Back to Journals » Clinical Ophthalmology » Volume 16
Pediatric Non-Viral Microbial Keratitis: Predisposing Factors, Microbiological Profile, Treatment Modalities, and Visual Outcome
Authors Alwohaibi NN , Bamashmoos M, Al Somali A
Received 4 June 2021
Accepted for publication 1 March 2022
Published 15 March 2022 Volume 2022:16 Pages 775—783
DOI https://doi.org/10.2147/OPTH.S323408
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Nada N Alwohaibi,1 Malak Bamashmoos,2 Abdulaziz Al Somali3
1Ophthalmology Department, King Fahad Hospital of the University, Khobar, Eastern Province, Kingdom of Saudi Arabia; 2Ophthalmology, Dhahran Eye Specialist Hospital, Dammam, Saudi Arabia; 3Department of Ophthalmology, College of Medicine, King Faisal University, Al Ahsa, Saudi Arabia
Correspondence: Nada N Alwohaibi, Tel +00966563229600, Email [email protected]
Purpose: To describe the predisposing factors, causative organisms, treatment modalities, and visual outcomes of childhood non-viral microbial keratitis in our region.
Patients and Methods: All cases with the clinical or microbiological diagnosis of non-viral microbial keratitis in patients ≤ 18 years presenting to Dhahran Eye specialist Hospital, a tertiary eye care hospital in Dhahran, Eastern Province, Saudi Arabia, between 2010 and 2020 were included. A retrospective chart review was conducted. Demographic data, predisposing factors, clinical characteristics, isolated microorganisms, and visual outcomes were recorded.
Results: Fifty-nine patients were included in this study, of which three cases were bilateral. The mean age was 9.3 ± 6.3 years (range: 14 days - 18 years). Predisposing factors were identified in 89.8% (n=53) of cases. Contact lens wear was the leading cause 35.6% (n=21), followed by trauma 27.1% (n=16), ocular diseases 11.9% (n=7), systemic diseases 10.2% (n=6), and ocular surgery 5.1% (n=3). Out of all cases, 66.1% (n=39) have undergone corneal scraping, out of which 43.6% (n=17) showed positive growth. Gram-negative organisms accounted for 47.1% (n=8) isolates of all culture-positive cases. Pseudomonas aeruginosa was the most common pure isolate, which accounted for 41.2% (n=7) of culture-positive cases, followed by Staphylococcus aureus 11.8% (n=2). The most common complication was corneal scar in 71.2% (n=42). Nineteen (32.2%) patients had poor outcome. Seven patients (11.9%) required further intervention, these included penetrating keratoplasty (n=1), deep lamellar keratoplasty (n=3), Photorefractive keratectomy (n=2), and Phototherapeutic keratectomy (n=1).
Conclusion: Childhood non-viral microbial keratitis is uncommon; however, it carries significant risks. Most cases were associated with preventable risk factors, with contact lens wear being the leading cause. Early detection and management are mandatory to reduce the risk of vision-threatening complications. The difficulty in assessment should not jeopardize proper evaluation and management of suspected cases.
Keywords: bacterial, keratitis, pediatric, contact lens, trauma
Introduction
Worldwide, it was estimated that 32.4 million people were blind in 2010.1 Childhood keratitis is among the most common causes of unilateral corneal blindness, accounting for 36.7% of cases.2 In Saudi, acquired corneal opacities accounted for 21.2% of pediatric keratoplasty, equally distributed between nontraumatic and traumatic etiologies.3 All nontraumatic cases were due to microbial keratitis, with the lowest overall graft survival, 27.8%, compared to other etiologies.3
Studies investigating childhood microbial keratitis are scarce, with a wide geographic variation in etiologic patterns and causative organisms. In developing countries, trauma was the leading cause, with Gram-positive predominance.4 Whereas in developed countries, Pseudomonas aeruginosa secondary to contact lens wear is often the leading pathogen.4 Fungal keratitis was common in tropical regions in association with trauma.4 A study in Riyadh showed that 67.8% of isolates were gram-positive, 38.2% were gram-negative bacteria, and none were fungal.5 Microbial keratitis in pediatric population differs from that in adults in many aspects; difficult examination, higher levels of inflammation, and difficulty administering and maintaining adequate corneal levels of topical medications in uncooperative children.6
Although infrequent, it is a preventable, vision-threatening condition with a high incidence of amblyopia. Adequate data on childhood keratitis in our region and standard treatment protocols are lacking.
This study aims to describe the predisposing factors, causative organisms, treatment modalities, and visual outcomes for cases of pediatric non-viral microbial keratitis in our region.
Materials and Methods
This retrospective study was conducted at Dhahran Eye Specialist Hospital, a tertiary eye care institute, between 2010 and 2020. All patients 18 years or younger with the clinical, microbiological, or both diagnoses of microbial keratitis were included. Patients were identified using hospital coding data. Patients above 18 years, cases of viral keratitis, and charts with inadequate data were excluded. The charts were retrospectively reviewed; relevant data was recorded. The recorded data included demographic data, predisposing factors, clinical data including visual acuity at presentation; slit-lamp examination to identify the size and site of infiltrate, presence of hypopyon and laterality; microbiological results of corneal scraping including gram stain and culture, treatment instituted, response to treatment, subsequent complications and interventions if present, and final visual acuities.
Clinical Characteristics
Corneal infiltrates and scars were classified according to size. Scars < 2 mm were categorised as small, 2–6 mm as medium and > 6 mm as large. Children were categorised into three age groups: younger than 7 years, aged 7 to 10 years and older than 10 years. Response to treatment was defined as the improvement of symptoms or reduced corneal infiltrate size in the first 48–72 hours after initiation of antibiotic therapy.
Outcomes
Patients were considered to have a poor outcome if their final visual acuity was less than 20/30, one or more lines of Snellen visual acuity were lost, a major complication such as perforation or endophthalmitis occurred or therapeutic keratoplasty was needed.
Statistical Analysis
Corrected distance visual acuity (CDVA) was converted to the logarithm of minimum angle of resolution (logMAR) for the purpose of analysis. Statistical analysis was performed with IBM SPSS for Windows (v.22; IBM Corp, Armonk, NY, USA). All figures were constructed with Microsoft Excel (2019, Microsoft Corp, USA). The normality of data was assessed by the Shapiro–Wilk test. Normally distributed initial and final visual acuities were compared with a paired t-test. Categorical data were compared with a chi-square test. A p-value less than 0.05 was considered statistically significant. Ethical approval from Dhahran Eye Specialist Hospital’s ethical committee was obtained. Parental consent was not required by the Institutional Review Board at Dhahran Eye Hospital, consent waiver for minimal risk, exclusively retrospective chart review was obtained, and the study was carried under the tent of the Declaration of Helsinki.
Results
Demographics
Among all patients presenting with nonviral microbial keratitis (n = 602), from January 2010 to December 2020, a total of 9.8% (n = 59) were in the paediatric age group. Thirty patients (50.8%) were male, and 29 (49.2%) were female. Three patients had bilateral microbial keratitis.
The mean age was 9.3 ± 6.3 years (range: 14 days–18 years). Twenty-five (42.4%) patients were < 7 years, eight (13.6%) were 7–10 years and 26 (44.1%) were older than 10 years.
Predisposing Factors
Factors predisposing to microbial keratitis were identified in 89.8% (n=53) of all cases. Predisposing factors were trauma in 27.1% (n=16), contact lens wear in 35.6% (n=21), ocular disease in 11.9% (n=7), systemic disease in 10.2% (n=6), ocular surgery in 5.1% (n=3), and unidentified in 10.2% (n=6) of all patients in this study [Table 1].
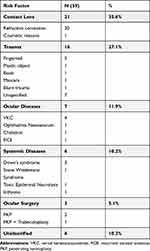 |
Table 1 Predisposing Factors for Pediatric Non-Viral Microbial Keratitis |
Contact lens wear was the most common cause of microbial keratitis in children > 10 years; trauma was the most common cause in children 7–10 years. Trauma, systemic diseases and ocular diseases were equally frequent in children < 7 years [Table 2]. The mean age was higher in contact lens-related cases, whereas the mean age was lower for patients with predisposing ocular surgery [Figure 1].
 |
Table 2 Predisposing Factors in Different Age Groups |
 |
Figure 1 Mean age for each predisposing factor. |
Clinical Characteristics
The time of presentation was morning in 44.1% (n=26), evening in 30.5% (n=18), afternoon in 22% (n=13), and night in 3.4% (n=2) of all patients. The ulcer size was small in 42.4% (n=25), medium in 54.2% (n=32), and large in 3.4% (n=2). The location of the ulcer was central in 42.4% (n=25), paracentral in 44.1% (n=26), and peripheral in 13.6% (n=8). Hypopyon was present in 25.4% (n=15) of all cases.
Microbiology
Among all cases, 66.1% (n=39) have undergone corneal scraping. Culture of the contact lens was performed in 3 cases. Sedation or general anesthesia was needed to perform corneal scraping in 53.8% (n=21) of cases. Seventeen (43.6%) of those who have undergone scraping showed positive culture.
All culture-positive cases were bacterial. Of all culture-positive cases, pure gram-negative isolates accounted for 47.1% (n=8), pure gram-positive isolates accounted for 23.5% (n=4), while 29.4% (n=5) showed mixed growth. All cases with mixed growth were related to contact lenses. Pseudomonas aeruginosa was the most common pure isolate, which accounted for 41.2% (n=7) of culture-positive cases, followed by Staphylococcus aureus 11.8% (n=2) of all culture-positive cases [Table 3].
 |
Table 3 Identified Isolates in Culture of Corneal Scraping or Contact Lens |
Treatment
Twenty-nine patients (49.2%) had used antibiotics before presentation. Antibiotics previously used included gatifloxacin (n = 2), a combination of tobramycin and dexamethasone (n = 3), moxifloxacin (n = 10), fusidic acid (n = 6), fortified cefazolin with vancomycin (n = 3), chloramphenicol (n = 1), fortified cefazolin with gentamycin (n = 3) and ciprofloxacin (n = 1).
After presentation, fortified antibiotics were used in 74.6% (n = 44) of all patients: cefazolin and ceftazidime (n = 24), vancomycin and ceftazidime (n = 19) and a combination of ceftazidime and gentamycin (n = 1). Fluoroquinolones were used in 20.3% (n = 12): moxifloxacin (n = 10), gatifloxacin (n = 1) and ciprofloxacin (n = 1). Two cases received fusidic acid besides fluoroquinolones. Three patients were kept on the same antibiotics that were used before presentation. Steroids were used in 55.9% (n = 33) of all cases and were initiated 2.1 ± 6.7 months after presentation.
Outcomes
The mean follow-up time was 23.4±28.9 months (range: 0.10–114.1 months). Fifty-four patients (91.5%) showed good response to medical treatment, while poor response was seen in 8.5% (n=5), predisposing factors in these cases were (n =3), ocular disease (n = 1), and ocular surgery (n = 1).
Initial and final visual acuities were recorded in 78% (n=46) and 74.6% (n=44) of all cases, respectively. The mean corrected distance visual acuity (CDVA) was 1.03±1.09 LogMAR (Snellen: 20/214) at presentation and 0.30±0.36 LogMAR (Snellen: 20/40) at the last visit, ie, vision improved seven lines in visual acuity (p<0.001) [Table 4].
 |
Table 4 Initial and Final Visual Acuities |
Complications were present in 78% (n=46) of cases. The most common complication was corneal scar in 71.2% (n=42) of all patients. Other complications included graft failure 3.4% (n=2), and astigmatism 3.4% (n=2) of all patients.
Out of all patients who developed corneal scar 78.6% (n=33) received steroid. In these cases, the scar was small in 42.4% (n=14), medium in 57.6% (n=19), and large in none. The scar was central in 48.5% (n=16) of patients who received steroids, paracentral in 39.4% (n=13), and peripheral in 12.1% (n=4). The mean CDVA in this group initially was 1.02±1.22 LogMAR (Snellen: 20/209) and 0.26±0.28 LogMAR (Snellen: 20/36) at the last visit, ie, improvement of 7.6 lines (paired t-test, p=0.004).
Nine patients (21.4%) of those who developed a corneal scar did not receive steroids. In these cases, the scar was paracentral and medium in size in 44.4% (n=4), small and central in 22.2% (n=2), medium-sized and central in 22.2% (n=2), while one patient had a large central scar. The mean CDVA was 2.10±1.03 LogMAR (Snellen: 20/2518) initially and 0.49±0.52 LogMAR (Snellen: 20/62) at the last visit ie, vision improved 16.1 lines (paired t-test, p=0.026).
Nineteen (32.2%) patients had poor outcome. Seven patients (11.9%) required further intervention, these included penetrating keratoplasty (n=1), deep lamellar keratoplasty (n=3), Photorefractive keratectomy (n=2), and Phototherapeutic keratectomy (n=1).
Discussion
In adults, contact lens use, systemic diseases, trauma, previous ocular surgery, topical steroids use, bullous keratopathy, exposure keratitis, keratitis sicca, and recurrent erosions are all risk factors for infectious microbial keratitis.6–8 Microbial keratitis in pediatric patients differs from that in adults in many aspects, including the more challenging examination, higher levels of inflammation, and difficulty delivering and maintaining adequate corneal levels of topical medications in uncooperative children.5,9,10
A wide geographic variation in predisposing factors and causative organisms was reported in previously conducted studies [Table 5]. The reported risk factors in the pediatric age group included trauma, contact lens wear, systemic diseases, and previous ocular conditions.6,11–13 Trauma is often the leading cause of pediatric microbial keratitis in developing countries.5,10,14 Whereas in developed countries, contact lens wear is a major risk factor.11,15
 |
Table 5 Geographics, demographics, and Risk Factors for Cases of Childhood Microbial Keratitis |
In Saudi, Alotaibi et al5 have reported trauma to be the leading cause of pediatric microbial keratitis, followed by systemic disease, which was in contrast to our study as contact lens wear was found to be the leading cause (35.6%), followed by ocular trauma (27.1%), whereas the other factors such as ocular disease, systematic disease, and ocular surgery were the least common predisposing factors. This could be attributed to the increasing popularity of contact lens use in older children. Additionally, the mean age in our study appears to be higher than that reported by Alotaibi et al,5 which might also explain the higher rate of contact lens-related infectious keratitis in this study.
In this series, contact lens wear was the most common cause for microbial keratitis in children older than ten years, while trauma was the most common predisposing factor in children from seven to ten years of age, whereas trauma, systemic and ocular disease were found to be equally frequent in children younger than seven years.
Bilateral cases in our series were associated with contact lens wear and systemic diseases; this finding seems consistent with other studies that reported that systemic diseases were associated with bilateral cases.4,15
A wide range of culture-positive cases was reported in previous studies,15 in which different laboratory facilities and prior use of antibiotics could explain this variability. In this study, out of all patients who underwent corneal scraping 43.6% (n=17) showed positive growth, which is lower than that reported in previous studies (48%–87%),15 this is likely to be due to the high frequency 49.2% (n=29) of prior antibiotic use in our series.
All culture-positive cases were bacterial with gram-negative predominance. The most commonly isolated microorganism was Pseudomonas aeruginosa, followed by Staphylococcus aureus, whereas the other organisms were less prevalent [Table 3]. In agreement with our findings, several studies have reported a gram-negative predominance, with the most common isolate being Pseudomonas aeruginosa which was associated with contact lens use,9,11,16 yet, other studies reported contrasting results.11,13,14 In Saudi, a gram-positive predominance was reported with Streptococcus pneumonia being the most common isolate, followed by Staphylococcus epidermidis, whereas Pseudomonas aeruginosa was found to be the predominant isolate of gram-negative and it was associated with contact lens-related microbial keratitis,5 which seems consistent with the varying frequency of predisposing factors among different populations; as Pseudomonal keratitis was associated with contact lens, whereas Staphylococcus aureus was associated with trauma.
Patients in the current study were treated with fortified antibiotics in 74.6% of all cases, while the rest received fluoroquinolone, predominantly used for small ulcers. The most commonly used combination was Cefazolin and Ceftazidime. In a previous Saudi study, fortified antibiotics were used empirically for all patients, where Gentamycin and Cefazolin was the most used combination.5 The treatment regimens varied among different studies and populations based on the causative agent and its sensitivity.4–15
In our study, poor visual outcomes were observed in patients with large, centrally located infiltrates and preexisting ocular diseases. Centrally located and larger infiltrates had poorer initial visual acuities; however, they were associated with better improvement in final CDVA. Similarly, Hsiao et al9 concluded that poor visual outcome was associated with polymicrobial infection, fungal keratitis, systemic and ocular disease.
Conclusion
Pediatric non-viral microbial keratitis is infrequent; yet, is significantly vision-threatening. In this study, 62.7% of cases were secondary to preventable risk factors, that is, contact lens, which was the major risk factor, and trauma. Education on proper hygiene among children using contact lenses is crucial. Our study implicated that Pseudomonas aeruginosa is the most common causative organism and was associated with contact lens wear; thus, corneal scraping using selective culture media and empirically treating all contact lens-related cases with topical fluoroquinolones or fortified antibiotics with gram-negative coverage should be considered.
Microbial keratitis in children carries a significant risk of amblyopia and ocular morbidity. Therefore, early detection and proper management are necessary to reduce the risk of vision-threatening complications. Good evaluation and management of suspected cases should not be compromised by the difficulty in evaluating and obtaining adequate scraping samples.
This study is limited by its retrospective nature, in addition to the relatively small sample size, given the lower prevalence of microbial keratitis in the pediatric age group compared to that in adults. Additionally, visual acuity recording was affected by the uncooperativeness of children. This study was conducted in a tertiary eye institute; children with predisposing systemic diseases and severely ill children do not usually present to our hospital, which may underestimate the prevalence of associated risk factors. Further research in different geographic areas is recommended, as a better understanding of childhood microbial keratitis may help prevent and manage subsequent complications.
Ethics Approval and Informed Consent
This study adheres to the Declaration of Helsinki and was approved by the Research Review Board at Dhahran Eye Specialist Hospital, Eastern Province, Saudi Arabia.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Stevens G, White R, Flaxman S, et al. Global prevalence of vision impairment and blindness. Ophthalmology. 2013;120(12):2377–2384. doi:10.1016/j.ophtha.2013.05.025
2. Dandona R. Corneal blindness in a southern Indian population: need for health promotion strategies. Br J Ophthalmol. 2003;87(2):133–141. doi:10.1136/bjo.87.2.133
3. Al-Ghamdi A, Al-Rajhi A, Wagoner M. Primary pediatric keratoplasty: indications, graft survival, and visual outcome. J Am Assoc Pediatric Ophthalmol Strabismus. 2007;11(1):41–47. doi:10.1016/j.jaapos.2006.09.012
4. Di Zazzo A, Antonini M, Fernandes M, Varacalli G, Sgrulletta R, Coassin M. A global perspective of pediatric non-viral keratitis: literature review. Int Ophthalmol. 2020;40(10):2771–2788. doi:10.1007/s10792-020-01451-z
5. Al Otaibi A, Allam K, Damri A, Al Shamri A, Kalantan H, Mousa A. Childhood microbial keratitis. Oman J Ophthalmol. 2012;5(1):28. doi:10.4103/0974-620x.94763
6. Ormerod L, Murphree A, Gomez D, Schanzlin D, Smith R. Microbial Keratitis in Children. Ophthalmology. 1986;93(4):449–455. doi:10.1016/s0161-6420(86)33717-5
7. Ormerod L, Hertzmark E, Gomez D, Stabiner R, Schanzlin D, Smith R. Epidemiology of Microbial Keratitis in Southern California. Ophthalmology. 1987;94(10):1322–1333. doi:10.1016/s0161-6420(87)80019-2
8. Cruz O, Sabir S, Capo H, Alfonso E. Microbial Keratitis in Childhood. Ophthalmology. 1993;100(2):192–196. doi:10.1016/s0161-6420(93)31671-4
9. Hsiao C. Pediatric Microbial Keratitis in Taiwanese Children. Arch Ophthalmol. 2007;125(5):603. doi:10.1001/archopht.125.5.603
10. Singh G, Palanisamy M, Madhavan B, et al. Multivariate analysis of childhood microbial keratitis in South India. Ann Acad Med Singap. 2006;35(3):185–189.
11. Young A, Leung K, Tsim N, Hui M, Jhanji V. Risk Factors, Microbiological Profile, and Treatment Outcomes of Pediatric Microbial Keratitis in a Tertiary Care Hospital in Hong Kong. Am J Ophthalmol. 2013;156(5):1040–1044.e2. doi:10.1016/j.ajo.2013.06.019
12. Song X, Xu L, Sun S, Zhao J, Xie L. Pediatric Microbial Keratitis: a Tertiary Hospital Study. Eur J Ophthalmol. 2012;22(2):136–141. doi:10.5301/ejo.2011.8338
13. Yu M, Höfling-Lima A, Furtado G. Microbiological and epidemiological study of infectious keratitis in children and adolescents. Arq Bras Oftalmol. 2016;79:5. doi:10.5935/0004-2749.20160084
14. Aruljyothi L, Radhakrishnan N, Prajna V, Lalitha P. Clinical and microbiological study of paediatric infectious keratitis in South India: a 3-year study (2011–2013). Br J Ophthalmol. 2016;100(12):1719–1723. doi:10.1136/bjophthalmol-2015-307631
15. Al-Otaibi A. Non-viral microbial keratitis in children. Saudi J Ophthalmol. 2012;26(2):191–197. doi:10.1016/j.sjopt.2011.10.002
16. Rossetto J, Cavuoto K, Osigian C, et al. Paediatric infectious keratitis: a case series of 107 children presenting to a tertiary referral centre. Br J Ophthalmol. 2017;101(11):1488–1492. doi:10.1136/bjophthalmol-2016-3101192
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
