Back to Journals » Therapeutics and Clinical Risk Management » Volume 14
Perceptions of heart failure symptoms, disease severity, treatment decision-making and side effects by patients and cardiologists: a multinational survey in a cardiology setting
Authors Bruce Wirta S, Balas B , Proenca CC, Bailey H , Phillips Z, Jackson J , Cotton S
Received 8 August 2018
Accepted for publication 26 October 2018
Published 16 November 2018 Volume 2018:14 Pages 2265—2272
DOI https://doi.org/10.2147/TCRM.S183200
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Sara Bruce Wirta,1 Bogdan Balas,2 Catia C Proenca,3 Hollie Bailey,4 Zoe Phillips,4 James Jackson,4 Sarah Cotton4
1Real World Evidence, Cardio-Metabolic Franchise, Novartis Sweden, Stockholm, Sweden; 2Real World Evidence, Cardio-Metabolic Franchise, Novartis Pharma, Basel, Switzerland; 3Wellmera AG, Basel, Switzerland; 4Real World Research, Adelphi Real World, Bollington, UK
Purpose: Explore the extent to which heart failure (HF) symptoms and side effects of HF treatment experienced by patients are recognized by cardiologists, and concordance between patient–cardiologist perceptions of HF severity and patients’ contributions to treatment decision-making.
Methods: A multinational, cross-sectional survey of cardiologists and patients with HF was conducted. Patient-record forms (PRFs) were completed by cardiologists for consecutive consulting patients with HF, who completed a patient self-completion questionnaire (PSC). Responses from PRFs with an associated PSC were analyzed to compare patient- and cardiologist-reported occurrences of HF symptoms and treatment side effects, patient-perceived severity of HF and cardiologists’ perceived risk of death within 12 months, and patient input into treatment decisions. Concordance was calculated as the number of response agreements between PSCs and PRFs for total number of matched pairs. Over- or underreporting of symptoms and side effects by cardiologists relative to patient-reported occurrences were calculated.
Results: Overall, 2,454 patient–cardiologist pairs were identified. High levels of concordance between matched pairs were observed for the occurrence of reported HF symptoms (93%), side effects (77%–98%) and degree of patient input into treatment decisions (74%); for perceived HF severity, concordance was 54%. Most symptoms (except dyspnea when active and fatigue/weakness, experienced by >50% of patients) were underreported by cardiologists. Of patients reporting to have been informed by their cardiologist that their HF was mild, 28% were perceived by their cardiologist to have a moderate–high/very high risk of death within 12 months. Treatment choice was not discussed with almost a third of patients. When discussed, 94% of patients (n=1,540) reported the cardiologist made the final decision. Cardiologists more often under- than overreported the occurrence of side effects reported by patients.
Conclusion: Improved patient–cardiologist dialogue and shared decision-making is required for optimizing patient care and outcomes in HF.
Keywords: patient influence, disease awareness, treatment decision-making, patient-reported outcomes, disease-specific program, real world
Introduction
Results of previously published patient surveys indicate that individuals with heart failure (HF) have limited knowledge of the severity of their condition and its prognosis.1,2 Almost half the participants in a survey of 52 patients <50 years old with HF thought that their disease was curable.2 Effective two-way communication between people with HF and health-care professionals (HCPs) is key to improving patients’ knowledge and understanding of their heart condition and associated with improvements in both adherence to HF-management plans3,4 and patient outcomes.5,6 Communication between patients and HCPs is also needed to aid decisions on pharmacological treatment. It is important that patients are involved in these decisions so that they understand how to take their medication (dose, time of day) and the benefits of treatment, and are able to recognize possible side effects of pharmacotherapy.7 Results of a qualitative, community-based interview study of individuals with HF indicated that patients did not feel involved in decision-making or encouraged to discuss treatment with their HCP.8 With recent advances in care, it is now well recognized that shared patient–HCP decision-making is central to optimized HF management.9
There is a lack of international quantitative evidence on how patient perceptions of their HF and treatment correspond with those of their cardiologists. We thus used a large, real-world, multinational survey to investigate the extent to which HF symptoms and side effects of HF treatment experienced by patients were recognized by cardiologists. We also evaluated levels of concordance on perceptions of HF-disease severity by patients (which were based on information provided by their cardiologists) and cardiologists, in addition to patient contributions to treatment decision-making.
Methods
Study design
Data were collected from the Adelphi HF Disease Specific Programme (DSP),10 a cross-sectional survey of cardiologists and their consulting patients with HF, conducted in 2016 in a real-world setting across ten countries (Argentina, Brazil, China, Colombia, France, Japan, Mexico, Russia, Saudi Arabia, and Turkey). The DSP comprises three main phases, details of which have been described previously: a preparatory phase involving development of survey materials and participant recruitment, a data-collection phase, and data analysis.10
Preparatory phase
The DSP comprised a face-to-face cardiologist interview, a patient-record form (PRF), and a patient self-completion questionnaire (PSC). These questionnaires were developed in English and translated into the language of the relevant study country by native speakers from a local DSP agency. An independent UK-based translation agency subsequently verified the translated materials. The questionnaires were developed empirically, and their pharmacometric properties were not systematically assessed.
Participant recruitment
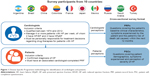
Cardiologists were identified from public lists of HCPs and invited to participate in the study, provided they had qualified as a cardiologist between 1974 and 2012, consulted with at least four patients with HF per week, and were personally responsible for drug-treatment decisions. To be eligible for inclusion, patients had to have a confirmed HF diagnosis and an associated cardiologist-completed PRF (Figure 1).
Cardiologists completed PRFs for consecutive consulting patients with HF (new or preexisting) using data from medical records. Patients with left ventricular ejection fraction (LVEF) <40% were classified as having HF with reduced ejection fraction (HFrEF), patients with LVEF 40%–49% were classified as having HF with midrange ejection fraction (HFmrEF), and those with LVEF ≥50% were classified as having HF with preserved ejection fraction (HFpEF). All patients were then invited to complete a PSC independently of their cardiologist immediately after their consultation. Patients gave informed consent to participate by ticking a box on the front page of the questionnaire to indicate that they had read the information provided and that they agreed to take part in the study.
Data collection and analysis
Information gathered from PRFs and PSCs included patient demographics and clinical characteristics, HF symptoms, and treatment aspects (including common side effects and input into treatment decision-making). Responses were anonymized to ensure confidentiality and avoid potential biases. Cardiologists were not able to see or influence patient responses.
Only responses from PRFs with an associated PSC were analyzed. Patient-reported and cardiologist-reported occurrences of individual HF symptoms were compared and concordance calculated as the number of response agreements between the PSC and PRF for the total number of patient–cardiologist matched pairs. Underreporting and overreporting of symptom occurrence by cardiologists relative to patient-reported occurrence was calculated by taking the patient’s perspective and evaluating how often a patient-reported symptom was not reported by their cardiologist in the PRF (underreported) and how often a cardiologist reported a symptom in the PRF that was not reported by the patient (overreported). Patient-perceived HF-disease severity (rated as mild, moderate, or severe) following information provided by their cardiologist was compared with the cardiologist’s perceived risk of death within 12 months (rated as low, moderate, or high). Patients and cardiologists recorded the degree of patient input into treatment decisions and the side effects experienced because of the patient’s current HF-treatment regimen. These data were compared, and concordance and cardiologist underreporting and overreporting were calculated as just described.
The questionnaires applied in this study follow guidelines outlined in the code of conduct published by the European Pharmaceutical Market Research Association.11 This code states that ethical approval within this context is not necessary, because the goal of research is to improve understanding, rather than to test hypotheses. The research was conducted in accordance with the US Health Insurance Portability and Accountability Act 1996 and European equivalents.11,12
Results
Study population
A total of 4,903 PRFs were received from 563 cardiologists. Subsequently, 2,454 patients with HF completed a PSC, amounting to the total number of matched patient–cardiologist pairs (Table 1). The study population had a mean age of 66.7±11.9 years, with more males (58%) than females, and most patients (66%) were retired. Of patients with available information on HF functional class (n=2,442), 50% had New York Heart Association (NYHA) class II, with similar proportions with NYHA classes I (24%) and III (22%), while 3% had NYHA class IV. The distribution of HF phenotypes across the studied population was HFpEF (43%), HFmrEF (32%), and HFrEF (26%; Table 1).
HF symptoms
A high level of concordance between matched patient–cardiologist pairs was observed for the occurrence of reported HF symptoms (n=2,379): overall, 93% of matched pairs reported similar occurrences of any HF symptom. High concordance was also observed for the occurrence of individual HF symptoms (ranging from 80% for palpitations to 92% for persistent cough [Figure 2A]). For symptoms reported by >50% of patients (ie, shortness of breath when active and fatigue/weakness [Figure 2B]), cardiologists more commonly overreported than underreported their occurrence, whereas for symptoms reported by <50% of patients, cardiologists were more likely to underreport than overreport their occurrence (Figure 2B).
HF severity
Overall concordance of patient-perceived and cardiologist-perceived severity of HF was 54% (n=2,260). Of 1,040 patients reporting to have been told by their cardiologist that their HF was mild, 28% were perceived by their cardiologist to have a moderate–high or very high risk of death within the next 12 months. This increased to 50% for the 888 patients who reported having been informed by their cardiologist that their HF was moderate and 75% for the 332 patients who reported having been informed that their HF was severe. Conversely, cardiologists perceived the risk of death in the next 12 months to be low or very low in 25% of the 332 patients who reported having been informed that their HF was severe.
Patient input on HF-treatment decision-making
Of matched patient–cardiologist pairs (n=2,228), almost a third (30%) of patients stated in the PSC that they had had no opportunity to influence the choice of their HF therapy. In total, 1,540 patients (69%) reported that they had had the opportunity to influence their treatment options. Most (n=1,442; 94%) of these recorded in the PSC that the cardiologist had made the final treatment decision, while 6% stated that they had made the final decision. A high level of concordance (74%) between matched patient–cardiologist pairs was observed for the degree of patient input into treatment decisions, though patient input was overreported and underreported by cardiologists for 14% and 12% of matched pairs, respectively.
HF-treatment side effects
Concordance was high between matched patient–cardiologist pairs (n=2,385) for the occurrence of side effects of HF treatment, ranging from 77% for fatigue/tiredness to 98% for gout, rash, and swelling of lips, tongue, throat, or face (Figure 3A; blue and gray bars). Individual side effects were reported by 1%–22% of patients (Figure 3B). Cardiologists more often underreported than overreported the occurrence of side effects of treatment reported by patients (Figure 3B). Results from HF-treatment side effects relied on patient and physician reporting, and a confirmed diagnosis could not be established by the methodology used in our research.
Discussion
This analysis covering ten countries was conducted to generate quantitative evidence on concordance of patient and cardiologist perceptions regarding HF symptoms, disease severity, treatment decisions, and treatment-associated side effects. We found that while cardiologists were aware of the most common HF symptoms experienced by patients (ie, dyspnea when active and fatigue/weakness), they tended to underreport the less common symptoms, such as need to urinate at night, swelling of the abdomen, and shortness of breath when lying flat. Moreover, cardiologists were aware of more common or potentially severe side effects of HF treatment, including tiredness and swelling of the lips, tongue, throat, or face, but frequently underreported their occurrence. However, caution should be taken in the interpretation of these findings, as these symptoms may not necessarily reflect a diagnosis of angioedema, and the methodology used in this study did not allow verification of the accuracy of diagnoses made in the clinic. The findings of this survey also indicated that having been informed of the severity of HF by their cardiologist, patients often underestimated it, indicating a misalignment in understanding. Importantly, almost a third of patients reported having no involvement (including any discussion) in the most recent decision regarding their HF treatment.
These results indicate a need for improvement in open communication between cardiologists and patients. Other studies have found misaligned perceptions between patients and HCPs with regard to symptoms experienced and disease severity.13–15 In a study by Rogers et al, patients tended to attribute their symptoms to the normal aging process or treatment side effects, and consulted their HCP only when such symptoms as breathlessness became unmanageable.13,14 Additionally, some patients were unaware of their unfavorable prognosis,13 a situation that has been reported in other studies in which patients did not conceptualize HF as an incurable condition.1,2
HF is a complex syndrome to manage: patients tend to be elderly, have additional chronic illnesses, and receive numerous pharmacotherapies.16 Findings from qualitative studies have also shown that patients often have concerns about their treatment, but fail to discuss such issues, trusting their physician.13,14 Shared decision-making is the central goal of patient-centered care,17 whereby the HCP educates the patient about their condition, available treatment options, possible outcomes and side effects of therapy, and considers the patient’s preferences, in order to reach an informed treatment decision by mutual consent.9 This process, which allows for an effective and open relationship between patient and HCP, is considered fundamental to optimized patient management.18 Indeed, there is evidence that collaborative HCP–patient communication is associated with achievement of treatment goals and improved patient satisfaction.19,20 Therefore, interventions that encourage the patient’s active participation (eg, increased questioning) during medical visits, as well as interventions aimed at improving cardiologists’ communication skills (eg, improvements in the degree of friendliness, sensitivity, and supportiveness) may enhance the collaborative relationship between patient and cardiologist.20 Encouragingly, the American College of Cardiology recently reported that 78% of 400 patients with heart disease surveyed actively engaged with their HCP during an office visit to clarify treatment issues or other personal illness-related problems, suggesting that the concept of shared decision-making is becoming more and more mainstream.21
From the standpoint of future research, the high concordance across all queried individual HF symptoms and treatment-associated side effects in matched patient–cardiologist responses holds good promise. When conducting research, collecting outcomes from both patients and HCPs can be challenging, while our results suggest that using one perspective is largely representative. The observed concordance could potentially have been further improved by additional validation of the questionnaires from the perspective of the respondents, eg, it is plausible that many patients adapted their activity levels owing to their disease, and so a question on shortness of breath when active might have been misinterpreted by the patient (stemming from the fact that they were not active).
Conclusion
Ensuring patient education and increasing patient–cardiologist dialogue and shared decision-making may lead to increase awareness of HF-associated risks and treatment-related side effects. Improved communication between patients and their cardiologists may encourage patients to seek help from HCPs earlier in their disease progression, leading to optimized HF treatment and outcomes.
Acknowledgments
Medical writing support was provided by Sharon Smalley of PharmaGenesis, London, UK and was funded by Novartis Pharma AG, Basel, Switzerland. Adelphi Real World was commissioned to conduct this study on behalf of Novartis Pharma, and has ongoing consulting and research relationships with Novartis Pharma AG. This research was funded by Novartis Pharma AG, Basel, Switzerland. These data were presented as a poster at Heart Failure 2018 and the World Congress on Acute Heart Failure, May 26–29, 2018, Vienna, Austria.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
SBW is an employee of Novartis Sweden. BB was an employee of Novartis Pharma at the time of the study, and is now an employee of Hoffmann La Roche, Basel, Switzerland. CCP is an employee of Wellmera AG, Switzerland. SC, JJ, HB, and ZP are employees of Adelphi Real World, UK. The authors report no other conflicts of interest in this work.
References
Hupcey JE, Kitko L, Alonso W. Patients’ perceptions of illness severity in advanced heart failure. J Hosp Palliat Nurs. 2016;18(2):110–114. | ||
Płotka A, Prokop E, Migaj J, Straburzyńska-Migaj E, Grajek S. Patients’ knowledge of heart failure and their perception of the disease. Patient Prefer Adherence. 2017;11:1459–1467. | ||
van der Wal MH, Jaarsma T, van Veldhuisen DJ. Non-compliance in patients with heart failure; how can we manage it? Eur J Heart Fail. 2005;7(1):5–17. | ||
van der Wal MH, Jaarsma T, Moser DK, Veeger NJ, van Gilst WH, van Veldhuisen DJ. Compliance in heart failure patients: the importance of knowledge and beliefs. Eur Heart J. 2006;27(4):434–440. | ||
Koelling TM, Johnson ML, Cody RJ, Aaronson KD. Discharge education improves clinical outcomes in patients with chronic heart failure. Circulation. 2005;111(2):179–185. | ||
Toback M, Clark N. Strategies to improve self-management in heart failure patients. Contemp Nurse. 2017;53(1):105–120. | ||
Ponikowski P, Voors AA, Anker SD. 2016 ESC guidelines for the diagnosis and treatment of acute and chronic heart failure: The task force for the diagnosis and treatment of acute and chronic heart failure of the European Society of Cardiology (ESC). Developed with the special contribution of the Heart Failure Association (HFA) of the ESC. Eur Heart J. 2016;37(27):2129–2200. | ||
Murray SA, Boyd K, Kendall M, Worth A, Benton TF, Clausen H. Dying of lung cancer or cardiac failure: prospective qualitative interview study of patients and their carers in the community. BMJ. 2002;325(7370):929. | ||
Kambhampati S, Ashvetiya T, Stone NJ, Blumenthal RS, Martin SS. Shared decision-making and patient empowerment in preventive cardiology. Curr Cardiol Rep. 2016;18(5):49. | ||
Anderson P, Benford M, Harris N, Karavali M, Piercy J. Real-world physician and patient behaviour across countries: Disease-Specific Programmes – a means to understand. Curr Med Res Opin. 2008;24(11):3063–3072. | ||
European Pharmaceutical Market Research Association (EphMRA). Code of conduct. 2017. Available from: https://www.ephmra.org/media/1785/ephmra-2017-code-of-conduct-october-2017.pdf. Accessed June 26, 2018. | ||
US Department of Health & Human Services. Summary of the HIPAA Privacy Rule, July 2013. Available from: https://www.hhs.gov/hipaa/for-professionals/privacy/laws-regulations/index.html. Accessed June 15, 2018. | ||
Rogers AE, Addington-Hall JM, Abery AJ, et al. Knowledge and communication difficulties for patients with chronic heart failure: qualitative study. BMJ. 2000;321(7261):605–607. | ||
Rogers A, Addington-Hall JM, Mccoy AS, et al. A qualitative study of chronic heart failure patients’ understanding of their symptoms and drug therapy. Eur J Heart Fail. 2002;4(3):283–287. | ||
Jourdain P, Funck F, Bellorini M, et al. Perception and understanding chronic heart failure by the patient. The impact of therapeutic education on the level of knowledge of patients. About 350 cases. Arch Mal Coeur Vaiss. 2007;100(3):163–174. | ||
Cobretti MR, Page RL, Linnebur SA, et al. Medication regimen complexity in ambulatory older adults with heart failure. Clin Interv Aging. 2017;12:679–686. | ||
Barry MJ, Edgman-Levitan S. Shared decision making – pinnacle of patient-centered care. N Engl J Med. 2012;366(9):780–781. | ||
Gupta C, Bell SP, Schildcrout JS, et al. Predictors of health care system and physician distrust in hospitalized cardiac patients. J Health Commun. 2014;19(2):44–60. | ||
Sommaruga M, Bettinardi O, Opasich C. Communication between physician and patient with chronic heart decompensation may help to reach the therapeutic objectives. How to make it efficient. Ital Heart J Suppl. 2001;2(9):945–957. | ||
Schoenthaler A, Kalet A, Nicholson J, Lipkin M. Does improving patient-practitioner communication improve clinical outcomes in patients with cardiovascular diseases? A systematic review of the evidence. Patient Educ Couns. 2014;96(1):3–12. | ||
American College of Cardiology. The patient perspective of shared decision making. Clinical spotlight. 2015. Available from: http://www.acc.org/latest-in-cardiology/articles/2015/12/08/11/56/clinical-spotlight-patient-perspective-shared-decision. Accessed June 15, 2018. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.