vertebral fractures.19 The therapeutic effect of PKP combined with ZOL infusion in the treatment of elderly OVCF is currently controversial. Therefore, the purpose of this retrospective study is to investigate the clinical efficacy of PKP/ZOL combined treatment for elderly osteoporotic patients and to determine the protective effect of ZOL against recompression fractures after initial PKP by comparing patients with OVCF treated with and without ZOL infusion.
Materials and methods
Study design and patients
This retrospective, single-center study was performed based on a protocol approved by the institutional review board of Wuhan No 1 Hospital and in accordance with the principles of the Declaration of Helsinki. We reviewed the medical records of elderly patients (mean age 77.4±5.4 years, range 67–88 years) with OVCF from June 2009 to June 2015. The informed consent to review medical records was obtained from every patient enrolled in this study. The inclusion criteria were a diagnosis of osteoporosis, defined as a BMD T-score of −2.5 or less at the lumbar spine measured by dual-energy X-ray absorptiometry (DXA) and additional diagnosis of OVCF based on symptoms of low back pain, difficult walking/standing and results of X-ray and magnetic resonance imaging.20 All the cases were single-level vertebral fractures and not conservatively treated, such as bed rest, pain medication, anti-osteoporosis drugs, physical therapy and bracing. The exclusion criteria included spinal tumor, infection, burst vertebral fractures, history of spinal surgery, history of anti-osteoporosis treatment, severe spinal deformities, uncorrected bleeding diatheses, renal insufficiency with creatinine clearance rate <35 mL/min and other metabolic disorders.
Grouping and treatments
A total of 95 elderly patients (44 women and 51 men) were enrolled in this study and they were divided into three groups, including ZOL group, PKP group and PKP+ZOL group. All patients received conservative treatment for OVCF, which consisted of daily 1,000 mg calcium and 800 IU vitamin D supplements prior to the ZOL infusion and/or PKP treatments. In the ZOL group, 32 patients received once-yearly intravenous ZOL infusion (Aclasta) as 5 mg dose in 100 mL solution infused over at least 15 minutes. In the PKP group, 34 patients underwent standard PKP treatments. In the PKP+ZOL group, 29 patients received ZOL 5 mg infusion 3 days after PKP and once-yearly thereafter. All patients continued receiving conservative treatment after ZOL infusion and/or PKP treatments. Nonsteroidal anti-inflammatory drugs and other pain killers were administered if necessary. All feasible and suitable therapeutic strategies were provided for every patient and they selected different treatments mainly based on the economic conditions and personal wishes without any mandatory requirement.
Surgical procedures
The indications for PKP included moderate to severe pain caused by radiologically confirmed compression fracture secondary to osteoporosis that did not improve with >4 weeks of conservative management including bed rest, nonsteroidal anti-inflammatory drugs, opioid analgesics and bracing. All surgical procedures of PKP were performed by the same group of spinal surgeons in our department. Patients were positioned prone on the operation table under general anesthesia. A small incision was made based on X-rays’ guidance localization and a probe was subsequently placed into the vertebral space at the fracture site. The bone was drilled, and a balloon (Kinetic Inc., Shanghai, China) was inserted on each side and the balloons were then inflated with contrast medium in order to obtain the desired height recovery. The spaces created by the balloons were then filled with bone cement carefully with fluoroscopic monitoring. After surgery, patients were monitored for 6 hours postoperatively.21
Radiographic and clinical assessment
Radiographic and clinical assessments were performed at admission and after treatment of PKP and/or ZOL infusion at 1 week, 3, 6, 12 and 24 months. As for the PKP group and the PKP+ZOL group, patients had to undergo preoperative magnetic resonance imaging and computed tomography assessments also for ensuring operative orientation and routes. Postoperative X-rays for these patients were assessed at 1 week, 3, 6, 12 and 24 months. The changes in vertebral body height and degrees of kyphotic deformity were measured by lateral radiographs. The incidence of recompression vertebral fracture (RVF) was also measured based on the result of radiography which was confirmed by the Genant et al semi-quantitative assessment method as a reduction of 20% (minimum 4 mm) from baseline in the height of any vertebra.22 Moreover, BMD of the spine was preoperatively measured as an indicator of osteoporosis (T-score ≤−2.5) with DXA and selected as baseline for each patient. Postoperative BMDs were detected at 12 and 24 months. Clinical outcome was evaluated using Visual Analog Scale (VAS) scores and Oswestry Disability Index (ODI) scores at before and after PKP/ZOL treatments. VAS scores (from 0, no pain to 10, worst pain) were applied for evaluating back pain. The ODI scores were used to assess functional capacity, indicating a better health status with a lower percentage. The ODI is the most commonly recommended condition-specific outcome measure in patients with acute and chronic low back pain with high test–retest reliability.23 Safety assessments of PKP and ZOL infusion were performed in the period of hospitalization and at each clinic visit during post-discharge follow-up. As for ZOL infusion, all documented adverse effects and acute-phase reactions (occurring within 3 days after ZOL infusion) were evaluated for its safety profile. As for PKP, operative complications, including cement leakage, bleeding, infection, cardiac arrest, pulmonary embolism and stroke, were recorded for safety assessments.
Statistical analysis
Statistical analysis was performed using Statistical Product and Service Solutions software (SPSS 19.0; IBM Corporation, Armonk, NY, USA). The results were presented as mean±SD. Student’s t-test and one-way analysis of variance were applied for continuous data, and chi-square test for categorical data. A value of P<0.05 was considered statistically significant.
Results
Demographic characteristics
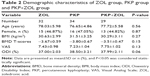
A total of 95 elderly patients of age 65 and above were diagnosed with OVCF based on BMD (T-score ≤−2.5), radiography (a reduction of ≥20% from baseline in the height of any vertebra) and clinical manifestations. Table 1 shows the different locations of vertebral fractures. Patients were divided into three groups based on different therapeutic strategies, including the ZOL group (once-yearly 5 mg ZOL infusion, n=32), the PKP group (standard PKP treatment, n=34) and the PKP+ZOL group (once-yearly 5 mg ZOL infusion after standard PKP treatment, n=29). All patients completed 24 months of follow-up and received radiological and clinical evaluations at 1 week, 3, 6, 12 and 24 months after PKP surgery and/or ZOL infusion. Table 2 shows the demographic characteristics of patients before PKP and/or ZOL infusion. There were no significant differences in patients’ age, gender, body mass index, lumbar spine BMD T-scores, baseline of VAS scores and ODI scores.
Radiological outcomes
The vertebral body height before PKP and/or ZOL infusion was 11.66±1.34 mm in the ZOL group, 11.29±1.28 mm in the PKP group and 11.32±1.30 mm in the PKP+ZOL group. There were no significant differences among the three groups (P>0.05). The vertebral body heights of patients with OVCF after ZOL infusion at 1 week, 3, 6, 12 and 24 months were 11.70±1.35, 11.33±1.37, 11.12±1.48, 10.76±1.47 and 10.38±1.33 mm, respectively. The postoperative vertebral body heights after PKP at 1 week, 3, 6, 12 and 24 months were 23.70±3.03, 22.46±2.93, 21.17±2.96, 20.84±2.38 and 20.13±2.41 mm, respectively, which were significantly higher than those of the ZOL group (P<0.05). As for the PKP+ZOL group, the postoperative vertebral body heights were 24.30±3.13, 24.12±2.97, 23.82±3.01, 23.76±2.67 and 23.31±2.59 mm at 1 week, 3, 6, 12 and 24 months, respectively, after PKP and ZOL infusion, which were also significantly higher than those of the ZOL group (P<0.05). Moreover, the postoperative vertebral body heights in the PKP+ZOL group were significant higher than those of the PKP group at 3, 6, 12 and 24 months after PKP operations (Figure 1).
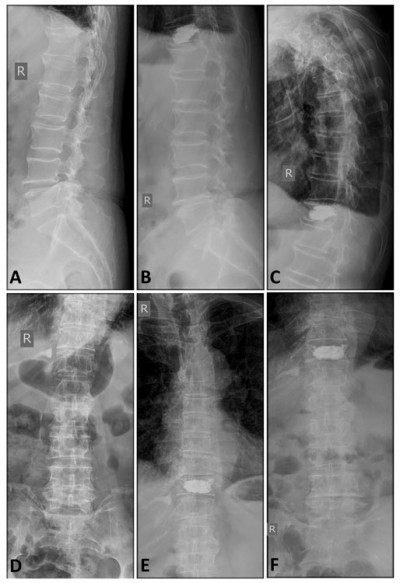
The pretreatment kyphotic wedge angle was 15.35°±5.33° in the ZOL group, 17.23°±5.83° in the PKP group and 16.83°±5.33° in the PKP+ZOL group. There were no significant differences among the three groups (P>0.05). The kyphotic wedge angles of patients with OVCF after ZOL infusion at 1 week, 3, 6, 12 and 24 months were 15.66°±6.54°, 15.83°±6.31°, 15.94°±4.98°, 16.11°±4.81° and 16.30°±5.43°, respectively. The postoperative kyphotic wedge angles after PKP at 1 week, 3, 6, 12 and 24 months were 8.84°±4.31°, 9.33°±4.91°, 9.59°±4.19°, 10.01°±3.19° and 10.64°±3.89°, respectively, which were significantly lower than those of the ZOL group (P<0.05). As for the PKP+ZOL group, the postoperative kyphotic wedge angles were 8.13°±4.11°, 8.19°±3.72°, 8.20°±3.28°, 8.23°±4.83° and 8.45°±4.29° at 1 week, 3, 6, 12 and 24 months, respectively, after PKP and ZOL infusion, which were also significantly lower than those of the ZOL group (P<0.05). Moreover, the postoperative kyphotic wedge angle in the PKP+ZOL group was significantly lower than that of the PKP group at 24 months after PKP operations (Figure 2). A representative case of a 71-year-old female patient with T11 OVCF who received the combination treatment of PKP and ZOL infusion has been shown in Figure 3.
The baseline of lumbar spine BMD T-score was −3.80±0.67 in the PKP group, −3.67±0.59 in the ZOL group and −3.51±0.47 in the PKP+ZOL group. There were no significant differences among the three groups (P>0.05). The BMD T-scores of patients with OVCF after only PKP at 12 and 24 months were −3.74±0.81 and −3.82±0.59, respectively, indicating no significant differences compared with baseline (P>0.05). However, after ZOL infusion, the BMD T-scores were significantly higher than baseline, which were −3.03±0.72 after 12 months and −2.78±0.48 after 24 months in the ZOL group (P<0.05). Similarly, in the PKP+ZOL group, the BMD T-scores of lumbar spine at post-12 months and post-24 months were −2.91±0.57 and −2.59±0.64, respectively, which were significantly higher than the baseline (P<0.05; Figure 4).
Clinical outcomes
The baseline of pretreatment VAS was 7.43±0.98 in the ZOL group, 7.23±1.04 in the PKP group and 7.75±1.02 in the PKP+ZOL group. There were no significant differences among the three groups (P>0.05). The VAS scores of patients with OVCF after ZOL infusion at 1 week, 3, 6, 12 and 24 months were 3.30±1.32, 5.31±1.27, 4.82±1.03, 4.57±0.98 and 4.33±1.19, respectively, indicating that the VAS scores were significantly less than the baseline (P<0.05). As for the PKP group, the VAS scores after PKP at 1 week, 3, 6, 12 and 24 months were 2.48±1.09, 1.62±0.63, 2.83±0.71, 3.11±1.10 and 3.42±0.97, respectively, which were significantly lower than the baseline (P<0.05) and those of the ZOL group (P<0.05). Similarly, the VAS scores of the PKP+ZOL group were 2.13±0.84, 1.42±0.76, 2.73±0.80, 2.81±0.67 and 3.08±1.03 at 1 week, 3, 6, 12 and 24 months, respectively, after PKP and ZOL infusion. Compared with the baseline, the VAS scores of PKP+ZOL group had significantly decreased (P<0.05) and were found to be significantly lower than those of ZOL group (P<0.05). However, the differences in VAS scores between the PKP group and the PKP+ZOL group were not statistically significant (P>0.05; Figure 5).
The baseline of pretreatment ODI was 37.00%±2.03% in the ZOL group, 38.50%±3.21% in the PKP group and 37.99%±2.11% in the PKP+ZOL group. There were no significant differences among the three groups (P>0.05). The ODI scores of patients with OVCF after ZOL infusion at 1 week, 3, 6, 12 and 24 months were 33.21%±2.31%, 29.96%±1.91%, 31.20%±2.14%, 32.79%±2.29% and 34.34%±1.83%, respectively, suggesting that the ODI scores were significantly less than the baseline (P<0.05). As for the PKP group, the ODI scores after PKP at 1 week, 3, 6, 12 and 24 months were 24.37%±2.98%, 21.19%±3.34%, 26.74%±3.09%, 28.97%±2.73% and 30.13%±2.44%, respectively, which were significantly lower than the baseline (P<0.05) and those of the ZOL group (P<0.05). Similarly, the ODI scores of the PKP+ZOL group were 21.32%±2.47%, 17.38%±1.89%, 19.74%±2.08%, 21.76%±2.81% and 24.98%±3.01% at 1 week, 3, 6, 12 and 24 months, respectively, after PKP and ZOL infusion. Compared with the baseline, the ODI scores of PKP+ZOL group had significantly decreased (P<0.05) and were found to be significantly lower than those of ZOL group (P<0.05). Moreover, the ODI scores of the PKP+ZOL group were significantly lower than those of the PKP group at 6, 12 and 24 months after treatments (P<0.05; Figure 6).
Safety profile
The complications of PKP operations and the adverse events (AEs) of ZOL infusion were evaluated in this study (Table 3). The postoperative radiography of PKP-treated vertebral body indicated that the cement extravasation occurred in 10 patients, including 6 patients in the PKP group and 4 patients in the PKP+ZOL group. The differences in cement extravasation rates between two groups were not statistically significant (P>0.05). Cement leakages appeared in the paravertebral soft tissues, venous plexus and adjacent intervertebral disks. Other major complications of PKP, such as bleeding, infections, cardiac arrest, pulmonary embolism, stroke or spinal canal leakage, were not observed in this research. No major complications of PKP, such as pulmonary embolism or spinal canal leakage, were observed. The incidence of RVF in the PKP group was 14.7% within the 2-year follow-up period. However, there was no patient with RVF in the PKP+ZOL group and the differences in RVF incidences between the two groups were statistically significant (P<0.05). The most common AEs observed with ZOL infusion were acute-phase reactions, including flu-like symptoms, fever, headache, nausea, myalgia and arthralgia. Table 3 shows that there were no significant differences in the AEs between the PKP+ZOL group and the ZOL group.
Discussion
Osteoporosis had affected an estimated 22.61 million elderly persons aged over 65 in China, which brought serious burden to the public health and social economy. The most prominent feature of osteoporosis was low BMD, which was accompanied by the impairment of bone microarchitecture, increase in bone fragility and a propensity to fractures. Oral nitrogen-containing bisphosphonates, such as alendronate, risedronate and ibandronate, were proved to be the first-line agents for osteoporosis treatment by reducing the bone turnover rate, increasing BMD and decreasing the risk of osteoporotic fractures.24 Recently, a once-yearly intravenous infusion of 5 mg ZOL, the third generation of nitrogen-containing bisphosphonate, has been approved by the US Food and Drug Administration for the treatment of postmenopausal osteoporosis, male osteoporosis and glucocorticoid-induced osteoporosis.25 Particularly, ZOL infusion was useful for patients with serious gastrointestinal intolerance and malabsorption to oral bisphosphonates. Moreover, ZOL infusion exhibited strong inhibition of bone resorption, effective improvement of secondary mineralization for refilling of remodeling space and significant decrease in vertebral/non-vertebral fractures.19 As we all know, osteoporotic fracture was one of the most serious complications of osteoporosis, which was more prevalent in the elderly patients. The significant clinical manifestations of patients with OVCF included chronic disabling pain, spinal kyphosis, psychological disorders, decreased QoL, loss of mobility and even death. Percutaneous vertebral augmentations, such as PVP and PKP, were the most popular treatments for OVCF for quickly relieving pain and achieving spinal stabilization. Compared with PVP, PKP could provide more injected cement volume, shorter-term pain relief, significant improvement of vertebral height and kyphotic wedge angle. However, the occurrence of RVF after PKP seemed to be inevitable and its possible mechanism was that PKP increased the stiffness of treated vertebral body which changed the biomechanical properties of adjacent vertebral body. Overall, the effect of ZOL infusion on PKP for treating elderly OVCF was still unclear and it was urgent to resolve the relationship between the two important treatments for osteoporosis.
In this study, within 24-year follow-up period, the radiographic and clinical outcomes of elderly patients with OVCF who received ZOL infusion after PKP, including vertebral height, kyphotic wedge angle, BMD, VAS, ODI and the incidence of RVF, were compared with those of patients treated by only ZOL infusion or PKP. The postoperative vertebral heights of patients with OVCF after PKP and PKP+ZOL were 23.70±3.03 and 24.30±3.13 mm, respectively, which were significantly higher than that of ZOL group. The reduction in degrees of kyphotic deformity in the PKP and PKP+ZOL groups was corrected to 8.4° and 8.7°, respectively. Similarly, Liu et al21 reported that PKP could restore the vertebral height to 2.04±0.41 cm and 8.0° reduction in wedge angle. Moreover, Saxena et al26 retrospectively investigated 199 kyphoplasty procedures in 135 patients and reported that the kyphotic angle corrected from 17.41° to 10.59°. The effective restoration of vertebral height and kyphotic angle indicated excellent therapeutic effects of PKP for elderly patients with OVCF in this study. Noticeably, the restoration of vertebral height and kyphotic angle was more effective in the PKP+ZOL group than that in the PKP group, indicating that ZOL infusion might inhibit deterioration of bone mass after PKP surgery for maintaining the vertebral height and delaying kyphotic deformity. Moreover, according to DXA results, the BMD T-scores of patients with OVCF in the ZOL group and PKP+ZOL group were significantly higher than those in the PKP group, indicating that ZOL could significantly improve the BMD of lumbar spines. In the HORIZON-Pivotal Fracture Trial (HORIZON-PET), after ZOL infusion, the risk of vertebral fractures was significantly reduced by 77% and the BMD of lumbar spine significantly increased by 6.7%, which were similar with our results.27 As for the VAS and ODI scores, our results showed that PKP and PKP+ZOL treatment could provide better pain relief and functional recovery than single ZOL conservative treatment for elderly patients with OVCF. Moreover, we suggested that PKP+ZOL combination treatment might promote the recovery of spinal cord function, compared with PKP or ZOL infusion. Similarly, Tu et al28 retrospectively analyzed 64 patients with osteoporosis who underwent lumbar interbody fusion surgery (LIFS) and reported that the ZOL infusion could significantly improve the VAS and ODI scores. According to the results of complications and the AE of PKP or ZOL treatment, the rate of cement leakage in the PKP was ~15%. Sun et al23 performed a retrospective study of 89 cases for PKP in treating osteoporotic occult vertebral fracture and they reported that the rate of cement extravasation was 8.45% and the new fractures of adjacent vertebral body was 5 (3.52%). In this study, the incidence of RVF was about 7.9% (5/63) in all PKP-treated patients. Interestingly, all five RVFs occurred in the PKP group without ZOL infusion and there was no RVF in the PKP+ZOL group. It indicated that ZOL infusion might reduce the risk of RVFs in elderly osteoporotic patients with OVCF after initial PKP treatment. Tu et al28 reported that ZOL infusion could significantly reduce the incidences of final subsequent vertebral compression fractures, pedicle screw loosening and cage subsidence >2 mm. They suggested that ZOL treatment caused beneficial effects on instrumented LIFS and recommended ZOL treatment for osteoporosis patients undergoing LIFS. Lin et al29 conducted a retrospective review of osteoporotic patients who underwent vertebroplasty and divided them into two groups according to whether or not they received ZOL infusion and they reported that only 4% of the patients (2/51) required a second vertebroplasty, compared to 13% (206/1,595) in the non-ZOL-treated group (P=0.032), indicating that osteoporotic patients who undergo vertebroplasty are significantly less likely to require reoperation if treated with ZOL infusion. Moreover, Chen et al30 preformed a randomized, placebo-controlled and triple-blinded study for exploring the effects of ZOL on the healing process in osteoporotic patients following spinal fusion and no adjacent vertebral compression fractures were observed in the ZOL-treated group, indicating that ZOL treatments for osteoporotic patients with spinal fusion could shorten the time to fusion, improve the fusion rate, prevent subsequent adjacent vertebral compression fractures and improve the clinical outcomes. Xue and Ye31 suggested that PVP combined with ZOL could significantly relieve pain, improve vertebral stability and bone density, and reduce the incidence of adjacent vertebral fractures in patients with osteoporotic vertebral compression fractures. Zhang et al32 reported that PVP combined with zoledronic acid was a highly effective and safe combination therapy to relieve pain and improve QoL in patients with osteolytic spinal metastases from breast cancer. Therefore, we suggest that once-yearly 5 mg ZOL infusion could reduce the risk of RVFs for elderly osteoporotic patients with OVCF after PKP treatment probably by increasing BMD and inhibiting bone resorption.
One of the limitations of this study was the small sample size of 95 elderly patients with OVCF and only 29–34 patients enrolled in each group receiving three different therapeutic strategies. Although the majority of results reached statistical significance, it would be presumptuous to draw broad, all-encompassing conclusions from this study. However, this study showed a clear difference between these treatments, which would be helpful to formulate treatment decisions. Since elderly female patients would be more likely to suffer from osteoporosis, the analysis of differences in these outcomes measured in only female elderly patients had important clinical significance; however, this study could not provide these results. Moreover, our follow-up period was 24 months, which was considered to be short-term data only. A long-term follow-up (2–5 years) could provide more valuable results. Also, a prospective study could be more useful than this retrospective study.
Conclusion
In summary, this study showed the efficacy of once-yearly 5 mg ZOL infusion in maintaining the vertebral height, reducing kyphotic deformity, relieving back pain, promoting the recovery of spinal cord function and decreasing the risk of RVFs in elderly osteoporotic patients with OVCF after PKP. Therefore, once-yearly 5 mg ZOL infusion in combination with PKP could be recommended for treating elderly osteoporotic patients with OVCF.
Disclosure
The authors report no conflicts of interest in this work.
References
Curtis EM, Moon RJ, Dennison EM, Harvey NC, Cooper C. Recent advances in the pathogenesis and treatment of osteoporosis. Clin Med. 2016;16(4):360–364. | ||
Lin X, Xiong D, Peng YQ, et al. Epidemiology and management of osteoporosis in the People’s Republic of China: current perspectives. Clin Interv Aging. 2015;10:1017–1033. | ||
Ballane G, Cauley JA, Luckey MM, El-Hajj Fuleihan G. Worldwide prevalence and incidence of osteoporotic vertebral fractures. Osteoporos Int. 2017;28(5):1531–1542. | ||
Kung AW. Epidemiology and diagnostic approaches to vertebral fractures in Asia. J Bone Miner Metab. 2004;22(3):170–175. | ||
Cauley JA, Chalhoub D, Kassem AM, Fuleihan Gel-H. Geographic and ethnic disparities in osteoporotic fractures. Nat Rev Endocrinol. 2014;10(6):338–351. | ||
Lange A, Kasperk C, Alvares L, Sauermann S, Braun S. Survival and cost comparison of kyphoplasty and percutaneous vertebroplasty using German claims data. Spine. 2014;39(4):318–326. | ||
Chang W, Zhang X, Jiao N, et al. Unilateral versus bilateral percutaneous kyphoplasty for osteoporotic vertebral compression fractures: a meta-analysis. Medicine (Baltimore). 2017;96(17):e6738. | ||
Yang H, Liu T, Zhou J, Meng B, Wang G, Zhu X. Kyphoplasty versus vertebroplasty for painful osteoporotic vertebral compression fractures-which one is better? A systematic review and meta-analysis. Int J Spine Surg. 2013;7:e45–e57. | ||
Garfin SR, Yuan HA, Reiley MA. New technologies in spine: kyphoplasty and vertebroplasty for the treatment of painful osteoporotic compression fractures. Spine. 2001;26(14):1511–1515. | ||
Wang H, Sribastav SS, Ye F, et al. Comparison of percutaneous vertebroplasty and balloon kyphoplasty for the treatment of single level vertebral compression fractures: a meta-analysis of the literature. Pain Physician. 2015;18(3):209–222. | ||
Kim YY, Rhyu KW. Recompression of vertebral body after balloon kyphoplasty for osteoporotic vertebral compression fracture. Eur Spine J. 2010;19:1907–1912. | ||
Yi X, Lu H, Tian F, et al. Recompression in new levels after percutaneous vertebroplasty and kyphoplasty compared with conservative treatment. Arch Orthop Trauma Surg. 2014;134(1):21–30. | ||
Wang E, Yi H, Wang M, Huang C. Treatment of osteoporotic vertebral compression fractures with percutaneous kyphoplasty: a report of 196 cases. Eur J Orthop Surg Traumatol. 2013;23(Suppl 1):S71–S75. | ||
Tella SH, Gallagher JC. Prevention and treatment of postmenopausal osteoporosis. J Steroid Biochem Mol Biol. 2014;142:155–170. | ||
Jansen JP, Bergman GJ, Huels J, Olson M. The efficacy of bisphosphonates in the prevention of vertebral, hip, and nonvertebral-nonhip fractures in osteoporosis: a network meta-analysis. Semin Arthritis Rheum. 2011;40(4):275–284. | ||
Byun JH, Jang S, Lee S, et al. The efficacy of bisphosphonates for prevention of osteoporotic fracture: an update meta-analysis. J Bone Metab. 2017;24(1):37–49. | ||
Huang S, Lin H, Zhu X, Chen X, Fan L, Liu C. Zoledronic acid increases bone mineral density and improves health-related quality of life over two years of treatment in Chinese women with postmenopausal osteoporosis. Endokrynol Pol. 2014;65(2):96–104. | ||
Maricic M. The role of zoledronic acid in the management of osteoporosis. Clin Rheumatol. 2010;29(10):1079–1084. | ||
Seeman E, Martin TJ. Co-administration of antiresorptive and anabolic agents: a missed opportunity. J Bone Miner Res. 2015;30(5):753–764. | ||
Boonen S, Reginster JY, Kaufman JM, et al. Fracture risk and zoledronic acid therapy in men with osteoporosis. N Engl J Med. 2012;367(18):1714–1723. | ||
Liu JT, Liao WJ, Tan WC, et al. Balloon kyphoplasty versus vertebroplasty for treatment of osteoporotic vertebral compression fracture: a prospective, comparative, and randomized clinical study. Osteoporos Int. 2010;21(2):359–364. | ||
Genant HK, Wu CY, van Kuijk C, Nevitt MC. Vertebral fracture assessment using a semiquantitative technique. J Bone Miner Res. 1993;8(9):1137–1148. | ||
Sun ZY, Li XF, Zhao H, et al. Percutaneous Balloon Kyphoplasty in treatment of painful osteoporotic occult vertebral fracture: a retrospective study of 89 Cases. Med Sci Monit. 2017;23:1682–1690. | ||
Sunyecz JA. Zoledronic acid infusion for prevention and treatment of osteoporosis. Int J Womens Health. 2010;2:353–360. | ||
Dhillon S. Zoledronic Acid (Reclast®, Aclasta®): a review in osteoporosis. Drugs. 2016;76(17):1683–1697. | ||
Saxena BP, Shah BV, Joshi SP. Outcome of percutaneous balloon kyphoplasty in vertebral compression fractures. Indian J Orthop. 2015;49(4):458–464. | ||
Eastell R, Boonen S, Cosman F, et al. Relationship between pretreatment rate of bone loss and bone density response to once-yearly ZOL: HORIZON-PFT extension study. J Bone Miner Res. 2015;30(3):570–574. | ||
Tu CW, Huang KF, Hsu HT, Li HY, Yang SS, Chen YC. Zoledronic acid infusion for lumbar interbody fusion in osteoporosis. J Surg Res. 2014;192(1):112–116. | ||
Lin TY, Yang SC, Tsai TT, et al. Correlation between zoledronic acid infusion and repeat vertebroplasty surgery in osteoporotic patients. Curr Med Res Opin. 2016;32(5):921–927. | ||
Chen F, Dai Z, Kang Y, Lv G, Keller ET, Jiang Y. Effects of zoledronic acid on bone fusion in osteoporotic patients after lumbar fusion. Osteoporos Int. 2016;27(4):1469–1476. | ||
Xue F, Ye YS. Bone cement combined with zoledronic acid to repair osteoporotic vertebral compression fractures. Chinese Journal of Tissue Engineering Research. 2015;19(25):3937–3941. | ||
Zhang J, Wang Y, Han K, et al. Percutaneous vertebroplasty combined with zoledronic acid for the treatment of painful osteolytic spinal metastases in patients with breast cancer. J Vasc Interv Radiol. 2013;24(12):1861–1867. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.