Back to Journals » Vascular Health and Risk Management » Volume 18
Pediatric Stroke from Bench to Bedside: A Single-Center Experience in Saudi Arabia
Authors Al-Sharydah AM , Al-Arfaj HK, Al-Suhibani SS , Al-Safran FS, Al-Abdulwahhab AH , Al-Jubran SA, AlSaflan AA
Received 23 March 2022
Accepted for publication 8 July 2022
Published 13 July 2022 Volume 2022:18 Pages 529—540
DOI https://doi.org/10.2147/VHRM.S367452
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Pietro Scicchitano
Abdulaziz Mohammad Al-Sharydah,1 Hussain Khalid Al-Arfaj,2 Sari Saleh Al-Suhibani,1 Fahad Safran Al-Safran,2 Abdulrahman Hamad Al-Abdulwahhab,1 Saeed Ahmad Al-Jubran,1 Abdulhadi Ahmad AlSaflan3
1Diagnostic and Interventional Radiology Department, Imam Abdulrahman Bin Faisal University, King Fahd Hospital of the University, Khobar City, Eastern Province, Saudi Arabia; 2Medical Imaging Department, King Fahd Specialist Hospital, Dammam City, Eastern Province, Saudi Arabia; 3Anesthesia Department, King Fahd Hospital of the University, Khobar City, Eastern Province, Saudi Arabia
Correspondence: Abdulaziz Mohammad Al-Sharydah, Diagnostic and Interventional Radiology Department, Imam Abdulrahman Bin Faisal University, King Fahd Hospital of the University, P.O. Box: 31952 (4398), Khobar City, Eastern Province, Saudi Arabia, Tel +966 566473111, Fax +966 13-8676697, Email [email protected]
Purpose: Stroke is a leading cause of severe long-term disability and death worldwide. This study aimed to determine the genetic background, causative factors, and diagnostic and outcome measures of pediatric stroke in an area endemic to sickle cell disease (SCD).
Patients and Methods: This retrospective review analyzed pediatric patients with acute stroke who were admitted to King Fahd Hospital of the University, Eastern Province, Saudi Arabia, between January and June 2019. We assessed 49 cases based on computed tomography (CT) and magnetic resonance imaging (MRI) findings. Patients with incomplete records or unavailable radiological images were excluded.
Results: A high likelihood of familial coexistence of stroke was detected in patients with affected siblings (33%). Among various central nervous system manifestations, motor weakness (28.6%) and headache (20.4%) were the most common symptoms/signs. Hypoxic-ischemic encephalopathy (HIE) (28.6%), SCD (22.5%), and moyamoya disease (14.3%) were the most prevalent underlying etiologies. CT without intravenous contrast was the most used initial imaging technique (92.5%). An arterial blockage was more prevalent (53.4%) than a venous infarct (46.6%) (p = 0.041), while arterial ischemic stroke was more prevalent (56.5%) than hemorrhagic stroke (43.5%). The middle cerebral artery (MCA) was most affected (63.5%), followed by the anterior cerebral artery (22.7%) and posterior cerebral artery (13.6%). Most patients were managed with medical treatment (86.1%). No mortalities occurred during the initial hospital stay. The mean length of hospital stay was 12 days.
Conclusion: HIE was the most prevalent etiology of pediatric stroke. Motor weakness and headache were the most common initial manifestations. Arterial ischemic stroke was more prevalent than venous or hemorrhagic stroke. Considering the rarity of pediatric stroke, future studies should be performed with a aborative effort nationally and internationally.
Keywords: magnetic resonance imaging, computed tomography, ischemic stroke, hemorrhagic stroke, paediatric
Plain Language Summary
Children and young adult patients with stroke survive longer than older patients with stroke. To maximize their life spans, clinicians must optimize their management and identify remediable barriers to long-term functional independence. Herein, we review the epidemiology and risk factors for stroke in pediatric patients and outline consensus-based practices in its evaluation and management. Moreover, we discuss the outcomes observed in the SCD-prevalent population. Although many uncertainties persist, the ongoing development of specialized centers and the dedication of pediatric stroke investigators will improve care for these patients.
Introduction
Stroke is relatively rare in children; however, it is a possible cause of morbidity and mortality. Children with stroke often have unique risk factors that predict their outcomes.1 Despite the increased incidence of pediatric stroke, its diagnosis is often delayed, and many cases are under or misdiagnosed. Acute arterial ischemic stroke (AIS) accounts for half of all strokes in children as opposed to 80–85% in adults.1 More than half of the pediatric survivors have a long-term neurological impairment, and 10–20% suffer recurrent strokes.2 Estimated average costs for acute hospital care are US$20,972 per child, and a 5-year direct cost is US$130,000 per patient in the US; these costs place significant financial burdens on families, communities, and the health system.2
In addition, children have multiple risk factors for stroke, such as congenital heart disease, sickle cell disease (SCD), and leukemia, that significantly differ from those in adults, including hypertension, diabetes, and atherosclerosis.3 The combined prevalence of ischemic and hemorrhagic stroke in the pediatric Saudi population is 1.2–13 cases per 100,000 children aged <18 years. Stroke is more common among boys than among girls and more common among black children than those of other races.3 Based on the International Pediatric Stroke Study (IPSS), 94% of young patients with stroke have at least one risk factor.4 An estimated 10–25% of children with stroke will die. Moreover, up to 25% of children with stroke will have a recurrence, while up to 66% will have persistent neurological deficits or manifest subsequent convulsive disorders or developmental delay.5 The IPSS broadly defines arterial stroke etiologies using six congenital cardiac disease categories, which account for up to one-third of all stroke cases;6 other causes include hematologic disease, arteriopathy, moyamoya disease, infection, metabolic syndromes, and drugs.6
Non-contrast brain computed tomography (CT) is sensitive to acute ischemia or bleeding and is used in an emergency to exclude stroke. Magnetic resonance venography (MRV) can identify hemorrhagic stroke, and 10% of hemorrhages in children are due to cerebral venous sinus thrombosis (CVST).1 The use of magnetic resonance arteriography (MRA) and MRV is paramount in confirming vessel patency and defining the basic vascular anatomy.1 Catheter-directed angiography, which can provide better resolution and more precise images of the vascular network, is superior to MRA and CT angiography (CTA) for the visualization of tertiary branches and small cerebral arteries and can be performed in combination with percutaneous endovascular management.1
Overall, only a few studies have examined the management of pediatric stroke.1 The thrombolytic therapy guidelines for children with AIS published by the American Heart Association (AHA) Stroke Council suggest that tissue plasminogen activator (tPA) should be considered in selected children with CVST, but they do not make further recommendations about the feasibility of applying adult guidelines to adolescents meeting adult eligibility criteria.7 However, based on availability and unanimity statements, such generalizations and recommendations can still be made.1 Notably, more aggressive interventions are warranted in children because the absence of brain atrophy might aggravate the effect of expanding hematoma in terms of the enhanced space-occupying lesion effect.1
Moreover, neurological deficits in children can be subtle, and parents might delay the search for care.8 The median time between symptom onset and the diagnosis of AIS exceeds 20 h.9 Thus, it is important to evaluate the age of onset of pediatric stroke and identify its correlation with clinical and diagnostic measures. We aimed to determine the demographic factors, frequency, cause, diagnostic measures, available therapies, and outcome predictors of pediatric stroke in an SCD-prevalent area.
Materials and Methods
Study Setting
This study was conducted at King Fahd Hospital of the University (KFHU), a tertiary-care 650-bed university teaching hospital located in the Eastern Province of Saudi Arabia. This teaching hospital is accredited by the Joint Commission International and the Saudi Central Board for Accreditation of Healthcare Institutions.
Study Design and Participants
We used the keyword “stroke” of the International Classification of Diseases-10 coding system to obtain charts of interest from the electronic medical files. A review of the radiology database was performed to filter the data. Our sample size was determined from the beginning based on G*Power version 3.1.9.7. software to ensure sufficient statistical power. We included all pediatric patients aged ≤14 years who were admitted in the last 3 years. Moreover, our chart review included all brain imaging analyses of these pediatric patients. We excluded patients with incomplete records or unavailable radiological data. For a better outcome assessment, patients who were transferred to other centers were also excluded. We eventually examined the data of 49 pediatric patients diagnosed clinically and confirmed the occurrence of stroke radiologically for patients whose brain imaging was performed between June 2019 and June 2021.
Imaging Studies
Image Acquisition
Brain MRI for each patient was performed on a 3T whole-body system (Magnetom Skyra; Siemens Medical Solutions, Erlangen, Germany) with a 20-channel phased-array head coil. Brain CT imaging was acquired using a 128-slice dual-source CT system (Somatom Definition FLASH, Siemens Healthcare, Forchheim, Germany) with a 64-channel multidetector CT scanner.
Image Interpretations
Two certified diagnostic radiologists (AS and FS) reviewed MR and CT images, and four neuroradiology consultants (HA, SS, SJ, and AA) reviewed the data by sampling the cases to check the consistency of the findings with subsequent agreement on all the results. Cronbach’s alpha coefficient was 0.949, indicating adequate reliability. The kappa test was conducted to evaluate the obtained agreement significance (> 80%).
Statistical Analysis
Statistical analyses were conducted using IBM SPSS version 22.0 (IBM SPSS, Armonk, NY, US). Univariate logistic regression analyses were used to identify possible correlations between different groups. The one-way analysis of variance was used to identify significant differences among independent means, and the paired t-test was used to compare dependent means. Bonferroni adjustments were applied to multiple logistic regression analyses using backward elimination to analyze potential predictors of unfavorable outcomes based on the Pediatric Stroke Outcome Measure (PSOM) of neurological impairment after stroke.10 The patients in the study contributed one or more data points (individual stroke events). The stepwise elimination process included variables with a significance level. The mean ± standard deviations were obtained, and a p-value < 0.05 was considered statistically significant.
Ethical Statement
The Imam Abdulrahman Bin Faisal University Institutional Review Board granted approval for the study to be conducted at King Fahd Hospital of the University (IRB‐2019‐01‐366).
Results
Demographic Analysis
We reviewed 49 confirmed cases of pediatric-onset stroke. The mean age of onset was 7.0 ± 5.31 years, with a predominance of boys (21 [52.5%]) over girls (19 [47.5%]). The average height was 92 ± 46.2 cm, while the average weight was 21.5 ± 15.9 kg. Most patients were Saudis (75.5%) (Table 1).
 |
Table 1 Initial Vital Signs Analysis of Pediatric Stroke Patients During Admission |
Role of Inheritance
No family history of stroke was documented in 54% of the charts. However, the likelihood of familial coexistence of strokes was seen more frequently in patients with affected siblings (33%) than those with affected mothers or fathers (9% and 4%, respectively). Additionally, parental consanguinity was a less frequently observed factor (9.09%).
Etiology
Hypoxic-ischemic encephalopathy (HIE, 28.6%) was the most prevalent etiology, followed by SCD (22.5%) and moyamoya disease (14.3%). Of note, thromboembolic episodes caused only (10%) of cases. (Figure 1).
 |
Figure 1 Percentage of different etiologies of stroke among pediatric patients in the study population. (Other etiologies ie, drugs; toxins.). |
Symptomatology
Among various central nervous system manifestations, motor weakness (28.6%) and headache (20.4%) were the most common symptoms/signs. The average motor weakness score was grade 3 ± 1 for all evaluated patients. Statistical results for other potential predictive factors are summarized in (Table 2).
 |
Table 2 Odds Ratios and 95% Confidence Intervals of Independent Variables for the Occurrence of Stroke Among Pediatric Patients |
Neuroimaging
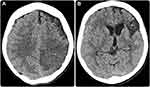
CT without intravenous contrast was the most used initial imaging technique (92.5%), followed by MRI with intravenous contrast (43.6%). Most MRI scans were performed with a diffusion-weighted sequence (92%) (Figure 2). Infarcted vasculature had more arterial blockages than venous infarcts (53.4% vs 46.6%, respectively; p = 0.041). Moreover, ischemic stroke was a more prevalent type (56.5%) than hemorrhagic stroke (43.5%) (Figures 3 and 4; Table 3).
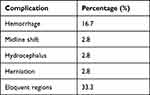 |
Table 3 Associated Radiological Features Complicating the Occurrence of Stroke Among Pediatric Patients |
Stroke Topography
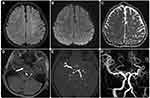
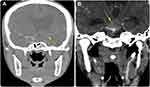
There was a slight predilection for strokes to occur on the left side over the right side (43 [36.8%] vs 39 [33.3%], respectively). The frontal lobe was the most affected (38 [32.5%]), followed by the temporal lobe (25 [21.4%]). Regarding the affected regions, the supratentorial hemisphere (52.1%) and basal ganglia (31.4%) were the most affected regions, with no substantial differences between these two regions (p > 0.05). Additionally, the middle cerebral artery (MCA) was the most affected (63.5%), while the anterior cerebral artery (22.7%) and posterior cerebral artery (13.6%) were less frequently involved. Most stroke events exhibited solitary lesions (103 [88.0%]), but multicenter/multifocal lesions were also observed (14 [12.0%]) (Figures 3 and 5–6; Table 3).
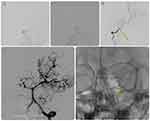 |
Figure 6 Representative case of an 8-year-old girl referred with paralysis (same patient as in Figure 5). Catheter based cerebral angiogram (A and B) shows stigmata of moyamoya disease in the form of prominent primary and secondary collaterals (asterisks). No flow was detected along the left ICA angiogram which revealed occlusion at the carotid terminus (not shown). (C) However, there is cross flow from the left posterior communicating artery (primary collaterals) which supply part of MCA (arrow). (D) AP view of left vertebral artery angiogram showed basilar tip and P1 segment of left posterior cerebral artery wide neck and shallow ruptured aneurysm (asterisk). (E) This was treated successfully by exclusion of aneurysmal sac using a flow diverting stent (arrow). |
Treatment and Outcomes
Ultimately, in most cases (86.1%), medical management was the primary treatment plan, but a few patients underwent endovascular or surgical interventions (11.11%). No mortalities were registered during the hospital stay. The average duration of hospitalization was 12 days. Male patients, those who were younger, and those with a prolonged interval between the development of symptoms and presentation to the hospital had poorer outcomes with a higher total PSOM score. These patients strongly correlated with poorer social wellbeing, including impairments in cognitive ability, adaptive behavior, and social participation.
Discussion
Strokes are more common in adults but also occur in the pediatric population, and they are considered medical emergencies that require prompt diagnosis and treatment to maximize favorable outcomes. Unfortunately, most treatment strategies for pediatric stroke have been adapted from adult guidelines, and no optimal treatment has been ascertained for pediatric stroke patients. This study extensively reviewed cases of pediatric stroke and the most recent treatment recommendations.
Risk factors associated with neonatal stroke, including biological or environmental factors relating to the mother, pregnancy, delivery, or neonatal comorbidities, may account for high and variable rates of AIS (Table 2).11,12 The etiologies of childhood and adolescent stroke are diverse and include, but are not limited to, cardiac causes, arteriopathies (intracranial or extracranial), vasculitis and autoimmune disorders (eg, systemic lupus erythematosus), hematologic disorders (eg, SCD), and traumatic injury.12,13 Contrary to our expectations, parental history of stroke or consanguinity did not significantly contribute to the incidence of strokes in our study.
The risk factors for pediatric stroke are likewise diverse. Stroke seems to be a common presentation in patients with moyamoya disease (Figures 4–6). In young children, symptoms that suggest a compromise of the posterior cerebral vasculature appear to be an important risk factor for high stroke burdens and unfavorable outcomes.14 Infants under the age of 2 months and boys from disadvantaged socioeconomic backgrounds have a much higher risk of stroke. Hemorrhagic stroke has a much higher death rate than ischemic stroke. Over half of children with stroke have underlying diseases, and the risk factors for hemorrhagic stroke differ significantly from those for ischemic stroke.15 Notably, thromboembolic disorders were not noted in this study as contributing factors among the patients who met our selection criteria.
In the present study, we found that CT was the favorable initial imaging modality. However, a pediatric stroke specialist survey-based study by Harrar et al16 in the United States and Canada revealed that most institutions preferred MRI over CT and used abbreviated MRI protocols for pediatric acute stroke imaging. A neuroimaging-based core-penumbra approach must recognize the incapability of children under mechanical circulatory support to endure MRI.17 The cerebral perfusion thresholds of the ischemic core and penumbra in adults may not be implemented in pediatric patients due to differences in cerebrovascular circulation.17
Arterial wall imaging (AWI) is a non-invasive, high-resolution MR technique and can be successfully used in children with stroke. Patterns of AWI enhancement are recognizable and associated with specific AIS pathogeneses.18 In this study, we found that strong vessel wall enhancement in acute childhood AIS was associated with progressive arteriopathy. Further studies are needed to evaluate the additional diagnostic usefulness of AWI compared to that of routine vascular imaging techniques in infant AIS.19
Among pediatric stroke patients in the SCD-prevalent area, the solitary supratentorial frontal lobe was the most frequent region of stroke, and the MCA was usually involved. A recent national study from Saudi Arabia showed that hemorrhagic stroke had better functional outcomes than AIS.20
Acute ischemic stroke could be managed by mechanical thrombectomy due to large vessel occlusion (internal carotid artery [ICA] terminus, M1, basilar artery) in patients aged 1–18 years (Level C evidence; Class IIb recommendation).21 Mechanical recanalization has lower complication rates and better long-term neurologic outcomes than mechanical thrombectomy. However, the content of the existing database is likely affected by multiple selection bias.21 The feasibility of endovascular thrombectomy in pediatric patients with ischemic stroke and large vessel occlusion has been demonstrated by The Save Childs study with the largest retrospective, multicenter cohort.21 A multinational study is recommended to confirm the present findings.22
To date, no studies have reported prospective data on the use of intra-arterial thrombolysis in managing stroke in children, and randomized controlled trials are impracticable in such a small patient population.23 Owing to the lack of clinical evidence, the AHA has no level I recommendations for pediatric acute stroke care.23 However, it recommends conservative management (eg, control of fever and maintenance of good oxygenation, blood pressure, and glucose levels) with guidelines on antiplatelet or anticoagulation therapy.7,23 In our cohort, medical management was the option used in most cases.
Level II recommendations for IV tPA are available for selected children with stroke secondary to CVST; however, to date, there are no official AHA recommendations for endovascular therapy in children with AIS.7,23 Surgical bypass techniques, including direct superficial temporal artery-to-MCA bypass, indirect anastomosis encephalo-duro-arterio-syn-angiosis and encephalo-duro-arterio-myo-syn-angiosis, and combined direct/indirect procedures, have been recommended as effective revascularization options for moyamoya disease.24 A recent meta-analysis of 29 studies by Ravindran et al24 showed similar efficacy of combined/direct revascularization and indirect revascularization for pediatric moyamoya disease with comparable long-term clinical and angiographic outcomes.
Cobb et al23 performed a retrospective case review of endovascular management of pediatric AIS and found that the mechanical thrombectomy option showed better clinical and radiographic outcomes in AIS with fewer complications than did the fibrinolytic option. Notably, current treatment options in young stroke patients have focused on either removing or preventing acute thrombus, which perhaps does not play a role in the etiology of stroke (Figure 6).4
A recent retrospective analysis showed in-hospital mortality of 2.6% in pediatric AIS cases,25 and the Global Disease Burden Study20 reported higher mortality among boys with AIS. Interestingly, a recently published study with the largest pediatric AIS cohort revealed that larger infarct volume and younger age at the time of stroke are associated with poorer outcomes.26 Consistently, our study showed that male sex, younger age, and a prolonged interval between the development of symptoms and presentation to the hospital could predict poorer outcomes with higher total PSOM scores and were strongly correlated with lower social wellbeing, including lesser cognitive ability, reduced coping behavior, and less social involvement.
Our study has some limitations. Despite the gaps in the literature bridged by this study, further research in an international context is needed to confirm the present findings. Several uncertainties persist in our current knowledge of pediatric strokes. The ongoing development of specialized centers and dedication of investigators to pediatric stroke will improve effective care for these patients. Additionally, the present findings might have been influenced by limited data and small sample size. Finally, the univariate analysis was performed primarily using the conventional method, and this study achieved the main objective of potentially identifying predictive variables rather than testing a hypothesis. We considered the scientific plausibility and clinical meaningfulness of such associations, but a further application of our methodology in a larger sample is warranted.
Conclusions
Despite the lack of a sub-specialized multidisciplinary team caring for pediatric patients with stroke, HIE and SCD with or without moyamoya disease are the most prevalent underlying etiologies. Our results support the findings of previous studies regarding motor weakness and headache being the most common initial manifestations in these patients. AIS is more predominant than venous or hemorrhagic types of stroke. The distribution of the MCA is the worst-hit territory. Given the high prevalence and importance of multiple risk factors, an in-depth set of investigations, including hematological, neuroimaging, and metabolic analyses, should be considered in every child with stroke. Because of the low incidence of pediatric stroke, future research on stroke should be performed with a concerted national and international effort.
Abbreviations
AHA, American Heart Association; AIS, arterial ischemic stroke; AWI, arterial wall imaging; CT, computed tomography; CTA, computed tomography angiography; CVST, cerebral venous sinus thrombosis; HIE, hemorrhagic ischemic encephalopathy; MCA, middle cerebral artery; MRA, magnetic resonance arteriography; MRI, magnetic resonance imaging; MRV, magnetic resonance venography; SCD, sickle cell disease; tPA, tissue plasminogen activator.
Data Sharing Statement
The datasets generated and/or analyzed during the current study are not publicly available due to restrictions (eg, their containing information that could compromise the privacy of research participants). The anonymized data are available from the corresponding author on reasonable request. AS is the principal investigator and corresponding author for this project. AS should be contacted for data from this study ([email protected]).
Ethical Approval and Consent to Participate
The non-experimental study protocol was approved by the Imam Abdulrahman Bin Faisal University licensing committee of Institutional Review Board (IRB ‐2019‐01‐366), and granted approval for the study to be conducted at King Fahd Hospital University. This study was performed in accordance with the Helsinki Declaration of 1975 (revised in 1983). Anonymized data were ected, analyzed, and reported only in aggregate form, and no identifiable participant information (image, face, name etc.) was revealed in the study. Informed consent was obtained from all subjects and/or their legal guardian(s).
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; they took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study did not receive any specific grant from funding agencies in the public, commercial, or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in relation to this work.
References
1. Tsze DS, Valente JH. Pediatric stroke: a review. Emerg Med Int. 2011;2011:734506. doi:10.1155/2011/734506
2. Medley TL, Miteff C, Andrews I, et al. Australian clinical consensus guideline: the diagnosis and acute management of childhood stroke. Int J Stroke. 2019;14(1):94–106. doi:10.1177/1747493018799958
3. Salih MA, A-GM A-G, Al-Jarallah AA, et al. Stroke in Saudi children. Saudi Med J. 2006;27(1):S12–S20.
4. van Alebeek ME, Arntz RM, Ekker MS, et al. Risk factors and mechanisms of stroke in young adults: the FUTURE study. J Cerebral Blood Flow Metab. 2018;38(9):1631–1641. doi:10.1177/0271678X17707138
5. Lanni G, Catalucci A, Conti L, Di Sibio A, Paonessa A, Gallucci M. Pediatric stroke: clinical findings and radiological approach. Stroke Res Treat. 2011;2011:1–11. doi:10.4061/2011/172168
6. Lyle CA, Bernard TJ, Goldenberg NA. Childhood arterial ischemic stroke: a review of etiologies, antithrombotic treatments, prognostic factors, and priorities for future research. Semin Thromb Hemost. 2011;37(7):786–793. doi:10.1055/s-0031-1297169
7. Roach ES, Golomb MR, Adams R, et al. Management of stroke in infants and children: a scientific statement from a special writing group of the American Heart Association Stroke Council and the Council on Cardiovascular Disease in the Young. Stroke. 2008;39(9):2644–2691. doi:10.1161/STROKEAHA.108.189696
8. Baldovsky MD, Okada PJ. Pediatric stroke in the emergency department. J Am Coll Emerg Physicians Open. 2020;1(6):1578–1586. doi:10.1002/emp2.12275
9. Mallick AA, Ganesan V, Kirkham FJ, et al. Diagnostic delays in paediatric stroke. J Neurol Neurosurg Psychiatry. 2015;86(8):917–921. doi:10.1136/jnnp-2014-309188
10. Lo W, Gordon AL, Hajek C, et al. Pediatric stroke outcome measure: predictor of multiple impairments in childhood stroke. J Child Neurol. 2014;29(11):1524–1530. doi:10.1177/0883073813503186
11. Munoz D, Hidalgo MJ, Balut F, et al. Risk factors for perinatal arterial ischemic stroke: a case–control study. Cell Med. 2018;10:2155179018785341. doi:10.1177/2155179018785341
12. Oleske DM, Cheng X, Jeong A, Arndt TJ. Pediatric acute ischemic stroke by age-group: a systematic review and meta-analysis of published studies and hospitalization records. Neuroepidemiology. 2021;55(5):331–341. doi:10.1159/000518281
13. Rambaud T, Legris N, Bejot Y, et al. Acute ischemic stroke in adolescents. Neurology. 2020;94(2):. doi:10.1212/WNL.0000000000008783
14. Hackenberg A, Battilana B, Hebeisen M, Steinfeld R, Khan N. Preoperative clinical symptomatology and stroke burden in pediatric moyamoya angiopathy: defining associated risk variables. Eur J Paediatr Neurol. 2021;35:130–136. doi:10.1016/j.ejpn.2021.10.007
15. Chiang K-L, Cheng C-Y. Epidemiology, risk factors and characteristics of pediatric stroke: a nationwide population-based study. QJM. 2018;111(7):445–454. doi:10.1093/qjmed/hcy066
16. Harrar DB, Benedetti GM, Jayakar A, et al. Pediatric acute stroke protocols in the United States and Canada. J Pediatr. 2021;242:
17. Lee S, Felling RJ. Moving toward a new horizon of pediatric stroke intervention. Am Heart Assoc. 2021;52:789–791.
18. Stence NV, Pabst LL, Hollatz AL, et al. Predicting progression of intracranial arteriopathies in childhood stroke with vessel wall imaging. Stroke. 2017;48(8):2274–2277. doi:10.1161/STROKEAHA.117.017922
19. Dlamini N, Yau I, Muthusami P, et al. Arterial wall imaging in pediatric stroke. Stroke. 2018;49(4):891–898. doi:10.1161/STROKEAHA.117.019827
20. Ullah S, Ayaz SB, Qureshi AZ, Tantawy SS, Flandez MF. Characteristics and functional outcomes of pediatric stroke survivors at a rehabilitation unit in Saudi Arabia. J Clin Neurosci. 2020;81:403–408. doi:10.1016/j.jocn.2020.10.014
21. Sporns PB, Sträter R, Minnerup J, et al. Feasibility, safety, and outcome of endovascular recanalization in childhood stroke: the save childs study. JAMA Neurol. 2020;77(1):25–34. doi:10.1001/jamaneurol.2019.3403
22. Bhatia K, Kortman H, Blair C, et al. Mechanical thrombectomy in pediatric stroke: systematic review, individual patient data meta-analysis, and case series. J Neurosurg Pediatr. 2019;24(5):558–571. doi:10.3171/2019.5.PEDS19126
23. Cobb MI-PH, Laarakker AS, Gonzalez LF, Smith TP, Hauck EF, Zomorodi AR. Endovascular therapies for acute ischemic stroke in children. Stroke. 2017;48(7):2026–2030. doi:10.1161/STROKEAHA.117.016887
24. Ravindran K, Wellons JC, Dewan MC. Surgical outcomes for pediatric moyamoya: a systematic review and meta-analysis. J Neurosurg Pediatr. 2019;24(6):663–672. doi:10.3171/2019.6.PEDS19241
25. Beslow LA, Dowling MM, Hassanein SM, et al. Mortality after pediatric arterial ischemic stroke. Pediatrics. 2018;141(5):e20174146. doi:10.1542/peds.2017-4146
26. Jiang B, Hills NK, Forsyth R, et al. Imaging predictors of neurologic outcome after pediatric arterial ischemic stroke. Stroke. 2021;52(1):152–161. doi:10.1161/STROKEAHA.120.030965
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.