Back to Journals » International Journal of Nanomedicine » Volume 18
Peptide-Based Therapeutic HPV Cancer Vaccine Synthesized via Bacterial Outer Membrane Vesicles
Authors Chen H, Zheng X, Li L, Huang L, Huang W, Ma Y
Received 5 May 2023
Accepted for publication 17 July 2023
Published 8 August 2023 Volume 2023:18 Pages 4541—4554
DOI https://doi.org/10.2147/IJN.S416706
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Haoqian Chen,1 Xiao Zheng,2,3 Lingjue Li,1 Lishuxin Huang,1 Weiwei Huang,2 Yanbing Ma2
1Key Laboratory of Chemistry in Ethnic Medicinal Resources, State Ethnic Affairs Commission & Ministry of Education, School of Ethnic Medicine, Yunnan Minzu University, Kunming, People’s Republic of China; 2Laboratory of Molecular Immunology, Institute of Medical Biology, Chinese Academy of Medical Sciences and Peking Union Medical College, Kunming, People’s Republic of China; 3School of Life Sciences, Yunnan University, Kunming, People’s Republic of China
Correspondence: Lishuxin Huang, Key Laboratory of Chemistry in Ethnic Medicinal Resources, State Ethnic Affairs Commission & Ministry of Education, School of Ethnic Medicine, Yunnan Minzu University, Kunming, 650504, People’s Republic of China, Tel/Fax +86 871 6592 6040, Email [email protected] Yanbing Ma, Laboratory of Molecular Immunology, Institute of Medical Biology, Chinese Academy of Medical Sciences and Peking Union Medical College, 935 Jiaoling Road, Kunming, 650032, People’s Republic of China, Tel +86 871 6833 9287, Fax +86 871 6833 4483, Email [email protected]
Background: Peptide-based vaccines have broad application prospects because of their safety, simple preparation, and effectiveness, especially in the development of personalized cancer vaccines, which have shown great advantages. However, the current peptide-based vaccines often require artificial synthesis and intricate delivery technology, which increases the cost and complexity of preparation.
Methods: Here, we developed a simple technique for combining a peptide and a delivery system using the natural secretion system of bacteria. Specifically, we biosynthesized an antigenic peptide in bacteria, which was then extracellularly released through the bacterial secretory vesicles, thus simultaneously achieving the biosynthesis and delivery of the peptide.
Results: The system utilizes the natural properties of bacterial vesicles to promote antigen uptake and dendritic cell (DC) maturation. Therefore, tumor-specific CD4+ Th1 and CD8+ cytotoxic T lymphocyte (CTL) responses were induced in TC-1 tumor-bearing mice, thereby efficiently suppressing tumor growth.
Conclusion: This research promotes innovation and extends the application of peptide-based vaccine biosynthesis technology. Importantly, it provides a new method for personalized cancer immunotherapy that uses screened peptides as antigens in the future.
Keywords: tumor immunization, peptide vaccine, outer membrane vesicle, delivery system
Introduction
Immunotherapy is considered a potential cancer treatment strategy,1 in which therapeutic cancer vaccines are an important factor.2 The effectiveness of therapeutic cancer vaccines has been demonstrated in a variety of cancer types. At present, the therapeutic cancer vaccine strategy targeting screened personalized neoantigens has gradually shown great application prospects.3 Enabling the simultaneous display of multiple tumor antigens by linking antigens using a plug-and-play system may be a valuable approach for the development of personalized tumor vaccines against complex and heterogeneous tumor antigens.4,5 Recently, peptide-based vaccines have received increasing attention in both infectious disease and cancer research fields because of their convenient preparation, high safety, and favorable immunogenicity. Peptide-based vaccines can be intelligently designed to target specific MHC molecules for efficient presentation, thereby enhancing B-cell and T-cell responses. An advantage of peptide-based vaccines is that they are easy to synthesize, eliminating the need for many tedious processes, such as the expression and purification of vaccine antigens and in vitro transcription of nucleic acid vaccines. Peptide-based vaccines can be easily obtained through artificial synthesis, and have high purity. However, although artificially synthesized peptides are convenient, they have a higher cost. In addition, peptide-based vaccines generally require a suitable delivery system to protect and deliver the peptide, which increases the cost and complexity of peptide-based vaccine preparation.
Currently, more than 500,000 women are diagnosed with cervical cancer each year, and the disease causes more than 300,000 deaths worldwide. In most cases, high-risk subtypes of human papilloma virus (HPV) are the cause of the disease. However the disease is largely preventable.6 We have also conducted some previous research on the treatment of this disease and we established a vaccine technology platform based on norovirus virus-like particles (VLPs) displaying personalized antigens.7 These peptides were artificially synthesized and then linked to the VLP vector. To avoid the complex operation of peptide synthesis and reconnection, here, we synthesized an antigenic peptide in bacteria, and the natural bacterial secretion system mediated the extracellular release of the peptide. As a result, we bypassed the artificial synthesis of peptide-based vaccines and the complicated delivery system packaging process. Using Escherichia coli, we simultaneously accomplished peptide biosynthesis and peptide encapsulation using the bacterial vesicle delivery system.
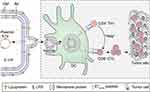
Bacterial outer membrane vesicles (OMVs) are spherical particles 20–250 nm in diameter that are naturally secreted by bacteria and produce OMVs when a small portion of the outer membrane projects from the cell and is released.8 It has been shown that the production of OMVs has been observed in various gram-negative bacteria at all stages of growth and in various growth environments (eg infected tissues).9 Bacterial outer membrane vesicles contain native bacterial material, including LPS, glycerophospholipids and outer membrane proteins, as well as closed outer membrane components.10 In particular, outer membrane vesicles have immunostimulatory pathogen-associated molecular patterns (PAMPs), including DNA, RNA, lipoproteins, LPS, and peptidoglycans. The PAMP component of outer membrane vesicles binds to host pattern recognition receptors (PRRs) and initiates proinflammatory signaling cascades leading to the production of cytokines, chemokines, and antimicrobial peptides,11 which have been well documented for their ability to promote immunity against cancer.12 Specifically, OMVs have been shown to act as agonists of TLR2/4/5,13 and TLR agonists are alternative strategies for inducing antitumor immune responses. Since the TLR located on the cell membrane has an extracellular structural domain containing leucine-rich repeat sequences that recognize different PAMPs and a Toll-interleukin 1 (IL-1) receptor (TIR) structural domain required for downstream signaling, this structural domain directs the activation of the transcription factor nuclear factor-κB (NF-κB) to induce proinflammatory cytokines and chemokines, as well as antigen presentation upregulation of costimulatory molecules on cells, such as macrophages (MΦ) and dendritic cells (DC), which in turn sensitize T cells to activation.14 In addition, the application of TLR agonists as adjuvants enhances the specificity and antigenicity of vaccine peptides and can induce strong T-cell and antibody-mediated responses, thus enhancing vaccine-induced long-term immune responses in recipients.15 On the other hand, vesicles are natural nanoscale carriers that can be easily loaded with various drugs. They can take advantage of their size to target antigen-presenting cells in lymph nodes for effective targeted transport and delivery in vivo.16 Therefore, bacterial vesicles are considered to be highly ideal therapeutic vaccine delivery systems.13
We have reported many studies based on bacterial outer membrane vesicle delivery systems.17–20 However, here, we show for the first time that short peptides can be enriched in outer membrane vesicles without any anchoring sequence. We think this is a very interesting and useful finding. Considering the natural ability of bacteria to synthesize antigens, we exploited the natural secretion system of bacteria to develop a simple technique that combines peptide biosynthesis and delivery system encapsulation. Specifically, we biosynthesized the antigenic peptide in bacteria, which was then released extracellularly through the natural secretion system of bacteria, thus achieving the biosynthesis and delivery of the peptide simultaneously. The system utilizes the natural properties of bacterial vesicles to promote antigen uptake and DC maturation. Therefore, HPV tumor-specific CD4+ Th1 and CD8+ CTL responses were induced in mice carrying TC-1 tumor cells, thereby efficiently inhibiting the growth of HPV tumors. With the technique we report, only one strain of bacteria is needed to effectively obtain an HPV tumor vaccine that automatically encapsulates the antigen in bacterial vesicles. The process is simple, and the production cost is very low. It saves the cost of artificially synthesizing peptide antigens and eliminates the complicated processing of linking antigens to bacterial vesicles. This research promotes innovation and extends the application of peptide-based vaccine biosynthesis technology. Importantly, this study provides a new method for personalized cancer immunotherapy using screened peptides as antigens in the future.
Materials and Methods
Ethics Statement
Animal experimentation and welfare procedures were approved by the Animal Care and Welfare Ethics Committee of the Institute of Medical Biology, Chinese Academy of Medical Sciences (Ethics Approval Number: DWSP201805008) in accordance with the Animal Ethics Guidelines of the National Health and Medical Research Council of China and the Laboratory Animal Management Office of Yunnan Province, China. All efforts were made to minimize animal suffering.
Plasmid Construction
The plasmid pET-28a was purchased from Sangon Bioengineering (Shanghai) Co., Ltd. The DNA fragment encoding the HPV16 E7 peptide was commercially synthesized by Sangon Bioengineering (Shanghai) Co., Ltd. The HPV16 E7 (amino acids 44–62) gene was introduced into the pET-28a vector between the Xho I and Nco I sites using gene recombination technology. The recombinant expression vector was transformed into competent BL21(DE3) ∆lpxm cells.
Expression of E7 Peptide and Preparation of OMVs
As previously described,5 the recombinant BL21(DE3) ∆lpxm cells were cultured in Luria–Bertani medium until the OD600 value was 0.4–0.6, and then isopropyl β-d-1-thiogalactoside (IPTG) was added at a final concentration of 1 mM to induce expression of the E7 peptide. After 16 h of induction, the cell-free culture supernatant was collected, centrifuged at 4°C and 14,000×g for 30 min, and then clarified by filtration through a 0.45 μm filter. The filtered supernatant was concentrated to 30 mL with a Pellicon XL tangential flow ultrafiltration membrane bag (PXB01MC50, Merck Millipore). The concentrate was further filtered through a 0.45 micron nylon membrane filter. After ultracentrifugation at 200,000×g and 4°C for 4 h (TiSW28 rotor, Beckman Coulter), the OMV pellet was finally resuspended in 400 μL PBS and stored at −20°C until use.
Protein Analysis
The total protein concentration of OMV preparations was determined with a Bradford kit (Sangon Biotech), and the result was defined as the OMV concentration. For SDS‒PAGE, samples were prepared in loading buffer containing β-mercaptoethanol and heated at 100°C for 5 min before electrophoresis on a 12% polyacrylamide gel. Finally the samples were stained with a Fast Silver Stain Kit (Beyotime).
Characterization of OMVs
The morphology of OMVs was observed and imaged by a Hitachi transmission electron microscope. In brief, 5 μL of the sample was dropped on a carbon-coated copper grid, and the excess liquid was blotted with filter paper. The OMV samples were stained and fixed with 0.75% (w/v) calcium carbonate buffer at 4°C. After drying for approximately 30 minutes, the cells were observed with a transmission electron microscope. The samples were analyzed for particle size using dynamic light scattering (DLS) (Zetasizer Nano ZS, Malvern Instruments) as previously described.21
Dot Blot Analysis
As previously described,22 the PVDF membrane was cut into a suitable size and marked, and then the membrane was activated in methanol for 5 minutes and soaked in transfer buffer. The membrane was dried on filter paper for 5 min at room temperature to eliminate residual moisture. Five microliters of OMV sample was dropped directly on the membrane, and the membrane was dried at 37°C for 5 minutes to fix the sample completely. The membranes were first blocked with 5% skim milk powder for 45 min, after which the peptides expressed in the OMV samples were tagged using mouse anti-HPV16 E7 antibody serum and horseradish peroxidase (HRP)-labeled anti-mouse IgG (H+L) (Invitrogen) as the primary antibody (1:500 dilution) and secondary antibody (1:10,000 dilution), respectively. The positive dots were developed using an enhanced chemiluminescence (ECL) reagent kit (Bio-Rad).
In vitro Bone Marrow-Derived Dendritic Cell (BMDC) Uptake
Femurs and tibias were isolated from mice. Bone marrow cells were flushed from the bones and treated with RBC lysis buffer (Solarbio). Cells were cultured in RPMI-1640 complete medium (with 10% fetal bovine serum and 1% penicillin‒streptomycin) supplemented with GM-CSF (20 ng/mL) (PeproTech). Dio (Beyotime)-labeled OMVs (0.05 mg/mL) were incubated with BMDCs for 4 h, and then stained with PE anti-mouse CD11c antibody (BioLegend). Imaging was performed using the Effector Cell Function Identification System (ImageXpress Micro Confocal, Molecular Devices).
Cell Lines and Mice
TC-1 tumor cells were derived from C57BL/6 mouse lung epithelial cells cotransformed with the HPV-16 oncoproteins E6 and E7 and the ras oncogene and were purchased from the Cancer Center, Chinese Academy of Medical Sciences. The TC-1 cells used expressed only mouse MHC molecules. Cells were cultured in RPMI-1640 containing 10% fetal bovine serum. Female C57BL/6 mice (6–8 weeks old, 16–18 g) were purchased from Beijing Weitong Lihua Experimental Animal Technology Co., Ltd. (China). All mice were housed under specific pathogen-free conditions at the Central Animal Care Center of the Institute of Medical Biology.
Flow Cytometry Analysis of Mature BMDCs
BMDCs (1×106/mL) were incubated with 50 μg/mL WT-OMVs, 50 μg/mL E7p-OMVs, and PBS for 24 h and then suspended in flow cytometry staining buffer. The cells were stained with PE anti-mouse CD11c antibody, FITC anti-mouse CD80 antibody, and FITC anti-mouse CD86 antibody (BioLegend) in the dark at 4°C for 30 min. Then, the expression levels of BMDC maturation markers were analyzed by flow cytometry.
OMVs Immune and HPV Tumor Model
Each mouse was subcutaneously injected with 5×105 TC-1 tumor cells in the right flank. When the tumor diameter reached 2–3 mm, the mice were randomly divided into 4 groups to receive immunotherapy: 10 μg E7p-OMV, 10 μg WT-OMV, 2.31 μg E7p (amino acids 44–62) and PBS control. Mice were injected subcutaneously every 10 days for a total of 3 injections.
Tumor volume was measured every few days starting from day 0. When the largest tumor reached the ethical cutoff point, the mice were executed and the tumor was first removed and weighed, after which the spleen and axillary lymph nodes were removed, and the corresponding lymphocytes were isolated. Briefly, splenocytes were first removed with a 70 μm cell strainer and suspended in 5 mL of lymphocyte separation medium (Shenzhen Dakwe Biotech Co., Ltd., China), and then 500 μL of RPMI-1640 culture medium was added. Immediately after centrifugation at 800×g for 30 min at room temperature, the lymphocyte layer was transferred to a new centrifuge tube. The cells were washed with fresh RPMI-1640 medium, and then centrifuged at 500×g for 5 min. Finally, the spleen lymphocytes at the bottom were suspended in complete RPMI-1640 medium for use. Lymph node fragments were incubated with 1 mg/mL collagenase (Merck Millipore) at 37°C for 1 h, and then the lymph node cells were removed with a 70 μm cell strainer, suspended in 5 mL of RPMI-1640 culture medium and washed once. Then, the samples were centrifuged at 500×g for 5 min at room temperature, and the lymph node lymphocytes at the bottom were suspended in complete RPMI-1640 culture medium for use.
Flow Cytometry Analysis of CTLs and Th1 Cells
Lymphocytes were stimulated with HPV16 E7 peptide (amino acids 44–62) for 3 hours, and Brefeldin A (BioLegend) was added and incubated for 4 hours to block the secretion of intracellular cytokines. Then, PerCP/Cy5.5 anti-mouse CD3 antibody, FITC anti-mouse CD4 antibody, APC anti-mouse CD8α antibody and PE anti-mouse IFN-γ antibody were used for corresponding CTL and Th1 antibody staining. Cells were analyzed using flow cytometry and data were analyzed using FlowJo Software.
Vaccine Tissue Pathology and Metabolic Disease Analysis
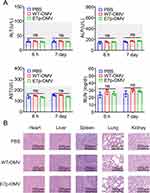
As previously described,23 10 days after the third immunization, the kidneys, hearts, lungs, livers, and spleens of the immunized mice were collected, and several samples were collected for pathological analysis in terms of tissue pathology. These samples were fixed with 10% formalin for 2 hours and sectioned for hematoxylin and eosin (H&E) staining. H&E images were acquired using a phase-contrast inverted fluorescence microscope (TS50, Nikon). To further evaluate the effect of E7p-OMV on metabolic aspects of the disease, 50 μg/50 μL E7p-OMV, WT-OMV or 6 μL PBS was subcutaneously injected into C57BL/6 mice (6–8 weeks old). Serum levels of alanine aminotransferase (ALT), aspartate aminotransferase (AST), alkaline phosphatase (ALP) and blood urea nitrogen (BUN) were measured using an automatic analyzer (Chemray-800, Rayto) 6 h and 7 d after injection.
Statistical Analysis
Statistical analysis was performed using Prism 8.0 (GraphPad software). Specific methods used to assess the statistical significance of differences between groups are shown in the figure legends. ****p < 0.0001, ***p < 0.001, **p < 0.01, *p < 0.05, ns, not significant.
Results
The HPV Cancer Peptide Antigen E7p Can Be Easily and Efficiently Loaded into Bacterial OMVs
Our goal was to achieve the total biosynthesis of peptides in bacteria, and to investigate whether these peptides synthesized in vivo can be extracellularly released through the bacterial efflux system (bacterial OMVs). Moreover, we wanted to achieve the simultaneous synthesis and delivery of antigens. We designed a prokaryotic expression plasmid without any tag (Figure 1A) to observe whether the expressed peptides would accumulate in the bacterial cell or be excreted. Since our aim was to complete peptide synthesis and vesicle encapsulation in one step, we focused on the ability of these peptides to be encapsulated and expelled by bacterial OMVs. In our experiments, it was found that the synthetic peptide was not enriched in bacteria, but was excreted by bacterial vesicles (Figure 1B). Dot immunoassays using anti-E7 antibodies demonstrated enrichment of this peptide. The massive enrichment of this peptide in bacterial vesicles suggested its great potential as a peptide vaccine. Compared with the loading capacity of peptide inside the bacteria (1.83%), the loading capacity of peptides in bacterial vesicles was 12.6 times that of bacterial cells, reaching 23.1%, specifically, 231 μg of E7p peptide was contained in every mg of secreted OMVs (Figure 1C). Since some peptides with membrane penetrating ability may damage the biomembrane, we investigated whether the loading of E7p would affect the structure of OMVs. The results of transmission electron microscopy showed that OMVs loaded with a large amount of peptides did not undergo structural damage (Figure 1D). However, through particle size analysis, we found that the particle size of OMVs loaded with E7p peptide became larger, from 145 nm to 163 nm, suggesting that peptide loading increased the space of the vesicle. To investigate the loading position of E7p on OMVs, we treated OMVs with proteinase K (PK), and the peptide inside was protected from degradation by the vesicle membrane, showing that E7p in E7p-OMVs was not decomposed. After destroying the membrane structure of OMVs with EDTA, PK decomposed the internal peptides (Figure 1F). These experiments demonstrated that the E7p peptide was mostly loaded into the interior of OMVs without being exposed on the surface. Compared with the delivery method of peptide exposure, the encapsulation method may have more advantages in stabilizing and activating cellular immunity. In summary, we designed a peptide antigen E7p for HPV cancer, that contained both CTL epitopes and Th cell epitopes. The E7p peptide gene expression plasmid was introduced into Escherichia coli cells, allowing Escherichia coli to synthesize a large amount of E7p peptides in the cells. The peptides were automatically excreted via OMVs. Thus, in vivo synthesis of the E7p peptide and E7p encapsulation using native bacterial OMVs was completed (Figure 1G).
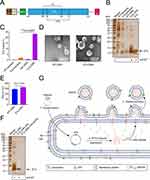 |
Figure 1 The HPV cancer peptide antigen E7p can be easily and efficiently loaded into the bacterial OMVs. Abbreviations: E7p, E7 peptide; CTL, cytotoxic T lymphocyte; Th, T helper cell; SDS‒PAGE, sodium dodecyl sulfate‒polyacrylamide gel electrophoresis; WT, wild-type; PK, proteinase K; EDTA, ethylene diamine tetraacetic acid; HPV, human papilloma virus; OMV, outer membrane vesicle. Notes: (A) Schematic diagram of the design of the E7p peptide and the construction of the expression plasmid. 49–57 represent CTL epitopes,24 48–54 represent Th epitopes,25 and others represent expression functional units on the plasmid. (B) The top side is a silver-stained SDS‒PAGE electrophoresis graph. Each lane shows Escherichia coli wild-type bacteria (WT-bacteria), bacteria transformed with the E7p short peptide (E7p-bacteria), and OMV released from the corresponding bacteria, with the E7p control being a synthetic peptide consistent with the E7p sequence. The figure below shows the dot blot analysis of protein samples reacted with anti-E7p antibody. (C) The loading levels of E7p in the whole samples were analyzed and column graph statistics were made using Bio-Rad Image Lab software. The experiment was repeated three times. (D) Representative transmission electron microscopy image of vesicles selected from three replicates. (E) Column graph statistics of vesicle sizes analyzed using a particle size analyzer. The experiment was repeated three times. (F) SDS‒PAGE and dot immunoassay results. Bacterial vesicles were treated with PK and PK with EDTA. (G) Schematic representation of the peptide antigen of HPV tumors efficiently loaded into the bacterial OMV through the natural secretion system of the bacteria. Data are presented as the mean ± standard deviation (SD). Statistical significance was calculated using one-way analysis of variance (ANOVA) (C) or Student’s t-test (E) to obtain p values. |
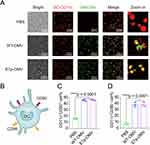
OMVs Loaded with Antigenic Peptides Can Increase the Uptake and Presentation of Antigens
Peptide-based vaccines are difficult for antigen-presenting cells (APCs) to take up, so the delivery system for transporting peptides is particularly important. Our experiments demonstrated that both WT-OMV and peptide-loaded E7p-OMV could be efficiently taken up by CD11c+ BMDC cells (Figure 2A). In addition, mature BMDCs exhibited increased expression of costimulatory molecules (Figure 2B), and the OMV vector also efficiently stimulated an increase in the number of CD80+ (Figure 2C) and CD86+ (Figure 2D) DC cells, suggesting that OMVs increased antigen presentation.
OMVs Loaded with Antigenic Peptides Can Suppress HPV Tumor Growth in Animal Models
We analyzed the tumor-suppressive effect of E7p-OMV in an HPV tumor-bearing mouse model established by TC-1 cells. After tumor establishment, HPV-tumor-bearing mice were given three subcutaneous injections of E7p-OMV (Figure 3A). We found that E7p-OMV exerted a very significant HPV tumor suppressive effect compared to that of the E7p peptide (Figure 3B and C), demonstrating the efficacy of this system as a therapeutic cancer vaccine delivery system.
OMVs Loaded with Antigenic Peptides Can Induce HPV Tumor Antigen-Specific Th1/CTL Responses
To clarify the tumor-suppressing mechanism of E7p-OMV, we analyzed the ability of E7p-OMV to induce antigen-specific CD4 and CD8 cells. The results showed that E7p-OMV induced significant specific Th1 (CD4+IFN-γ+) responses and specific CTL (CD8+IFN-γ+) responses in the spleen (Figure 4A) and lymph nodes (Figure 4B). It is suggested that this natural biosynthetic E7 antigen loaded inside OMVs can effectively induce an antigen-specific cellular immune response, and has broad application prospects.
OMVs Loaded with Antigenic Peptides Do Not Cause Tissue or Metabolic Diseases
The impact of OMVs on tissue and metabolic diseases is often a concern. Here, we used the BL21 strain with knockout of the virulence factor (LpxM) MsbB. This attenuated strain has proven its immunostimulatory activity and safety.26 Here, we proceeded with a preliminary evaluation of the metabolic impact of this peptide-loaded OMVs. From the blood biochemical analysis, 6 hours and 7 days after injection of OMVs, the AST, ALT, ALP, and BUN levels of the mice were all within the normal range, suggesting that OMVs does not cause metabolic disturbances. In addition, 10 days after 3 injections of OMVs, the organs were collected for H&E staining, and no obvious lesions were found. In summary, this peptide-based OMVs delivery system does not cause tissue disease or metabolic disorders.
Discussion
The effectiveness of therapeutic cancer vaccines has been demonstrated in a variety of cancer types.27–31 Cancer vaccines mainly use general tumor antigens, however, as a result of tumor heterogeneity, they often have poor efficacy.32 Personalized tumor vaccines can address individual differences. Personalized cancer vaccines, whether in the form of nucleic acid vaccines or peptide-based vaccines, use MHC-restricted epitope peptides as antigenic targets.33 The purpose of using peptides is that they are directly loaded onto the MHC-I molecules expressed by local submucosal dendritic cells (DCs) at the injection site,34 and then this antigen presentation induces antigen-specific CD8+ CTL cells to drain lymph nodes.35 The use of peptides as personalized vaccines has multiple advantages, such as relatively easy production, minimal induced toxic effects, and flexibility to combine with different and more effective immune adjuvants.36 Therefore, therapeutic cancer vaccines using peptides as antigens are of high research value. Currently, several HPV therapeutic vaccines have demonstrated good antitumor effects in animal models, but there is still no successful marketed HPV therapeutic vaccine, and continued exploration of new vaccine forms may give new hope for vaccine success. Here, we propose a new method of using biosynthesis to obtain peptide antigens. Only one strain of bacteria is needed to complete the simultaneous biosynthesis and delivery of peptide antigens. This method can be extended to other research fields investigating vaccine and drug delivery.
In this study and our previous investigations, corroborated by computer software analysis of bands from SDS‒PAGE electrophoresis and loading data obtained from WB-specific E7p-based reaction greyscale values, it was possible to find a low peptide load in bacteria and a relatively high peptide load in outer membrane vesicles (Figure 1B), which may have influenced the long-standing belief that peptides could only be expressed at low levels in bacteria. Our experiments prove that this peptide can be biosynthesized efficiently, but it is automatically excreted by bacterial vesicles. Therefore, many short peptides were successfully synthesized and effluxed through OMVs, resulting in very few peptides retained in the bacteria. We used this efflux mechanism as a new type of vaccine delivery system. OMVs loaded with peptide antigens were conveniently obtained, eliminating the need to prepare the vesicles first and then load the artificially synthesized antigens on the vesicles.37
The tumor suppressive effect of the HPV16 E7 (amino acids 44–62) peptide we tested here has been verified in our previous work.38,39 This peptide contains not only CTL epitopes but also several Th epitopes, so it can effectively activate specific CD4+ and CD8+ cell responses. However, the potential of this system extends beyond treating cervical cancer caused by HPV. Many peptide-based vaccines could potentially be effective using this system.
In this study, it was proven that vesicles expressing the E7p peptide were intact because the isoelectric point of this E7p peptide was predicted to be 5.38, and structural analysis indicated that the peptide was hydrophilic. In terms of specific discussion, the vesicle integrity was observed by transmission electron microscopy on the one hand, and by DLS light analysis to determine whether the OMV was intact, because if the OMV was fragmented, see our previous study,40 the OMV was clearly observed to occur differently under electron microscopy, and the particle size and distribution of the DLS would also appear different. Therefore OMV integrity can be confirmed in the experiment. However, when expanding the application of this system, it may be that the expressed protein can penetrate the membrane, which may lead to bacterial death or vesicle damage. Therefore, special attention should be given to the evaluation of the properties of the loaded peptide in the specific application of this system.
We demonstrated that E7p was encapsulated inside the vesicle (Figure 1F), which may be an advantage. Compared with exposed antigens, encapsulated antigens are less likely to be degraded41 and are more likely to cause cross-presentation,42 thereby promoting CTL responses.
Many peptide-loaded delivery systems have been reported, including liposomes,43 exosomes,44 chemical materials,45 two-dimensional materials,46 polysaccharides,47 iron metals,48 protein,49 poly(lactic-co-glycolic acid) (PLGA),50 virus-like particles (VLP)51 and nanofibers.52 Each delivery system has advantages and disadvantages. The advantage of the OMV delivery system is that surface PAMP molecules53 can be used to promote antigen uptake and stimulate DC maturation (Figure 2). Moreover, LPS on OMVs has the ability to induce Th1-related responses, so this system is particularly suitable for cancer immunity therapy. However, the toxicity of OMVs has always been a concern, and therapies using OMVs are still in the research and development phase, with insufficient general awareness and understanding of the various scenarios that ensure safety and efficacy.54 There is concern about administering treatments without full knowledge of them. If the technology needed for large-scale stable production of OMVs can be developed, this could lead to a variety of new therapeutic areas. As researchers, we will continue to work on the development of such technologies. Therefore, here, we used Escherichia coli with knocked out MsbB (lpxM). The low virulence of this modified Escherichia coli has been demonstrated many times,55–57 and based on the analysis of blood chemistry of mice to detect liver and kidney function it can be seen that the vaccine has no adverse effects on the metabolic function of the liver or kidney, while the analysis of histological sections also showed that the vaccine did not cause a significant inflammatory response (Figure 5), and these are the general toxicity tests for the vaccine.58 In addition to this, histological and other tests can actually be performed.59 In conclusion, the side effects of OMVs are relatively low. In addition, knocking out the pagP gene further resolved the safety problem of Escherichia coli vesicles,60 thus indicating that this bacterial vesicle delivery system has great application potential.
In summary, we have developed an HPV cancer vaccine method that rapidly synthesizes peptide antigens within bacteria and encapsulates them directly through the bacterial secretion system (OMVs). This bacterial vesicle can efficiently deliver peptide antigens to APCs, stimulate DC maturation, and induce peptide antigen-specific CD4+ Th1 and CD8+ CTL responses, thereby inhibiting HPV tumor development (Figure 6). This study details the simultaneous biosynthesis and delivery of peptide-based vaccines using bacterial biomaterials and represents the latest advances in synthetic biology, microbiology, tumor immunology, and nanoscience. This research will promote innovation and expand the application of peptide-based vaccine biosynthesis technology, providing new methods for cancer immunotherapy.
Conclusion
We synthesized antigenic peptides in bacteria, which were extracellularly released through the natural secretion system of bacteria. In this way, we bypassed the artificial synthesis of peptide-based vaccines and the complicated delivery system packaging process. Using Escherichia coli, we simultaneously achieved the biosynthesis and encapsulation of a peptide. This OMVs delivery system can promote antigen uptake and DC maturation, and induce HPV tumor-specific CD4+ Th1 and CD8+ CTL responses in tumor-bearing mice, thereby efficiently suppressing HPV tumor growth. However, this system of OMVs delivering tumor peptides also has challenges such as potential biotoxicity, lack of personalized immunogenicity and low natural yield. With the development of bioengineering technology and a better understanding of OMVs, the safety, immunogenicity and yield of OMVs can be further improved by manipulating the host bacteria. We believe that this study provides insights into the broad application potential of this bacterial-based natural secretion system.
Data Sharing Statement
The data that support the findings of this study are available from the corresponding author upon reasonable request.
Ethics Approval
All animal procedures were performed with ethical compliance and approved by the Animal Care and Welfare Ethics Committee, Institute of Medical Biology, CAMS (Ethics Approval Number: DWSP201805008).
Acknowledgments
This work was supported by the Science and Technology Project of Yunnan Province (202105AC160019, 202201AS070061, 202202AA100003, 202002AA100009, 2019FB045, 2016FA049) and the National Natural Science Foundation of China (81773270). The authors thank Mr Jingxian Zhou for excellent assistance with electron microscopy. H.C. and W.H. contributed equally to this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Zhang Y, Zhang Z. The history and advances in cancer immunotherapy: understanding the characteristics of tumor-infiltrating immune cells and their therapeutic implications. Cell Mol Immunol. 2020;17(8):807–821. doi:10.1038/s41423-020-0488-6
2. Saxena M, van der Burg SH, Melief CJM, Bhardwaj N. Therapeutic cancer vaccines. Nat Rev Cancer. 2021;21(6):360–378. doi:10.1038/s41568-021-00346-0
3. Liu Z, Lv J, Dang Q, et al. Engineering neoantigen vaccines to improve cancer personalized immunotherapy. Int J Biol Sci. 2022;18(15):5607–5623. doi:10.7150/ijbs.76281
4. Ragupathi G, Coltart DM, Williams LJ, et al. On the power of chemical synthesis: immunological evaluation of models for multiantigenic carbohydrate-based cancer vaccines. Proc Natl Acad Sci USA. 2002;99(21):13699–13704. doi:10.1073/pnas.202427599
5. Cheng K, Zhao R, Li Y, et al. Bioengineered bacteria-derived outer membrane vesicles as a versatile antigen display platform for tumor vaccination via Plug-and-Display technology. Nat Commun. 2021;12(1):2041. doi:10.1038/s41467-021-22308-8
6. Cohen PA, Jhingran A, Oaknin A, Denny L. Cervical cancer. Lancet. 2019;393(10167):169–182. doi:10.1016/S0140-6736(18)32470-X
7. Zheng P, Yang Y, Fu Y, et al. Engineered norovirus-derived nanoparticles as a plug-and-play cancer vaccine platform. ACS Nano. 2023;17(4):3412–3429. doi:10.1021/acsnano.2c08840
8. Kulp A, Kuehn MJ. Biological functions and biogenesis of secreted bacterial outer membrane vesicles. Annu Rev Microbiol. 2010;64:163–184. doi:10.1146/annurev.micro.091208.073413
9. McBroom AJ, Kuehn MJ. Release of outer membrane vesicles by Gram-negative bacteria is a novel envelope stress response. Mol Microbiol. 2007;63(2):545–558. doi:10.1111/j.1365-2958.2006.05522.x
10. Ellis TN, Kuehn MJ. Virulence and immunomodulatory roles of bacterial outer membrane vesicles. Microbiol Mol Biol Rev. 2010;74(1):81–94. doi:10.1128/MMBR.00031-09
11. Kaparakis-Liaskos M, Ferrero RL. Immune modulation by bacterial outer membrane vesicles. Nat Rev Immunol. 2015;15(6):375–387. doi:10.1038/nri3837
12. Sheikh A, Taube J, Greathouse KL. Contribution of the microbiota and their secretory products to inflammation and colorectal cancer pathogenesis: the role of toll-like receptors. Carcinogenesis. 2021;42(9):1133–1142. doi:10.1093/carcin/bgab060
13. Zhao X, Zhao R, Nie G. Nanocarriers based on bacterial membrane materials for cancer vaccine delivery. Nat Protoc. 2022;17(10):2240–2274. doi:10.1038/s41596-022-00713-7
14. Mukherjee S, Karmakar S, Babu SP. TLR2 and TLR4 mediated host immune responses in major infectious diseases: a review. Braz J Infect Dis. 2016;20(2):193–204. doi:10.1016/j.bjid.2015.10.011
15. Padma S, Patra R, Sen Gupta PS, Panda SK, Rana MK, Mukherjee S. Cell surface fibroblast activation protein-2 (Fap2) of fusobacterium nucleatum as a vaccine candidate for therapeutic intervention of human colorectal cancer: an immunoinformatics approach. Vaccines. 2023;11:3.
16. Kesty NC, Kuehn MJ. Incorporation of heterologous outer membrane and periplasmic proteins into Escherichia coli outer membrane vesicles. J Biol Chem. 2004;279(3):2069–2076. doi:10.1074/jbc.M307628200
17. Hua L, Yang Z, Li W, et al. A novel immunomodulator delivery platform based on bacterial biomimetic vesicles for enhanced antitumor immunity. Adv Mater. 2021;33(43):e2103923. doi:10.1002/adma.202103923
18. Long Q, Zheng P, Zheng X, et al. Engineered bacterial membrane vesicles are promising carriers for vaccine design and tumor immunotherapy. Adv Drug Deliv Rev. 2022;186:114321. doi:10.1016/j.addr.2022.114321
19. Yang Z, Hua L, Yang M, et al. RBD-modified bacterial vesicles elicited potential protective immunity against SARS-CoV-2. Nano Lett. 2021;21(14):5920–5930. doi:10.1021/acs.nanolett.1c00680
20. Huang W, Shu C, Hua L, et al. Modified bacterial outer membrane vesicles induce autoantibodies for tumor therapy. Acta Biomater. 2020;108:300–312. doi:10.1016/j.actbio.2020.03.030
21. Li P, Luo W, Xiang TX, et al. Horizontal gene transfer via OMVs co-carrying virulence and antimicrobial-resistant genes is a novel way for the dissemination of carbapenem-resistant hypervirulent Klebsiella pneumoniae. Front Microbiol. 2022;13:945972. doi:10.3389/fmicb.2022.945972
22. Madhusudana SN, Paul JP, Abhilash VK, Suja MS. Rapid diagnosis of rabies in humans and animals by a dot blot enzyme immunoassay. Int J Infect Dis. 2004;8(6):339–345. doi:10.1016/j.ijid.2004.02.006
23. Yang Z, Hua L, Yang M, et al. Polymerized porin as a novel delivery platform for coronavirus vaccine. J Nanobiotechnology. 2022;20(1):260. doi:10.1186/s12951-022-01469-8
24. Feltkamp MC, Smits HL, Vierboom MP, et al. Vaccination with cytotoxic T lymphocyte epitope-containing peptide protects against a tumor induced by human papillomavirus type 16-transformed cells. Eur J Immunol. 1993;23(9):2242–2249. doi:10.1002/eji.1830230929
25. Tindle RW, Fernando GJ, Sterling JC, Frazer IH. A “public” T-helper epitope of the E7 transforming protein of human papillomavirus 16 provides cognate help for several E7 B-cell epitopes from cervical cancer-associated human papillomavirus genotypes. Proc Natl Acad Sci U S A. 1991;88(13):5887–5891. doi:10.1073/pnas.88.13.5887
26. Han Y, Li Y, Chen J, Tan Y, Guan F, Wang X. Construction of monophosphoryl lipid A producing Escherichia coli mutants and comparison of immuno-stimulatory activities of their lipopolysaccharides. Mar Drugs. 2013;11(2):363–376. doi:10.3390/md11020363
27. Hashemi R, Montazer M, Salehi Z, Azadbakht L. Association of recent and long-term supplement intakes with laboratory indices in patients with COVID-19 in Tehran, Iran, During 2020. Front Nutr. 2022;9:834826. doi:10.3389/fnut.2022.834826
28. Sahin U, Oehm P, Derhovanessian E, et al. An RNA vaccine drives immunity in checkpoint-inhibitor-treated melanoma. Nature. 2020;585(7823):107–112. doi:10.1038/s41586-020-2537-9
29. Zhu B, Sun Y, Wei X, et al. Dendritic cell vaccine loaded with MG-7 antigen induces cytotoxic T lymphocyte responses against gastric cancer. J Healthc Eng. 2022;2022:1964081. doi:10.1155/2022/1964081
30. Schuster J, Lai RK, Recht LD, et al. A Phase II, multicenter trial of rindopepimut (CDX-110) in newly diagnosed glioblastoma: the ACT III study. Neuro Oncol. 2015;17(6):854–861. doi:10.1093/neuonc/nou348
31. Walter S, Weinschenk T, Stenzl A, et al. Multipeptide immune response to cancer vaccine IMA901 after single-dose cyclophosphamide associates with longer patient survival. Nat Med. 2012;18(8):1254–1261. doi:10.1038/nm.2883
32. Hollingsworth RE, Jansen K. Turning the corner on therapeutic cancer vaccines. NPJ Vaccines. 2019;4:7. doi:10.1038/s41541-019-0103-y
33. Sahin U, Tureci O. Personalized vaccines for cancer immunotherapy. Science. 2018;359(6382):1355–1360. doi:10.1126/science.aar7112
34. Rock KL, Reits E, Neefjes J. Present Yourself! By MHC Class I and MHC Class II Molecules. Trends Immunol. 2016;37(11):724–737. doi:10.1016/j.it.2016.08.010
35. Bijker MS, van den Eeden SJ, Franken KL, Melief CJ, Offringa R, van der Burg SH. CD8+ CTL priming by exact peptide epitopes in incomplete Freund’s adjuvant induces a vanishing CTL response, whereas long peptides induce sustained CTL reactivity. J Immunol. 2007;179(8):5033–5040. doi:10.4049/jimmunol.179.8.5033
36. Hu Z, Ott PA, Wu CJ. Towards personalized, tumour-specific, therapeutic vaccines for cancer. Nat Rev Immunol. 2018;18(3):168–182. doi:10.1038/nri.2017.131
37. Mehanny M, Lehr CM, Fuhrmann G. Extracellular vesicles as antigen carriers for novel vaccination avenues. Adv Drug Deliv Rev. 2021;173:164–180. doi:10.1016/j.addr.2021.03.016
38. Zhang Q, Huang W, Yuan M, et al. Employing ATP as a new adjuvant promotes the induction of robust antitumor cellular immunity by a PLGA nanoparticle vaccine. ACS Appl Mater Interfaces. 2020;12(49):54399–54414. doi:10.1021/acsami.0c15522
39. Li S, Zhu W, Ye C, et al. Local mucosal immunization of self-assembled nanofibers elicits robust antitumor effects in an orthotopic model of mouse genital tumors. Nanoscale. 2020;12(5):3076–3089. doi:10.1039/c9nr10334a
40. Wang S, Huang W, Li K, et al. Engineered outer membrane vesicle is potent to elicit HPV16E7-specific cellular immunity in a mouse model of TC-1 graft tumor. Int J Nanomedicine. 2017;12:6813–6825. doi:10.2147/IJN.S143264
41. Blum JS, Wearsch PA, Cresswell P. Pathways of antigen processing. Annu Rev Immunol. 2013;31:443–473. doi:10.1146/annurev-immunol-032712-095910
42. Wagner H. The immunogenicity of CpG-antigen conjugates. Adv Drug Deliv Rev. 2009;61(3):243–247. doi:10.1016/j.addr.2008.12.010
43. Haddadzadegan S, Dorkoosh F, Bernkop-Schnurch A. Oral delivery of therapeutic peptides and proteins: technology landscape of lipid-based nanocarriers. Adv Drug Deliv Rev. 2022;182:114097. doi:10.1016/j.addr.2021.114097
44. Barile L, Vassalli G. Exosomes: therapy delivery tools and biomarkers of diseases. Pharmacol Ther. 2017;174:63–78. doi:10.1016/j.pharmthera.2017.02.020
45. Langer R. Chemical materials and their regulation of the movement of molecules. Q Rev Biophys. 2015;48(4):424–428. doi:10.1017/S0033583515000165
46. Zhang H, Fan T, Chen W, Li Y, Wang B. Recent advances of two-dimensional materials in smart drug delivery nano-systems. Bioact Mater. 2020;5(4):1071–1086. doi:10.1016/j.bioactmat.2020.06.012
47. Dos Santos MA, Grenha A. Polysaccharide nanoparticles for protein and Peptide delivery: exploring less-known materials. Adv Protein Chem Struct Biol. 2015;98:223–261. doi:10.1016/bs.apcsb.2014.11.003
48. Behzadi M, Vakili B, Ebrahiminezhad A, Nezafat N. Iron nanoparticles as novel vaccine adjuvants. Eur J Pharm Sci. 2021;159:105718. doi:10.1016/j.ejps.2021.105718
49. Pham DT, Tiyaboonchai W. Fibroin nanoparticles: a promising drug delivery system. Drug Deliv. 2020;27(1):431–448. doi:10.1080/10717544.2020.1736208
50. Allahyari M, Mohit E. Peptide/protein vaccine delivery system based on PLGA particles. Hum Vaccin Immunother. 2016;12(3):806–828. doi:10.1080/21645515.2015.1102804
51. Boisgerault F, Moron G, Leclerc C. Virus-like particles: a new family of delivery systems. Expert Rev Vaccines. 2002;1(1):101–109. doi:10.1586/14760584.1.1.101
52. Loo Y, Zhang S, Hauser CA. From short peptides to nanofibers to macromolecular assemblies in biomedicine. Biotechnol Adv. 2012;30(3):593–603. doi:10.1016/j.biotechadv.2011.10.004
53. Gnopo YMD, Watkins HC, Stevenson TC, DeLisa MP, Putnam D. Designer outer membrane vesicles as immunomodulatory systems - Reprogramming bacteria for vaccine delivery. Adv Drug Deliv Rev. 2017;114:132–142. doi:10.1016/j.addr.2017.05.003
54. Tsuchiya A, Terai S, Horiguchi I, et al. Basic points to consider regarding the preparation of extracellular vesicles and their clinical applications in Japan. Regen Ther. 2022;21:19–24. doi:10.1016/j.reth.2022.05.003
55. Sugawara T, Onoue S, Takimoto H, Kawahara K. Modification of lipid A structure and activity by the introduction of palmitoyltransferase gene to the acyltransferase-knockout mutant of Escherichia coli. Microbiol Immunol. 2018;62(8):497–506. doi:10.1111/1348-0421.12631
56. Xu H, Ling J, Gao Q, et al. Role of the lpxM lipid A biosynthesis pathway gene in pathogenicity of avian pathogenic Escherichia coli strain E058 in a chicken infection model. Vet Microbiol. 2013;166(3–4):516–526. doi:10.1016/j.vetmic.2013.05.030
57. Gorzelak P, Klein G, Raina S. Molecular basis of essentiality of early critical steps in the lipopolysaccharide biogenesis in Escherichia coli K-12: requirement of MsbA, Cardiolipin, LpxL, LpxM and GcvB. Int J Mol Sci. 2021;22(10):5099. doi:10.3390/ijms22105099
58. Yoo MH, Lee AR, Moon KS. Characteristics of extracellular vesicles and preclinical testing considerations prior to clinical applications. Biomedicines. 2022;10(4):869. doi:10.3390/biomedicines10040869
59. Mukherjee S, Joardar N, Mondal S, et al. Quinolone-fused cyclic sulfonamide as a novel benign antifilarial agent. Sci Rep. 2018;8(1):12073. doi:10.1038/s41598-018-30610-7
60. Lee DH, Kim SH, Kang W, et al. Adjuvant effect of bacterial outer membrane vesicles with penta-acylated lipopolysaccharide on antigen-specific T cell priming. Vaccine. 2011;29(46):8293–8301. doi:10.1016/j.vaccine.2011.08.102
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.