Back to Journals » Patient Preference and Adherence » Volume 16
Perception of Adherence to Daily Human Growth Hormone Treatments Among Pediatric and Adolescent Patients in Japan: A Cross-Sectional Survey of Physicians and Caregivers
Authors Akazawa M , Shima D , Sato T , Shoji E, LoPresti M , Nishi R
Received 9 July 2022
Accepted for publication 18 October 2022
Published 10 November 2022 Volume 2022:16 Pages 3081—3094
DOI https://doi.org/10.2147/PPA.S380871
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Manabu Akazawa,1 Daisuke Shima,2 Takahiro Sato,2 Emi Shoji,2 Michael LoPresti,3 Ryosuke Nishi3
1Department of Public Health and Epidemiology, Meiji Pharmaceutical University, Tokyo, Japan; 2Medical Affairs, Rare Disease, Pfizer Japan Inc, Tokyo, Japan; 3Market Access, INTAGE Healthcare Inc, Tokyo, Japan
Correspondence: Takahiro Sato, Medical Affairs, Rare Disease, Pfizer Japan Inc, 3-22-7 Yoyogi, Shibuya-ku, Tokyo, 151-8589, Japan, Tel +81 90-2935-0848, Email [email protected]
Background: Poor adherence to daily human growth hormone (hGH) treatment has been shown to be associated with poor clinical outcomes for growth hormone deficiency (GHD) patients. However, few studies have examined the perception of adherence to hGH treatment among both physicians and caregivers in Japan.
Objective: The aim of this study is to examine the perception of adherence for daily hGH treatment among physicians and caregivers of pediatric and adolescent patients treated with GH in Japan. Moreover, we explore reasons for skipping treatment and the potential impact of a once-weekly treatment on adherence.
Methods: A cross-sectional survey was conducted in Japan among physicians that prescribe daily hGH treatment and caregivers that have administered daily hGH treatment to children/adolescents for 3 months or longer. The Morisky Medication Adherence Scale (MMAS-8) was used to gauge perceived adherence for both physician and caregiver groups. Caregivers were also questioned regarding reasons for missing injections. Moreover, both groups were asked about the impact of a once-weekly treatment on adherence.
Results: Responses were collected from 123 physicians and 112 caregivers. Physicians reported that 18.1% of patients have poor adherence based on the MMAS-8 instrument. In contrast, 32.1% of the caregivers reported poor adherence. “Simply forgetting”, “Patient refused/resisted”, and being “Busy with school activities, etc” were the most commonly selected reasons by caregivers for missing an injection. Physicians felt that a once-weekly injection could improve adherence for 64.5% of patients with poor adherence. Moreover, 56.9% of the caregivers that reported an experience of missed injections felt that a once-weekly injection would improve their adherence.
Conclusion: Approaches to improve adherence to hGH treatment in Japan are continuously needed. While further research is needed to understand factors most likely to improve adherence, availability of a once-weekly treatment is expected to help improve adherence.
Keywords: adherence, growth hormone, Japanese, recombinant, injections, MMAS
Introduction
Growth hormone deficiency (GHD) is a medical condition whereby the body does not produce enough growth hormone factor which can result in a noticeable reduction in height. It is the most common pituitary hormone deficiency in children.1 In Japan GHD is said to affect less than 1% of the pediatric population, but there are estimated to be about 2000 new GHD patients per year.2–4 Recombinant human growth hormone (rhGH) administered daily via subcutaneous injections has been available since 1988.3 Guidance on the treatment of GHD for pediatric patients in Japan recommends a dose of 0.175 mg of somatropin (recombinant) per kilogram of body weight per week by subcutaneous injection, in 6 or 7 divided doses (hereafter referred as “daily hGH treatment”).5 A once-weekly, long-acting rhGH (somatrogon) was approved in January 2022 for pediatric GHD (pGHD) in Japan.6 Researchers and healthcare professionals have suggested that a once-weekly formulation for hGH treatment may lead to an improvement in treatment adherence and a reduction in the burden of treatment.7,8 However, since a once-weekly treatment option has only been available in Japan since January 2022, its contribution to adherence is still unclear.
Adherence to treatment may be defined as the extent to which patients follow recommendations from healthcare professionals concerning treatment administration.9,10 Poor adherence can involve a number of intentional or unintentional actions to limit treatment, including a reduction in the total amount of the dose administered and skipping doses altogether by patients due to concerns about the safety of the medication or the risk of administering too much medication, for example.11–14 Lack of adherence to daily hGH treatment has been shown to be associated with a number of negative outcomes, including poorer clinical outcomes, poorer QOL, and higher treatment costs.15–21 In Japan, treatment outcomes are thought to be further hindered by a lower regulatory-approved posology of dosing relative to the US and Europe.22
Despite the potential benefit of daily hGH treatment and the positive relationship between adherence in treatment outcomes, adherence with treatment is thought to be suboptimal. A recent systematic review (SR) of publications that included adherence data for rhGH treatment found that adherence to rhGH treatment was high (<80%) for many treatment studies.23 However, the studies included in that SR varied in the way that they defined, measured, and reported adherence making comparability and generalizability of the studies included difficult. Another SR of studies on factors associated with non-adherence in pGHD published between 1985 and 2018 found that as many as 71% of the GHD patients may be non-adherent to treatment in some cases.14 However, estimates reported by the identified studies varied widely ranging from 7% to 71% poor adherence overall depending on the setting that the study was conducted in (eg, clinical setting versus non-clinical setting), the method of gauging adherence (eg, scale-based questionnaire approach versus database study), and the definition of adherence used. Moreover, the previously mentioned SRs did not include any studies in Japan, and they considered adherence with treatment for a wide range of underlying conditions that can affect stature, including GHD, Turner Syndrome, short stature homeobox-containing gene (SHOX) deficiency, etc; thus, it may not be considered relevant to the Japanese population with pGHD.
An online study conducted in 2015 among Japanese persons, who reported having been treated for GHD or who had children who were treated for GHD, found that nearly two-thirds (64.3%) had missed a treatment in the past.17 Moreover, about one out of four participants in that study reported that they do not always follow the instructions of their physician concerning administration of treatment. Specifically, 20% of the participants said that they follow the instructions of their physician only 70–89% of the time and 3.5% followed the instructions of their physician less than 70% of the time overall. Other studies conducted in Japan have reported very high levels of adherence to hGH treatment but were conducted in a clinical trial setting where treatment adherence is expected to be higher.24,25
While previous research suggests that adherence may be moderate to high among GHD patients and their caregivers in Japan, a lack of consistency in the clinical trial setting and the method used to ascertain adherence makes the results of previous research difficult to interpret.17,24,25 Also, no studies were found that use a similar scale to examine the perception of adherence between physicians and caregivers of patients undergoing daily hGH treatment in Japan. In this study, we examine adherence to daily hGH treatment from the perspective of Japanese physicians and caregivers and consider the difference in perceived adherence using a similar scale. We also consider reported reasons for not injecting among caregivers – including those reporting poor adherence and those reporting moderate or good adherence. Lastly, we consider the perceived contribution of a once-weekly injection for patients with poor adherence.
Methods
Study Design and Participants
Two prospective, cross-sectional surveys were conducted in September 2021. First, an online survey was completed by physicians that prescribe growth hormone treatment to pGHD patients aged 14 or younger. Next, an online survey was conducted among caregivers of patients being treated for pGHD or for being small for gestational age (SGA) that had been treated with growth hormones for 3 months or more. Caregivers were defined as parents or guardians of children with GHD who are currently undergoing daily hGH treatment. All caregivers or their spouse/partner were the primary person administering rhGH treatment for the patient.
Physicians were identified from a registered panel of Japanese physicians practicing in Japan that is maintained by Plamed Inc. (Tokyo, Japan), a subsidiary of INTAGE Healthcare. Plamed maintains a panel of approximately 81,000 physicians employed at healthcare facilities in Japan, including about 3000 pediatricians and 1750 diabetes/endocrinology specialists. Caregivers were identified from a registered panel of Japanese consumers maintained by INTAGE Inc. (Tokyo, Japan) and a separate consumer panel managed by Rakuten Insight, Inc.. INTAGE maintains a panel of nearly 4 million consumers in Japan that actively participate in online research. Among those, about 720,000 persons have been screened to participate in studies related to their health. Rakuten Insight maintains a panel of approximately 2.2 million consumers that actively access the Rakuten site. Among those, nearly 450,000 persons have been screened to participate in studies related to their health.
Data collection was completed from September 3 to 9, 2021 for physicians and from September 9 to 16, 2021 for caregivers. An online survey comprising 20 questions was conducted among physicians, and an online survey comprising 29 questions was conducted among caregivers. The survey instrument was developed by the authors after a targeted review of existing literature on adherence. The draft survey instrument was reviewed by medical affairs specialists at Pfizer Japan Inc. (Tokyo, Japan) that specialize in GHD to improve its comprehension. Physicians and caregivers were contacted to answer an initial online screening questionnaire. Those meeting the study inclusion criteria were immediately invited to participate in the main online survey.
This study was conducted in accordance with the ethical principles that have their origin in the Declaration of Helsinki and that are consistent with Good Pharmacoepidemiology Practices and the applicable laws and regulations of Japan. Moreover, this study adhered to the European Pharmaceutical Market Research Association (EphMRA) Code of Conduct.26 Ethical approval for this study was obtained from the ethical review board maintained by the Saga Memorial Hospital based in Saga, Japan, prior to starting data collection. Informed consent to participate in the study was obtained from all respondents prior to start of the survey.
Survey Content
The online survey completed by physicians included questions about their background such as their primary specialty, certifications, and type and size of facility. Moreover, physicians were asked about the number GHD patients aged 0–14 years that they currently prescribe hGH treatment by school-age group. The online survey completed by caregivers included various questions on the background of the person involved in administering treatment such as their age gender, employment status, annual household income, etc. The caregiver survey also included questions concerning the patients that they care for such as their age, gender, etc.
Both groups were asked to report about adherence to daily hGH treatment using the eight-item Morisky Medication Adherence Scale (MMAS-8). The MMAS-8 instrument is a structured self-report measure of medication-taking behavior that has been widely used in various countries for a number of different conditions.12,27–33 A Japanese version of the MMAS-8 is available from the license holder and has been used outside Japan among GHD and SGA patients, and has been used in Japan among non-GHD patients/caregivers.34,35 The items included in the MMAS-8 instrument are shown in Table 1. Physicians were asked about the number of their GHD patients undergoing daily hGH treatment that may be considered to have low, medium, or high adherence, based on the MMAS-8 instrument. Caregivers were asked to complete the MMAS-8 instrument and were also asked if they have ever skipped one or more injection during a 1-week period – and if so, how recently.
 |
Table 1 The 8-Item Morisky Medication Adherence Scale Items |
Lastly, both physicians and caregivers were asked about how the availability of a once-weekly treatment option may affect adherence to hGH treatment. Physicians were asked to assume that they treat 10 patients with poor adherence to hGH treatment. Then, they were asked how many of those patients would experience an improvement, worsening, or no effect on their adherence with the availability of a once-weekly treatment option. The number of total patients with poor adherence among all physicians that experienced an improvement, worsening, or no effect on adherence due to the availability of a once-weekly treatment option was then aggregated to establish an aggregate percentage for each potential impact. Caregivers who had experienced missing an injection were also asked if they felt that a once-weekly injection might improve, worsen, or not affect their adherence to treatment.
Statistical Analyses
Quantitative data collected were analyzed by INTAGE Healthcare using Microsoft Excel (2016) and statistical testing was conducted using R statistical software. A descriptive analysis of the data was performed using summary statistics for categorical and continuous data. For categorical data, frequencies and proportions are provided. Missing data were not an issue; the online survey method prevented its occurrence.
The primary outcome for this study was overall rates of adherence reported by physicians and caregivers based on the MMAS-8 instrument. Those with a score of less than 6 on the MMAS-8 instrument were considered to have poor adherence, those with a score of 6–7 were considered to have moderate adherence and those with a score of 8 were considered to have good adherence. This categorization has been used to described poor, moderate, and good adherences in previous studies that utilized the MMAS-8 instrument.12,29,34–38 For physicians, the number of patients treated with poor, moderate, and good adherence was divided by the total number of patients treated overall and for each school age group to determine the adherence rate. For patients, their score for the MMAS-8 instrument (1–8 points) was used to determine their adherence. The number of patients overall and for each school age group responding for each level of adherence was divided by the total number of patients (caregivers) overall and for each school age group to determine the adherence rate. A Chi-squared test was used to determine the significance of differences in responses about missing an injection and reasons for not injecting among those with poor adherence and those with moderate or good adherence. A p-value less than or equal to 0.05 was considered statistically significant.
Results
Characteristics of Study Participants
Table 2 shows the characteristics of those that completed the physician survey. The physician survey was completed by 123 physicians who prescribe growth hormone treatment to pGHD patients aged 14 or younger. Among those, 80.5% were male and most were aged 30–59 years (78.9%). This is in line with the overall population of physicians in Japan. Pediatrics was the primary specialty for 61.0% of the physicians interviewed, with the remainder being diabetes/endocrinology specialists. Most of the physicians interviewed (86.9%) were from hospitals with 100 or more beds, which is where gHD patients are mainly treated. The type of facility that physicians were primarily employed at varied with 59.3% being primarily employed at a national, municipal, or other public hospital and 40.6% at a private hospital or clinic. The 123 physicians interviewed treated 757 patients in total – or 6.2 pGHD patients, on average, per physician. Physicians reported that more than half of their pediatric patients undergoing hGH treatment (59.5%) were in first to sixth year of elementary school. Only about one out of six patients treated (15.9%) had not started elementary school yet. (While ages vary depending on the month that the child was born, those that have not started elementary school are generally under the age of 6 years, those in first to sixth year elementary school are generally aged 6 to 12 years and those in middle school are generally aged 12 to 15 years.)
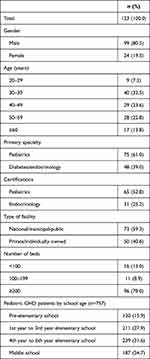 |
Table 2 Physician Baseline Characteristics |
Table 3 shows the characteristics of those that completed the caregivers survey. The caregiver survey was completed by 112 caregivers of patients being treated for pGHD or for being SGA that had been treated with growth hormones for 3 months or more. Over half (54.5%) of caregivers interviewed were female. Most caregivers (85.8%) were aged 30–49 years. About two-thirds (63.4%) of caregivers reported that their child was primarily being treated at a hospital and 80.4% were primarily being treated by a pediatrician. About two-thirds (66.1%) of GHD patients were male. More than half of GHD patients (56.2%) were in their first to sixth year of elementary school and about 29.5% of GHD patients were pre-elementary school-age patients.
 |
Table 3 Caregiver (and Patient) Baseline Characteristics |
Adherence to Daily hGH Treatment
Figure 1 shows a summary of responses concerning adherence to daily hGH treatment for physicians and caregivers overall. Physicians were asked about the percentage of their pediatric and adolescent patients undergoing daily hGH treatment (757 patients in total) that meet the criterion for poor adherence based on the MMAS-8 instrument. Physicians said that 18.1% of their pediatric and adolescent patients overall undergoing daily hGH treatment fit the criterion for poor adherence, defined as less than 6 points based on the MMAS-8 instrument. Among caregivers, however, nearly one-third (32.1%) reported poor adherence.
Figure 2 shows poor, moderate, and good adherence to daily hGH treatment for physicians and caregivers based on the MMAS-8 instrument by school age of the patient. For both physicians and caregivers, poor adherence was most commonly reported for pre-elementary school-age patients. Physicians suggested that 22.5% of the pre-elementary school-age patients have poor adherence and 48.5% of the caregivers of pre-elementary school-age patients reported poor adherence based on the MMAS-8 instrument. In contrast, physicians suggested that only 13.8% of fourth to sixth year elementary school-age patients and 18.7% of the middle school-age patients have poor adherence. Moreover, poor adherence reported by caregivers of older patients ranged from just 22.2% to 29.6% depending on the school age of the patient.
Table 4 shows the relationship between treatment adherence reported based on the MMAS-8 instrument and based on whether or not caregivers have experienced missing an injection once or more during a 1-week period. Nearly three out of five caregivers (58.0%) had experienced missing an injection once or more during a 1-week period. However, among those with poor adherence based on the MMAS-8 instrument, most caregivers reported that they had missed an injection once or more during a 1-week period (83.3%). The results of a Chi-square test also suggest a significant difference between those with poor adherence and those with moderate/good adherence based on the MMAS-8 instrument when it comes to missing an injection once or more during a 1-week period. Overall, this suggests a positive relationship between poor adherence as assessed by the MMAS-8 instrument and missing an injection once or more during a 1-week period.
 |
Table 4 Experience Having Missed an Injection Once or More During a 1-Week Period by Adherence Based on MMAS-8 Instrument |
Reasons for Not Injecting Daily hGH Treatment
Table 5 shows reasons for missing an injection of daily hGH treatment among caregivers that had experienced missing an injection once or more during a 1-week period (58.8% of the caregivers) overall and by adherence reported based on the MMAS-8 instrument. Among those caregivers, “Simply forgot” was the most commonly reason for not injecting with 52.3% of caregivers selecting it. Moreover, among caregivers that had experienced missing an injection once or more during a 1-week period AND reported poor adherence based on the MMAS-8 instrument, 70.0% selected “Simply forgot” as a reason for not injecting – which was significantly higher than those with moderate or good adherence (p=0.008). “Went somewhere and stayed overnight” was the second most commonly selected reason for missing an injection once or more during a 1-week period with 30.8% of caregivers selecting it. “Patient refused/resisted” was also commonly selected as a reason for missing an injection by caregivers with 18.5% selecting it overall. Among caregivers that had experienced missing an injection once or more during a 1-week period AND reported poor adherence based on the MMAS-8 instrument, 33.3% selected “Worry about side effects” and 23.3% selected “Worry about safety” as a reason for not injecting once or more often during a 1-week period – which was significantly higher than those with moderate or good adherence (p<0.001 and p=0.002, respectively).
“Busy with school activities, etc” was selected by 13.8% of the caregivers overall as a reason for missing an injection. Moreover, 13.3% of the caregivers had experienced missing an injection once or more during a 1-week period AND reported poor adherence based on the MMAS-8 instrument, selected “Ran out of medication/was unable to resupply/forgot to resupply” as a reason for missing an injection – which was significantly higher than those with moderate or good adherence (p=0.026).
Potential Impact of Less Frequent Injections
At the time of the survey, a once-weekly injection was not yet approved for treatment in Japan. All physicians (n=123) and caregivers that had missed an injection one or more times during a 1-week period (n=65) were also asked about the impact that an option for a once-weekly injection might have on adherence. Figure 3 shows the responses to those questions. Among 1230 hypothetical patients with poor adherence that were treated by physicians (10 per physician), the availability of a once-weekly injection was expected to improve adherence for 793 patients (64.5%). Physicians suggested that treatment adherence would not change for 322 patients (26.2%) and that it might worsen for 115 patients (9.3%) based on the availability of a once-weekly injection.
Among caregivers who had missed an injection once or more during a 1-week period, caregivers were asked more directly how their adherence to treatment may differ if there were a once-weekly treatment option available. Among those, 56.9% suggested that their number of missed injections would be fewer, 40.0% felt that their number of missed injections would not change, and 3.1% felt that their number of missed injections would increase.
Discussion
Findings from this study suggest that actual adherence to daily hGH treatment may be worse than the level of adherence that physicians perceive it to be in Japan. The results of the physician survey suggest that about 18.1% of the pediatric and adolescent patients in Japan undergoing hGH treatment are perceived by physicians to have poor adherence based on the MMAS-8 instrument. However, caregivers of patients undergoing hGH treatment reported a higher degree of poor adherence, with 32.1% reporting poor adherences based on the MMAS-8 instrument. Simply forgetting to administer treatment, busy travel schedules, concern about side effects, and concern about safety were the most common reasons given by caregivers for not injecting among those that had missed an injection during a 1-week period, and those reasons were particularly common among caregivers who reported poor adherence based on the MMAS-8 instrument.
For this study, both physicians and caregivers who had missed an injection one or more times during a 1-week period felt that a once-weekly treatment option is likely to improve adherence for those with poor adherence, so the availability of that treatment option is expected to improve adherence in Japan for those that miss injections. In a previous study conducted among adult GHD patients at a treatment center in Germany, 36% of the patients said they want to start or switch to a once-weekly treatment and 44% considered it a possibility.39 Moreover, patients with childhood-onset GHD (CoGHD) more commonly responded that they would be willing to switch to a once-weekly treatment compared to adult-onset GHD (AoGHD) patients (57% vs 23%). As such, the perceived benefit of a once-weekly treatment may differ depending on the age of onset of GHD.
The findings from this study are important in that, to our knowledge, they are the first such findings in Japan that consider adherence to treatment for those undergoing daily hGH treatment based on a commonly used adherence assessment scale – ie, the MMAS-8 instrument. While there are several ways to gauge adherence to treatment, the MMAS-8 instrument has been commonly used across numerous countries and disease areas which allows for greater comparison based on a common scale.12,29–38 Moreover, this is the first study that considered adherence for daily hGH treatment in Japan from the perspective of both physicians and caregivers of patients. These findings have highlighted a clear difference in the perception of treatment adherence for those two groups in Japan based on the MMAS-8 instrument which should be considered.
The adherence level observed for the present study is substantially lower than levels reported for previous studies that examined adherence to rhGH treatment in Japan.24,25 However, those previous studies were conducted in a more controlled clinical setting and which may have led to higher levels of adherence. Findings from previously conducted SRs that examined studies related to adherence to rhGH treatment have also suggested a higher degree of adherence ranging from about 71% to 80%.14,23 However, the way that the studies included in those reviews defined and measured adherence and the setting in which they observed adherence differed which makes comparison difficult. For example, most studies have relied on self-reporting of number of non-adherent days over a defined period and some have been conducted in a clinical setting which may lead to higher adherence due to the more controlled treatment. Previous studies that have considered adherence to treatment for rhGH treatment using the MMAS-8 instrument, on the other hand, have reported lower levels of adherence than those observed for the present study.12,29 Findings from Mohseni et al, for example, suggested that 43% of hGH treatment users have poor adherence based on the MMAS-8 instrument that was conducted in a non-clinical setting. That level is closer to the levels observed for the present study compared to those reported in previous studies that used different measures and different settings.
Simply forgetting to administer treatment, busy travel schedules, concern about side effects, and concern about safety were the most common reasons given for missing injections. Previous studies also identified degree of forgetfulness and experience with treatment side effects as factors associated with non-adherence to daily hGH treatment.14,16,27,28 Other patient characteristics identified by previous studies as being associated with adherence for daily hGH treatment include the education level of the patient or their caregiver, the patient’s age/pubertal stage, duration (years) since treatment was started, and/or ability to handle devices in general.27,28 Other treatment factors identified by previous studies to be associated with adherence for daily hGH treatment include the type of device used and its ease-of-use or issues with its use, injection frequency, experience with treatment side effects, and/or dissatisfaction with treatment efficacy.16,27 Moreover, the physician–patient relationship (eg, poor communication), patient choice in device selection, discomfort or pain associated with treatment, psychological issues with the patient, lack of support from family or caregivers, and the perceived benefit and/or risk following from treatment have also been shown in previous studies to be associated with adherence for daily hGH treatment.27
Given the gap in perceived and reported adherence among physicians and caregivers, it may be the case that physicians are unaware of the challenges and concerns that patients and caregivers face when it comes to regularly and frequently administering treatment. In fact, previous studies have suggested that better physician and patient communication can improve adherence for GH treatment and treatment for other diseases that require regular treatment such as osteoporosis and hypertension.40–42 As such, programs or services that help patients remember to administer treatment and/or that can alleviate concerns about the side effects and safety related to treatment through comprehensive and ongoing updates may lead to an overall improvement in treatment adherence. A multicenter prospective cohort study conducted in Japan among patients undergoing GH treatment found that the use of a digital app to support with treatment can lead to an improvement in adherence.43 Moreover, better tools for physicians to measure and address the burden and concerns that patients and caregivers have with regard to treatment might help improve adherence to treatment. Once-weekly injections as opposed to more regular injections have also been shown to improve adherence to treatment for some diabetes medications, for example.44,45 Therefore, the recent approval of a once-weekly treatment option may help improve adherence.
In some cases, less frequent injections may lead caregivers or patients to forget to administer treatment, but physicians felt that, in the case of hGH treatment, it would lead to an improvement in adherence for more than half those that exhibit poor adherence, and for most others there would be no negative impact. Caregivers who had experienced missing injections in the past responded similarly when asked about the impact of a once-weekly treatment option, with most suggesting that the net benefit of a once-weekly treatment option is likely to be an overall improvement in treatment adherence, if that treatment option was available.
Study Limitations
Although this study is unique, it has a number of limitations. First, although this study was designed to gauge differences in the perception of adherence to treatment and reasons for not injecting daily hGH treatment among physicians and caregivers in Japan, we were unable to link the responses of physicians to their specific patients. When considering gaps in the perception among physicians and patients (or caregivers), a method that allows linking the responses of physicians directly with patients that they personally manage would be ideal in terms of interpreting differences and considering underlying issues that may lead to those differences. However, that method was not possible for this study due to the challenge of recruiting caregivers through physicians or healthcare facilities. Regardless, we were able to observe some clear differences in the aggregate without directly linking physicians to caregivers.
Next, although this study only included physicians that treat pediatric GHD patients and caregivers that support with administering rhGH injections, in some cases, patients may administer treatment themselves. An initial screening of caregivers of GHD patients conducted by INTAGE Healthcare indicated that about one out of three patients primarily administer treatment themselves and most of those patients were 13 years or older. For this study, however, we only included caregivers of GHD patients who were the primary person administering treatment or whose spouse/partner was the primary person administering treatment. This is primarily due to the fact that in Japan, in order to interview persons under the age of 15, consent is required from their parent/guardian and that was difficult to achieve using an online consumer panel to recruit respondents. Future studies may examine the adherence of treatment among older patients that primarily administer treatment themselves.
Lastly, it is important note that while the MMAS-8 instrument is a widely used and validated instrument and has been used for numerous studies in Japan, relatively few cases were found where it has been used to consider adherence to treatment for daily hGH treatment and where it has been used among caregivers. For this study, most of the caregiver respondents were likely to have been the parent (mother or father) of the patient and were confirmed to play a direct role in hGH treatment administration for the patient. As such, they are more directly responsible for medication adherence than the patient themselves. Moreover, the MMAS-8 instrument and similar survey-based instruments are not the only method for ascertaining adherence. A database method that examines prescribing and refills such as the medication possession ratio (MPR), the proportion of days covered (PDC), or direct measurement using a “brown bag” review of dispensing in cooperation with pharmacies may yield less biased results given that those approaches do not only rely self-reporting.46,47 However, we chose to use a self-reported, survey-based study design in order to explore the reasons for low adherence and attitudes towards treatment in more detail.
Conclusion
Adherence to treatment was found to be an issue for about one-third of caregivers who administer daily hGH treatment to patients in Japan when measured using the MMAS-8 instrument in a non-clinical setting. However, physicians felt that fewer patients meet the criterion for poor adherence based on the MMAS-8 instrument, suggesting a substantial gap in the perception of adherence between physicians and caregivers. As such, approaches to improve adherence in Japan – particularly for pre-elementary school age children – are needed. Additional research may help understand specifically what aspects are most likely to improve adherence, but a long-acting hGH treatment option was reported as likely to help improve adherence for patients in Japan. Moreover, programs to reduce concerns about side effects or safety may improve adherence even further.
Acknowledgments
We thank Michele Stewart, Jane Loftus, and Monica Nijher from Pfizer Inc. for their support with the review of this manuscript and advice concerning the content. The MMAS-8 instrument, content, name, and trademarks are protected by US copyright and trademark laws. Permission for use of the scale and its coding is required. A license agreement is available from Donald E. Morisky, ScD, ScM, MSPH; [email protected].
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This research was funded by Pfizer Japan Inc.
Disclosure
MA is a full-time employee of Meiji Pharmaceutical University and has received some payments from Pfizer Japan Inc for lectures. DS, TS and ES are full-time employees of Pfizer Japan Inc. ML and RN are full-time employees of INTAGE Healthcare Inc., which received funding from Pfizer Japan Inc. to conduct, analysis and reporting for this study. The authors report no other conflicts of interest in this work.
References
1. Merck Sharp & Dohme Corp. Merck Manual Professional Version. New Jersey: Merck Sharp & Dohme Corp; 2021.
2. Tanaka T. Growth hormone treatment in Japan: past, present, and future. Pediatr Endocrinol Rev. 2012;10(Suppl 1):89–97.
3. Yokoya S, Tanaka T. The history of growth hormone treatment for GHD in Japan. Pediatr Endocrinol Rev. 2017;14(Suppl 1):201–208. doi:10.17458/per.vol14.2017.yt.historygrowthhormone
4. Ministry of Health, Labor and Welfare. Research on the practical infrastructure for the promotion of measures against chronic specific diseases in children. Policy Research Project on Intractable Diseases (Policy Research Project on Intractable Diseases). Study Report. FY2008-2010. Ministry of Health, Labor and Welfare. Available from: https://www.shouman.jp/research/pdf/23_2830/2830_18.pdf.
5. Ministry of Health, Labor and Welfare. A guide to the diagnosis and treatment of interstitial pituitary dysfunction. FY2008 health and labor sciences research grants for intractable diseases policy research project research: research on pituitary gland dysfunction. Ministry of Health, Labor and Welfare; 2008.
6. Yakushokushinn / Daiichi-bukai 5seihin no shounin ryoshou koukesshoban-yaku efeint no kyoketsusei noukekkan shougai-go no saihatsu yokusei no kouno tsuika mo [The First Committee of the Pharmaceutical and Food Safety Council approved five products, including an additional indication for the antiplatelet agent Effient to prevent recurrence of ischemic cerebrovascular disease]. Mikusu. Japanese. Available from: https://www.mixonline.jp/tabid55.html?artid=72170.
7. Lal RA, Hoffman AR. Perspectives on long-acting growth hormone therapy in children and adults. Arch Endocrinol Metab. 2019;63(6):601–607. doi:10.20945/2359-3997000000190
8. Yuen KCJ, Miller BS, Boguszewski CL, et al. Usefulness and potential pitfalls of long-acting growth hormone analogs. Front Endocrinol. 2021;24(12):637209. doi:10.3389/fendo.2021.637209
9. Horne R. Compliance, adherence, and concordance: implications for asthma treatment. Chest. 2006;130:65S–72S. doi:10.1378/chest.130.1_suppl.65S
10. National Institutes for Health and Care Excellence. Medicines Adherence: Involving Patients in Decisions About Prescribed Medicines and Supporting Adherence. London: National Institutes for Health and Care Excellence; 2009.
11. Norgren S. Adherence remains a challenge for patients receiving growth hormone therapy. Pediatr Endocrinol Rev. 2009;6(Suppl 4):545–548.
12. Mohseni S, Heydari Z, Qorbani M, et al. Adherence to growth hormone therapy in children and its potential barriers. J Pediatr Endocrinol Metab. 2018;31(1):13–20. doi:10.1515/jpem-2017-0157
13. Osterberg L, Blaschke T. Adherence to medication. N Engl J Med. 2005;353:487–497. doi:10.1056/NEJMra050100
14. Graham S, Weinman J, Auyeung V. Identifying potentially modifiable factors associated with treatment non-adherence in paediatric growth hormone deficiency: a systematic review. Horm Res Paediatr. 2018;90:221–228. doi:10.1159/000493211
15. Tanaka T, Sato T, Yuasa A, et al. Patient preference for growth hormone treatment in Japanese children. Pediatr Int. 2021;63(10):1185–1191. doi:10.1111/ped.14760
16. Graham S, Neo S, Auyeung V, et al. What potentially modifiable factors are associated with treatment nonadherence in pediatric growth hormone deficiency? A quantitative study. Endocr Pract. 2021;27(2):146–151. doi:10.4158/EP-2020-0543
17. Nishinaga H, Kishimoto S, Nishi Y. Survey of growth hormone treatment in clinical practice and patient choice of device. Prog Med. 2015;35:1348–1352.
18. Kapoor R, Burke S, Sparrow S, et al. Monitoring of concordance in growth hormone therapy. Arch Dis Child. 2008;93:147–148. doi:10.1136/adc.2006.114249
19. Cutfield W, Derraik J, Gunn A, et al. Non-compliance with growth hormone treatment in children is common and impairs linear growth. PLoS One. 2011;6:e16223. doi:10.1371/journal.pone.0016223
20. Hartmann K, Ittner J, Müller-Rossberg E, et al. Growth hormone treatment adherence in prepubertal and pubertal children with different growth disorders. Horm Res Paediatr. 2013;80:1–5. doi:10.1159/000351800
21. Foo J, Maghnie M, Colao A, et al. Cost-consequence analysis of human recombinant growth hormone (r-hGH) treatment administered via different devices in children with growth hormone deficiency in Italy. Clinicoecon Outcomes Res. 2019;11:525–537. doi:10.2147/CEOR.S195265
22. Tanaka T. International comparison of adult height in children with growth hormone deficiency and limitations of growth hormone treatment in Japan. Pediatr Endocrinol Rev. 2017;14(Suppl 1):216–221. doi:10.17458/per.vol14.2017.t.internationalcomparison
23. Gomez R, Ahmed SF, Maghnie M, et al. Treatment adherence to injectable treatments in pediatric growth hormone deficiency compared with injectable treatments in other chronic pediatric conditions: a systematic literature review. Front Endocrinol. 2022;13:795224. doi:10.3389/fendo.2022.795224
24. Fumio O, Takahashi Y, Tahara S, Ogawa Y, Højby Rasmussen M, Takano K. Similar safety and efficacy in previously treated adults with growth hormone deficiency randomized to once-weekly somapacitan or daily growth hormone. Clin Endocrinol. 2020;93:620–628. doi:10.1111/cen.14273
25. Tanaka T, Susumu Y, Hoshino Y, et al. Long-term safety and efficacy of daily recombinant human growth hormone treatment in Japanese short children born small for gestational age: final report from an open and multi-center study. Clin Pediatr Endocrinol. 2018;27:145–157. doi:10.1297/cpe.27.145
26. Japan Marketing Research Association. EphMRA code of conduct (Japanese edition). Japan Marketing Research Association; 2018. Available from: https://www.jmra-net.or.jp/LinkClick.aspx?fileticket=L%2F3co0zdIpM%3D&tabid=466.
27. Sultan S, El-Hourani M, Rondeau E, et al. Categorizing factors of adherence to parenteral treatment in growth hormone deficiencies and hemophilia: what should be the targets for future research? Patient Prefer Adherence. 2018;12:2039–2063. doi:10.2147/PPA.S177624
28. Maggio M, Vergara B, Porcelli P, et al. Improvement of treatment adherence with growth hormone by easypod™ device experience of an Italian centre. Ital J Pediatr. 2018;44:113–121. doi:10.1186/s13052-018-0548-z
29. Giavoli C, Profka E, Giancola N, et al. Growth hormone therapy at the time of Covid-19 pandemic: adherence and drug supply issues. Eur J Endocrinol. 2020;183:L13–L15. doi:10.1530/EJE-20-0481
30. Morisky DE, Ang A, Krousel-Wood M, et al. Predictive validity of a medication adherence measure in an outpatient setting. J Clin Hypertens. 2008;10(5):348–354. doi:10.1111/j.1751-7176.2008.07572.x
31. Beriowitz DR, Foy CG, Kazis LE, et al. Impact of intensive bIood pressure therapy on patient- reported outcomes. Outcomes results from the SPRINT study (for the SPRINT Study Research Group). N Engl J Med. 2017;377(8):733–744. doi:10.1056/NEJMoa1611179
32. Bress AP, Bellows BK, King J, et al. Cost-effectiveness of intensive versus standard bIood pressure control. N Engl J Med. 2017;377(8):745–755. doi:10.1056/NEJMsa1616035
33. Orimo H, Sato M, Kimur S, et al. Understanding the factors associated with initiation and adherence of osteoporosis medication in Japan: an analysis of patient perceptions. Osteoporos Sarcopenia. 2017;3:174–184. doi:10.1016/j.afos.2017.10.002
34. Tanaka M, Kawakami A, Maeda S, et al. Validity and reliability of the Japanese version of the Morisky Medication Adherence Scale-8 in patients with ulcerative colitis. Gastroenterol Nurs. 2021;44(1):31–38. doi:10.1097/SGA.0000000000000533
35. Furue M, Takeuchi S, Murota H. Poor adherence to oral and topical medication in 3096 dermatological patients as assessed by the Morisky Medication Adherence Scale-8. Br J Dermatol. 2015;172(1):272–275. doi:10.1111/bjd.13377
36. Ichiyama S, Ito M, Funasaka Y. Assessment of medication adherence and treatment satisfaction in Japanese patients with psoriasis of various severities. J Dermatol. 2018;45(6):727–731. doi:10.1111/1346-8138.14225
37. Ikeda N, Kono A. The relationship between health habits, medication adherence, kidney function, and QOL among kidney transplant patients. J Jpn Acad Nurs Sci. 2018;38:365–373. doi:10.5630/jans.38.365
38. Miyazaki M, Nakashima A, Nakamura Y, et al. Association between medication adherence and illness perceptions in atrial fibrillation patients treated with direct oral anticoagulants: an observational cross-sectional pilot study. PLoS One. 2018;13(9):e0204814. doi:10.1371/journal.pone.0204814
39. Amereller F, Schillbach K, Schopohl J, et al. Adherence, attitudes and beliefs of growth hormone deficient patients – a questionnaire-based cohort study. Exp Clin Endocrinol Diabetes. 2019;129(2):112–117. doi:10.1055/a-0956-1919
40. Acerini CL, Wac K, Bang P, et al. Optimizing patient management and adherence for children receiving growth hormone. Front Endocrinol. 2017;8:313. doi:10.3389/fendo.2017.00313
41. Goldshtein I, Rouach B, Shamir-Stein N, et al. Role of side effects, physician involvement, and patient perception in non-Adherence with oral bisphosphonates. Adv Ther. 2016;33(8):1374–1384. doi:10.1007/s12325-016-0360-3
42. Świątoniowska-Lonc N, Polański J, Tański W, et al. Impact of satisfaction with physician–patient communication on self-care and adherence in patients with hypertension: cross-sectional study. BMC Health Serv Res. 2020;20(1):1046. doi:10.1186/s12913-020-05912-0
43. Urakami T. Effectiveness of a smartphone application on medication adherence in children with short stature receiving GH therapy: a multicenter prospective cohort study (GTL-App). Clin Pediatr Endocrinol. 2021;30(2):85–92. doi:10.1297/cpe.30.85
44. Uzoigwe C, Liang Y, Whitmire S, et al. Semaglutide once-weekly persistence and adherence versus other GLP-1 RAs in patients with type 2 diabetes in a US real-world setting. Diabetes Ther. 2021;12(5):1475–1489. doi:10.1007/s13300-021-01053-7
45. Qiao Q, Ouwens MJNM, Grandy S, et al. Adherence to GLP-1 receptor agonist therapy administered by once-daily or once-weekly injection in patients with type 2 diabetes in Germany. Diabetes Metab Syndr Obes. 2016;9:201–205. doi:10.2147/DMSO.S99732
46. Anghel LA, Farcas AM, Oprean RN. An overview of the common methods used to measure treatment adherence. Med Pharm Rep. 2019;92(2):117–122. doi:10.15386/mpr-1201
47. Akazawa M, Nomura K, Kusama M, et al. Drug utilization reviews by community pharmacists in Japan: identification of potential safety concerns through the brown bag program. Value Health Reg Issues. 2012;1(1):98–104. doi:10.1016/j.vhri.2012.03.001
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




