Back to Journals » OncoTargets and Therapy » Volume 14
PD-L1 Expression and Outcome in Patients with Metastatic Non-Small Cell Lung Cancer and EGFR Mutations Receiving EGFR-TKI as Frontline Treatment
Authors Chang CY , Lai YC, Wei YF , Chen CY , Chang SC
Received 27 November 2020
Accepted for publication 7 January 2021
Published 31 March 2021 Volume 2021:14 Pages 2301—2309
DOI https://doi.org/10.2147/OTT.S290445
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Sanjeev K. Srivastava
Cheng-Yu Chang,1,* Yi-Chun Lai,2,3,* Yu-Feng Wei,4,5,* Chung-Yu Chen,6,7,* Shih-Chieh Chang2,8
1Division of Chest Medicine, Department of Internal Medicine, Far Eastern Memorial Hospital, New Taipei City, Taiwan; 2Division of Chest Medicine, Department of Internal Medicine, National Yang Ming Chiao Tung University Hospital, Yi-Lan, Taiwan; 3Faculty of Medicine, College of Medicine, National Yang Ming Chiao Tung University, Taipei, Taiwan; 4School of Medicine for International Students, College of Medicine, I-Shou University, Kaohsiung, Taiwan; 5Department of Internal Medicine, E-Da Cancer Hospital, Kaohsiung, Taiwan; 6Department of Internal Medicine, National Taiwan University Hospital Yunlin Branch, Yunlin County, Taiwan; 7College of Medicine, National Taiwan University, Taipei, Taiwan; 8Department of Critical Care Medicine, National Yang Ming Chiao Tung University Hospital, Yi-Lan, Taiwan
*These authors contributed equally to this work
Correspondence: Shih-Chieh Chang
Division of Chest Medicine, Department of Internal Medicine, National Yang Ming Chiao Tung University Hospital, No. 169, Siaoshe Road, Yi-Lan, 260, Taiwan
Tel +886-3-932-5192
Fax +886-3-936-5432
Email [email protected]
Background: Epidermal growth factor receptor (EGFR) mutations are most common in Eastern Asia, and frequencies of 30– 50% have been reported. EGFR-tyrosine kinase inhibitors (TKIs) are recommended as first-line therapeutic options for non-small cell lung cancer (NSCLC) with sensitizing EGFR mutations. Several immune checkpoint inhibitors have been successful in improving the outcomes of advanced lung cancer. The expression of programmed cell death-ligand 1 (PD-L1) on tumor cells plays an important role in predicting the efficacy of programmed cell death protein 1/PD-L1 inhibitors. The role of PD-L1 expression in tumors with EGFR mutation and its influence on clinical outcomes remain controversial.
Methods: Patients with newly diagnosed metastatic NSCLC with sensitizing EGFR mutations who received the standard treatment, ie, EGFR-TKIs for mutant adenocarcinoma as the first-line treatment, were enrolled in this retrospective study. EGFR mutations and PD-L1 expression levels were detected by Cobas RT-PCR and Dako 22C3 immunohistochemistry staining, respectively.
Results: From January 2011 to February 2019, 114 patients were enrolled. The average age was 62 years (range 34– 92), and 45 (39.5%) patients were male. Among these patients, EGFR mutation analysis revealed exon 19 in-frame deletion in 55 (48.2%) patients, exon 21 L858R in 53 (46.5%) patients, and uncommon mutations in 6 (5.3%) patients. Among these patients with EGFR mutations, PD-L1 expression levels by tumor proportion score (TPS) were < 1% in 54 (46.9%) patients, 1– 49% in 50 (44.2%) patients, and ≥ 50% in 10 (8.8%) patients. All patients received EGFR-TKIs as first-line treatment, and in the Kaplan-Meier analysis, progression-free survival was not significantly different among groups with different PD-L1 expression status.
Conclusion: For patients with metastatic NSCLC and EGFR mutations, PD-L1 expression is not uncommon, but no significant influence on clinical outcomes was observed in patients receiving standard initial treatment.
Keywords: programmed death-ligand 1, epidermal growth factor receptor mutation, epidermal growth factor receptor tyrosine kinase inhibitors
Introduction
Lung cancer is the leading cause of cancer mortality worldwide. In the era of precision medicine, targeted therapy is the first choice in non-small cell lung cancer (NSCLC). Examples are gene mutations of epidermal growth factor receptor (EGFR) treated with gefitinib, erlotinib, or afatinib or gene translocations of the anaplastic lymphoma kinase treated with crizotinib or alectinib.1 In recent years, immune checkpoint inhibitors have been a new therapeutic choice. Recent clinical trials revealed that agents disrupting programmed cell death protein 1 (PD-1)/programmed cell death-ligand 1 (PD-L1) signaling provide survival benefit in advanced NSCLC treatment, including nivolumab, pembrolizumab, and atezolizumab.2–5 Studies in animal models demonstrated that the expression of EGFR mutations induces PD-L1 expression and that EGFR tyrosine kinase inhibitors (TKIs) reduce this PD-L1 expression.6 The relationships between PD-L1 expression and EGFR mutations were also investigated in humans. However, the findings were paradoxical, indicating the relationship of PD-L1 high expression means better survival is uncertained.7,8
We, thus, investigated the association between PD-L1 expression and the outcome of metastatic EGFR mutation-expressing NSCLC treated with EGFR-TKI as a frontline treatment.
The study was conducted in compliance with the Declaration of Helsinki.
Methods
This multicenter study was conducted in one medical center (Far Eastern Memorial Hospital) and two regional teaching hospitals (National Yang-Ming University Hospital and E-DA Hospital) in Taiwan. The study was approved by the Institutional Review Board of the Research Ethics Committee of the study hospitals (No. 2017A034). Informed consent was waived by the Ethics Committee due to the retrospective nature of the study. The study was conducted in compliance with Declaration of Helsinki and the data was anonymized for the privacy of the participants.
In this retrospective study, we enrolled patients with NSCLC of newly diagnosed stage IV according to the American Joint Committee on Cancer (AJCC) classification, 7th edition or postoperative tumor recurrence who received gefitinib, erlotinib, or afatinib as their first-line treatment.9 Another inclusion criterion was the presence of EGFR exon 18–21 sensitizing mutations which were confirmed in tumor tissue obtained from surgical resection, core-needle biopsy, or pleural fluid samples using the Cobas real-time PCR test (Roche Molecular Systems Inc., Branchburg, NJ, USA). PD-L1 expression was determined using the Dako 22C3 pharmDx systems (Agilent Technologies Inc., Santa Clara, CA, USA) assay and is presented as a tumor proportion score.10 Exclusion criteria included inadequate tissue samples for further PD-L1 immunohistochemistry staining.
The enrolled patients initially received gefitinib 250 mg, erlotinib 150 mg, or afatinib 40 mg once daily, and the subsequent dose de-escalation was determined by the treating physician. The combination with 7.5 or 15 mg/kg bevacizumab every three weeks was allowed. The treatment response was evaluated using the Response Evaluation Criteria of Solid Tumors version 1.1.11 The primary outcome was progression-free survival (PFS), which was the period calculated from the initiation of the EGFR-TKI treatment to disease progression or death. Other outcomes included the best objective response and overall survival (OS).
Statistical Analysis
We used SPSS (version 22; IBM Corporation, Armonk, NY, USA) for the statistical analysis of clinical data. Data were calculated as frequencies for categorical variables and median (standard deviation) for continuous variables. Categorical variables were compared using the chi-square test or Fisher’s exact test, and continuous variables were compared using the independent unpaired t-test. PFS and OS were assessed by Kaplan-Meier survival curves, and statistical differences were calculated using the Log rank test. p-values of less than 0.05 were considered statistically significant.
Results
From January 2011 to February 2019, 114 eligible patients were enrolled in this study. The average age of the study population was 62 years (range 34–92), and 45 (39.5%) patients were male. The demographic data are summarized in Table 1. Before initiation of the EGFR-TKI treatment, 33 (29.2%) patients had a poor Eastern Cooperative Oncology Group performance status (ECOG score 2–4), and 84 (73.7%) patients had at least one extrathoracic distant metastasis (M1b status in the AJCC classification, 7th edition). Among these patients with EGFR mutations, PD-L1 expression levels by TPS were <1% in 54 (46.9%) patients, 1–49% in 50 (44.2%) patients, and ≥50% in 10 (8.8%) patients.
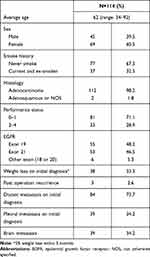 |
Table 1 Demographic Data |
As their frontline treatment, the patients had received gefitinib (n=42, 36.8%), erlotinib (n=36, 31.6%), or afatinib (n=36, 31.5%). Of those, 13 (11.5%) patients received a combined anti-angiogenesis treatment with bevacizumab or ramucirumab. The most common EGFR mutation was exon 19 in-frame deletion (48.2%), followed by exon 21 L858R point mutation (46.5%). For patients with EGFR mutations receiving EGFR-TKIs, PFS was not statistically different between groups with different PD-L1 expression status. For the groups with PD-L1 <1%, 1–49%, and ≥50%, the median PFS was 13.6, 18.4, and 15.7 months, respectively (p=0.738). A similar result was observed for the parameter OS (Table 2). Although the PD-L1 status was not associated with PFS of the first-line EGFR-TKI treatment, poor ECOG performance status score (2–4) and distant metastasis on initial diagnosis were associated with shortened PFS and OS in both univariate and multivariate analyses. Patients who initially received afatinib had better PFS than those receiving gefitinib (28.3 vs 12.1 months, hazard ratio 0.463, 95% confidence interval (CI) 0.226 to 0.952, p=0.036) in the multivariate analysis (Tables 3 and 4). In the multivariate analysis of the OS, the three EGFR-TKIs with or without additional anti-angiogenesis medication were not significantly different.
 |
Table 2 Univariate Analysis of PFS and OS |
 |
Table 3 Multivariate Analysis of PFS |
 |
Table 4 Multivariate Analysis of OS |
Discussion
Preclinical studies have reported that EGFR activation can induce PD-L1 expression, thereby facilitating immune escape, and that EGFR-TKIs can significantly downregulate PD-L1 expression in EGFR-mutant NSCLC cells.6 Several clinical studies have reported that NSCLCs harboring EGFR mutations are associated with poor clinical outcomes in groups with high PD-L1 expression levels.8,12 Another recent study demonstrated that PD-L1 expression tended to be correlated with CD8+ tumor-infiltrating lymphocytes, concomitant KRAS mutation, and poor survival in surgically resected EGFR-mutant NSCLCs. The authors pointed out that PD-L1 expression was neither a predictive nor a prognostic factor in advanced EGFR-mutant NSCLC patients treated with EGFR-TKIs.13
In our study, the PD-L1 expression status was not associated with PFS and OS in patients positive for EGFR mutation who received TKIs. At least two studies also suggest that PD-L1 expression is associated with inconsistent survival outcomes.14,15 The most important prognostic factors for OS are performance status and distant metastasis on initial diagnosis after multivariate analysis in our study. Although brain metastasis is also an important factor for OS in the univariate analysis (p=0.008), the effect was decreased in the multivariate analysis. As previously reported for NSCLC with EGFR mutation, patients with brain metastasis have poorer prognoses.16 Another study showed that patients with EGFR mutations were more susceptible to brain metastasis than those with wild-type EGFR, especially during the course of the disease.17
The ECOG performance status was another independent prognostic factor for OS and PFS in our study. The ECOG performance status, besides metastatic site, smoking status, and age, has also been proposed by other studies as a prognostic factor to predict the survival of patients harboring activating EGFR mutations.18,19 A real-world practice study in Taiwan also found that ECOG performance status, smoking index, hepatic metastasis on initial diagnosis, disease status (newly diagnosed or postoperative recurrence), and chronic hepatitis C virus infection were independent prognostic factors for OS.20
Some studies in NSCLC patients receiving EGFR-TKI treatment reported that the exon 19 deletion predicted a better OS rate than the L858R mutation.21 This was not observed in our study. In our study, the median OS of patients with exon 19 deletion and L858R mutation was 33.4 months and 33.3 months (95% CI 20.46–46.40 and 25.03–41.49, respectively; p=0.79), whereas the median PFS was 18.4 months and 14.8 months (95% CI 11.51–25.29 and 9.78–19.81, respectively; p=0.736).
Another specific finding of our study was that patients who initially received afatinib had longer PFS than those receiving gefitinib. Afatinib is an ErbB receptor blocker that is approved for the treatment of EGFR mutation-positive NSCLC. Pivotal randomized clinical studies demonstrated that afatinib significantly prolonged PFS compared to platinum-based chemotherapy (LUX-Lung 3 and LUX-Lung 6) and gefitinib (LUX-Lung 7), with manageable unwanted effects.22 Real-world studies consistently indicate that afatinib has similar or improved efficacy compared with first-generation EGFR-TKIs across a broad range of patients treated in diverse clinical practice settings.23,24
There are some limitations to our study. First, this study was retrospective in design, and the sample size was relatively small. Second, we did not exclude patients who received immunotherapy and third-generation TKIs (osimertinib). Most of these patients eventually develop secondary resistance to first- and second-generation TKIs with EGFR-T790M mutations. The incidence of T790M in tumors that have developed resistance to EGFR-TKIs ranges from 51% to 68%.25 In the AURA III study, PFS and OS were affected in patients receiving third-generation TKIs such as osimertinib.26 Third, we had a small number of patients who additionally received anti-angiogenic agents. Tumors with EGFR mutations show a significantly higher VEGF expression compared to EGFR wild-type tumors.27 Although combination of anti-angiogenic agents and EGFR-TKIs did not correlate with better PFS or OS in our patients, recent Phase III studies showed that the combination of EGFR-TKI (erlotinib) and an anti-angiogenic agent significantly prolonged PFS in advanced NSCLC with EGFR mutation.28,29 Objective determination of PD-L1 protein levels in NSCLC reveals heterogeneity within tumors and prominent interassay variability or discordance. This could be due to different antibody affinities, limited specificity, or distinct target epitopes.30 Malignant pleural effusion samples is feasible for PD-L1 IHC analysis. The PD-L1 levels of malignant pleural effusion cell blocks were comparable with paired tumor tissues, however, heterogeneity was found between these two media. Gene alterations based on NGS of malignant pleural effusion samples could contribute to select the samples that with different PD-L1 expression.31
Conclusion
In NSCLC patients with EGFR mutation and EGFR-TKI treatment, three major prognostic factors were associated with significantly prolonged PFS: afatinib use, good performance status, and no distant metastasis on initial diagnosis. Patients with good performance status and no distant metastasis on initial diagnosis also had longer OS. The PD-L1 expression was in our study not associated with the survival of patients. Whether PD-L1 expression is a reliable biomarker for the EGFR-TKI treatment of advanced NSCLC patients with EGFR mutations requires further investigation.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Oxnard GR, Binder A, Jänne PA. New targetable oncogenes in non-small-cell lung cancer. J Clin Oncol. 2013;31:1097–1104. doi:10.1200/JCO.2012.42.9829
2. Borghaei H, Paz-Ares L, Horn L, et al. Nivolumab versus docetaxel in advanced nonsquamous non-small-cell lung cancer. N Engl J Med. 2015;373:1627–1639. doi:10.1056/NEJMoa1507643
3. Brahmer J, Reckamp KL, Baas P, et al. Nivolumab versus docetaxel in advanced squamous-cell non-small-cell lung cancer. N Engl J Med. 2015;373:123–135. doi:10.1056/NEJMoa1504627
4. Herbst RS, Baas P, Kim DW, et al. Pembrolizumab versus docetaxel for previously treated, PD-L1-positive, advanced non-small-cell lung cancer (KEYNOTE-010): a randomised controlled trial. Lancet. 2016;387:1540–1550. doi:10.1016/S0140-6736(15)01281-7
5. Rittmeyer A, Barlesi F, Waterkamp D, et al. Atezolizumab versus docetaxel in patients with previously treated non-small-cell lung cancer (OAK): a Phase 3, open-label, multicentre randomised controlled trial. Lancet. 2017;389:255–265. doi:10.1016/S0140-6736(16)32517-X
6. Akbay EA, Koyama S, Carretero J, et al. Activation of the PD-1 pathway contributes to immune escape in EGFR-driven lung tumors. Cancer Discov. 2013;3:1355–1363. doi:10.1158/2159-8290.CD-13-0310
7. Lin C, Chen X, Li M, et al. Programmed death-ligand 1 expression predicts tyrosine kinase inhibitor response and better prognosis in a cohort of patients with epidermal growth factor receptor mutation-positive lung adenocarcinoma. Clin Lung Cancer. 2015;16:E25–35. doi:10.1016/j.cllc.2015.02.002
8. Tang Y, Fang W, Zhang Y, et al. The association between PD-L1 and EGFR status and the prognostic value of PD-L1 in advanced non-small cell lung cancer patients treated with EGFR-TKIs. Oncotarget. 2015;6:14209–14219. doi:10.18632/oncotarget.3694
9. Edge S, Byrd D, Compton C, et al. AJCC Cancer Staging Manual.
10. Roach C, Zhang N, Corigliano E, et al. Development of a companion diagnostic PD-L1 immunohistochemistry assay for pembrolizumab therapy in non-small-cell lung cancer. Appl Immunohistochem Mol Morphol. 2016;24:392–397. doi:10.1097/PAI.0000000000000408
11. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45:228–247. doi:10.1016/j.ejca.2008.10.026
12. Li J, Chen Y, Shi X, et al. A systematic and genome-wide correlation meta-analysis of PD-L1 expression and targetable NSCLC driver genes. J Thorac Dis. 2017;9:2560–2571. doi:10.21037/jtd.2017.07.117
13. Bai Y, Chen X, Hou L, et al. PD-L1 expression and its effect on clinical outcomes of EGFR-mutant NSCLC patients treated with EGFR-TKIs. Cancer Biol Med. 2018;15:434–442. doi:10.20892/j.issn.2095-3941.2018.0223
14. Zhang Y, Kang S, Shen J, et al. Prognostic significance of programmed cell death 1 (PD-1) or PD-1 ligand 1 (PD-L1) expression in epithelial-originated cancer: a meta-analysis. Medicine. 2015;94:e515. doi:10.1097/MD.0000000000000515
15. Velcheti V, Schalper KA, Carvajal DE, et al. Programmed death ligand-1 expression in non-small cell lung cancer. Lab Invest. 2014;94:107–116. doi:10.1038/labinvest.2013.130
16. Magnuson WJ, Lester-Coll NH, Wu AJ, et al. Management of brain metastases in tyrosine kinase inhibitor-naïve epidermal growth factor receptor-mutant non-small-cell lung cancer: a retrospective multi-institutional analysis. J Clin Oncol. 2017;35:1070–1077. doi:10.1200/JCO.2016.69.7144
17. Li L, Luo S, Lin H, et al. Correlation between EGFR mutation status and the incidence of brain metastases in patients with non-small cell lung cancer. J Thorac Dis. 2017;9:2510–2520. doi:10.21037/jtd.2017.07.57
18. Kim MH, Kim HR, Cho BC, et al. Impact of cigarette smoking on response to epidermal growth factor receptor (EGFR)-tyrosine kinase inhibitors in lung adenocarcinoma with activating EGFR mutations. Lung Cancer. 2014;84:196–202. doi:10.1016/j.lungcan.2014.01.022
19. Mitchell P, Mok T, Barraclough H, Strizek A, Lew R, van Kooten M. Smoking history as a predictive factor of treatment response in advanced non-small-cell lung cancer: a systematic review. Clin Lung Cancer. 2012;13:239–251. doi:10.1016/j.cllc.2011.08.003
20. Yao ZH, Liao WY, Ho CC, et al. Real-world data on prognostic factors for overall survival in EGFR mutation-positive advanced non-small cell lung cancer patients treated with first-line gefitinib. Oncologist. 2017;22:1075–1083. doi:10.1634/theoncologist.2016-0331
21. Riely GJ, Pao W, Pham D, et al. Clinical course of patients with non-small cell lung cancer and epidermal growth factor receptor exon 19 and exon 21 mutations treated with gefitinib or erlotinib. Clin Cancer Res. 2006;12:839–844. doi:10.1158/1078-0432.CCR-05-1846
22. Park K, Ta’ EH, O’Byrne K, et al. Afatinib versus gefitinib as first-line treatment of patients with EGFR mutation-positive non-small-cell lung cancer (LUX-Lung 7): a phase 2B, open-label, randomised controlled trial. Lancet Oncol. 2016;17:577–589. doi:10.1016/S1470-2045(16)30033-X
23. Tu CY, Chen CM, Liao WC, et al. Comparison of the effects of the three major tyrosine kinase inhibitors as first-line therapy for non-small-cell lung cancer harboring epidermal growth factor receptor mutations. Oncotarget. 2018;9:24237–24247. doi:10.18632/oncotarget.24386
24. Kim Y, Lee SH, Ahn JS, Ahn MJ, Park K, Sun JM. Efficacy and safety of afatinib for EGFR-mutant non-small cell lung cancer, compared with gefitinib or erlotinib. Cancer Res Treat. 2019;51:502–509. doi:10.4143/crt.2018.117
25. Cortot AB, Jänne PA. Molecular mechanisms of resistance in epidermal growth factor receptor-mutant lung adenocarcinomas. Eur Respir Rev. 2014;23:356–366. doi:10.1183/09059180.00004614
26. Mok TS, Wu YL, Ahn MJ, et al. Osimertinib or platinum-pemetrexed in EGFR T790M-positive lung cancer. N Engl J Med. 2017;376:629–640. doi:10.1056/NEJMoa1612674
27. Reinmuth N, Jauch A, Xu EC, et al. Correlation of EGFR mutations with chromosomal alterations and expression of EGFR, ErbB3 and VEGF in tumor samples of lung adenocarcinoma patients. Lung Cancer. 2008;62:193–201. doi:10.1016/j.lungcan.2008.03.011
28. Saito H, Fukuhara T, Furuya N, et al. Erlotinib plus bevacizumab versus erlotinib alone in patients with EGFR-positive advanced non-squamous non-small-cell lung cancer (NEJ026): interim analysis of an open-label, randomised, multicentre, phase 3 trial. Lancet Oncol. 2019;20:625–635. doi:10.1016/S1470-2045(19)30035-X
29. Nakagawa K, Garon EB, Seto T, et al. Ramucirumab plus erlotinib in patients with untreated, EGFR-mutated, advanced non-small-cell lung cancer (RELAY): a randomised, double-blind, placebo-controlled, phase 3 trial. Lancet Oncol. 2019;20:1655–1669. doi:10.1016/S1470-2045(19)30634-5
30. Mc Laughlin J, Han G, Schalper KA, et al. Quantitative assessment of the heterogeneity of PD-L1 expression in non-small-cell lung cancer. JAMA Oncol. 2016;2(1):46–54.
31. Song Z, Cheng G, Zhang Y. PD-L1 expression in malignant pleural effusion samples and its correlation with oncogene mutations in non-small cell lung cancer. J Thorac Dis. 2020;12(4):1385–1392. doi:10.21037/jtd.2020.02.06
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
