Back to Journals » OncoTargets and Therapy » Volume 15
PAX8 as a Potential Target for Ovarian Cancer: What We Know so Far
Authors Di Palma T, Zannini M
Received 30 June 2022
Accepted for publication 13 October 2022
Published 21 October 2022 Volume 2022:15 Pages 1273—1280
DOI https://doi.org/10.2147/OTT.S361511
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Arseniy Yuzhalin
Tina Di Palma, Mariastella Zannini
IEOS - Institute of Experimental Endocrinology and Oncology ‘G. Salvatore’, National Research Council, Napoli, 80131, Italy
Correspondence: Mariastella Zannini, IEOS - Institute of Experimental Endocrinology and Oncology ‘G. Salvatore’, National Research Council, via S. Pansini 5, Napoli, 80131, Italy, Tel +39-081-5465530, Fax +39-081-2296674, Email [email protected]
Abstract: The Fallopian tube epithelium harbors the origin cells for the majority of high-grade serous ovarian carcinomas (HGSCs), the most lethal form of gynecologic malignancies. PAX8 belongs to the paired-box gene family of transcription factors and it is a marker of the FTE secretory cell lineage. Its role has been investigated in migration, invasion, proliferation, cell survival, stem cell maintenance, angiogenesis and tumor growth. In this review, we focus on the pro-tumorigenic role of PAX8 in ovarian cancer; in this context, PAX8 possibly continues to exert its transcriptional activity on its physiological targets but may also function on newly available targets after the tumorigenic hits. Acquiring new insights into the different PAX8 mechanism(s) of action in the tumor microenvironment could uncover new viable therapeutic targets and thus improve the current treatment regimen.
Keywords: PAX8, ovarian cancer, transcription factor, Fallopian tube, STICs
Introduction
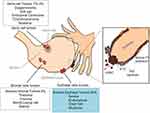
Ovarian cancer (OC) represents the fifth leading cause of cancer-related death in women and, like other cancers, is a spectrum of diseases.1 OC includes epithelial, germ cell and sex cord-stromal tumors originating from surface epithelial cells, from the germ cell or the oocyte and from stromal and primitive ovarian sex cord cells, as shown in Figure 1. The sex cord-stromal and germ cells types contribute to 8% and 3–7% of the malignant ovarian tumors, respectively; and given their histological and molecular heterogeneity, it is not easy to establish criteria for making the distinction between benign and malignant tumors. Most stromal tumors and germ cell tumors have a good prognosis.2 The epithelial tumors (90%) can be divided in four major histotypes: serous, mucinous, endometrioid and clear cell3 (Figure 1). Of these, HGSC is the most common and lethal histotype and is associated with mutations in BRCA1 or BRCA2 and p53.4,5 Although it was originally believed that HGSC arises from the ovarian surface epithelium (OSE), it has been recently suggested by various experimental evidences that it may instead arise from the Fallopian tube fimbria.6 In particular, the serous tubal intraepithelial carcinomas (STICs), which are identified in the distal end (fimbria) of the Fallopian tube and arise from p53 mutations, have been indicated as the primary lesions that evolve through subsequent oncogenic events into HGSC6,7 (Figure 1). This hypothesis is further supported by genetically engineered murine models that mimic the transformation from Fallopian tubal secretory epithelial cells to HGSC.8,9 In addition, gene profiling studies showing that the gene expression profile of HGSC is more closely related to the Fallopian tubes than to the ovarian surface epithelium supported the Fallopian tubes rather than ovarian surface epithelium as the site of origin of HGSC.10 It was indeed observed that STICs and HGSC share similar histomorphological and expression profiles and 92% of the STICs express TP53 mutations that are identical between these precursor lesions and the matched invasive ovarian carcinomas.11 Among the evidence that reinforce the tubal origin of the HGSC, there is also the transcription factor PAired boX8 (PAX8) that is expressed in the Fallopian tube secretory cells but not in OSE and is conserved in HGSC.9,12 This transcription factor is a marker of the Fallopian tube secretory cell lineage and its expression is retained in 96% of the serous ovarian carcinomas, in 89% of the endometrioid and 100% of the clear cell carcinomas, whilst it is not detected in the mucinous carcinoma.13 PAX8 is crucial for the organogenesis of the thyroid gland, kidney, nervous system and Müllerian system.14,15 In previous studies, we have shown that PAX8 plays a critical role in cell cycle progression and cell survival of thyroid differentiated epithelial cells.16 In tumors, PAX8 positivity has been displayed in gliomas, renal, thyroid, ovarian, Wilms and well-differentiated pancreatic neuroendocrine cancers.17–21 Moreover, PAX8 represents a useful marker for the diagnosis of primary or metastatic neoplasms, given its lack of expression in mammary, lung and mesothelial primary tumors.22–24 In the scenario of OC, the Cancer Genome Atlas (TGCA) Project indicated PAX8 as a survival gene essential for the proliferation of ovarian cancer cells.13 Overall, PAX8 belongs to a class of lineage-survival genes that are required for both normal development of specific tissues and for cancer cell proliferation/survival. In this review, we outline PAX8’s function and its role in OC; moreover, we discuss PAX8 regulatory mechanism(s) and possible therapeutic approaches.
PAX8 Structure and Function
PAX8 is a member of the PAired boX (PAX) gene family that is composed of nine genes in mammals tightly regulated in both temporal and spatial expression patterns.25 These genes, as described in details in Table 1, have been sub-classified into four subgroups based on shared structural motifs and similar expression pattern during embryogenesis.26 The PAX8 gene is located on chromosome 2 at position 2q12-14. Alternative splicing of PAX8 generates five different transcripts that give rise to equally distinct isoforms that are distinguished in their functional properties.27,28 PAX8A with 450aa is the longest and most common isoform that includes all the codons from exon 2 to 12. PAX8 as the other members of subgroup II is characterized by the presence of a Paired-domain (PD), an octapeptide (OP) and a partial homeodomain (HD) (Table 1). The PD is a conserved 128 amino-acid DNA-binding domain composed by two helix-turn-helix motif subdomains, PAI and RED.29 The OP and HD exert positive or negative regulation of transcription depending on the cellular context and availability of cofactors. Most of the current insight regarding PAX8 function during embryonic development and in adults came from studies in the thyroid. PAX8 is expressed during thyroid development and it is involved in sporadic and familial cases of CH with TD.30,31 Its expression plays a key role in determining the thyroid differentiated phenotype,32 typically seen in adults. Pax8 knockout mice show hypoplastic thyroid deprived of the follicular cells and die within 2–3 weeks after weaning.33 Exogenous thyroid hormone replacement allow female Pax8 null mice to survive, but they are infertile due to atresia of the uterus, closed vaginal openings and hydrosalpinx.34 In the male reproductive tissues, seminal vesicle and epididymis are PAX8 positive. The same treatment with exogenous thyroxine in male Pax8 null mice restores the general deficits of CH, but also these mice are infertile due to abnormal development of the efferent ducts, epididymis and complete absence of spermatozoa.35 PAX8 is also involved in the organogenesis of the kidney, but because of co-expression of PAX2, a severe phenotype is obvious only in the double mutant mice.36 Summarizing, in line with its expression pattern, PAX8 is essential for proper development of different body organ systems, especially the thyroid gland, the renal system and the Müllerian system.
 |
Table 1 Overview of PAX Transcription Factors |
Biological Activity of PAX8 in Cancers
It is well known that PAX genes are responsible for specifying positional information during embryonic development; in cancers, their over-expression or aberrant expression confers a strong advantage to cancer cell growth, tumor progression and maintenance, but they are not sufficient to initiate tumor.37 Based on this, the PAX8 transcription factor seems to be crucial for the proliferation/survival of thyroid differentiated epithelial cells16 and thyroid cancer cells.38
Kang et al39 evidenced that transforming growth factor-β1 (TGF-β1) exerts its function through PAX8, regulating normal and pathological processes in thyroid. Moreover, several studies reported that in a subset of human follicular thyroid carcinomas (FTC) the PAX8/PPARγ fusion protein (PPFP), composed of the paired and partial homeobox DNA binding domains of PAX8 fused to the coding exons of PPARγ, caused by a chromosomal translocation, represents a plausible early event in the development or progression of the tumor.40–43 The chimeric PPFP, whose expression is under the transcriptional regulation of the PAX8 promoter,44 exerts a dominant-negative effect on wild-type PPARγ and stimulates transcription of PAX8-responsive promoters.43 PAX8 is also expressed in papillary thyroid carcinoma (PTC) and anaplastic thyroid carcinoma (ATC) with ranging positive expression.45–47 Since PAX8 acts as a master gene for thyroid differentiation32 and represents an essential player for function and survival of adult thyroid cells,48 it is likely that variations in its expression level may affect the downstream molecular cascades (ie target genes) and in turn crucial pathways in thyroid tumorigenesis.49 In addition to the thyroid, the expression of PAX8 in neoplastic tissues has been analyzed in several cancers, including Wilms’ tumor, renal carcinomas,17,50 well-differentiated pancreatic neuroendocrine tumors,18 gliomas,19 endometrial and ovarian carcinomas.51–53 According to its expression pattern, PAX8 is involved in numerous pathways that contribute to carcinogenesis. In glioma cell lines, it plays a critical role for maintenance of telomeres, regulating the expression of telomerase RNA (hTR).19 Recently, it has been shown that PAX8 plays an oncogenic role in renal cell carcinoma (RCC).54 To date, several immunohistochemical analyses denote PAX8 expression in RCC, endometrial, cervical and various epithelial cancers.50,55–57 In 2021, numerous and representative studies on PAX8 expression and role in epithelial carcinomas were thoroughly and accurately reviewed by Khizer et al.58 Overall, PAX8 emerges as an important factor for cancer cell growth and viability/survival through transcriptional regulation of key factors such as Bcl2,59 E2F1,60 p53 and p21.61 However, to improve our knowledge on the role of this transcription factor in malignancies, it will be necessary to define the molecular pathways regulated by PAX8 and the multiprotein complexes it is part of in different contexts.
PAX8 Mechanism of Action in HGSC
In OC, PAX8 was initially used as a histological marker but Bowen et al in 200752 suggested a functional role of this transcription factor in the uncontrolled growth of epithelial ovarian cancer (EOC) and in the physiological development of the oviduct. Few years later, in 2011 Cheung et al13 using a genome-wide approach identified PAX8 as one of 54 genes specifically essential for proliferation and viability of ovarian cancer cells. Subsequently, our studies provided strong evidence of PAX8 involvement in OC tumorigenicity in vitro and in vivo.62 We demonstrated that PAX8 affects cell growth, migration and invasion of ovarian cancer cells and tumor growth in nude mice. Moreover, we suggested a possible role of PAX8 in the peritoneal dissemination of ovarian cancer cells by modulating the susceptibility to anoikis of cancer cells and their interaction with the extracellular matrix.63 In HGSC PAX8 acts as positive regulator of mutated TP53, that in turns induces p21 to promote proliferative and anti-apoptotic effect.64 On the contrary, in endometrial carcinoma mutated p53 and cytoplasmic p21 can independently mediate the pro-proliferative role of PAX8.61 To better understand PAX8 function and to gain new insights on the transcriptional mechanism(s) regulated by PAX8 leading to OC, RNA-seq analysis before and after PAX8 knockdown were performed in Fallopian tube and ovarian cancer cell lines by several research groups. In 2016, our group compared the transcriptome of normal Fallopian tube secretory epithelial cells (FT-194) with that of ovarian cancer cells (SKOV-3) before and after PAX8 silencing, to identify genes and pathways modified during the transformation process and regulated by this transcription factor.65 We highlighted that about 60% of the genes were differentially expressed between FT-194 and SKOV-3 and upon PAX8 silencing; the pathways most affected in both FT194 and SKOV-3 cells were TNFa signaling, inflammatory response, apoptosis, UV response and epithelial mesenchymal transition, estrogen response and p53 pathway. Elias et al66 demonstrated that the gene expression changes between Fallopian tube and serous ovarian cancer cells were due to PAX8 cistrome reprogramming. RNA-Seq and ChIP-Seq analysis were used to determine that the genes that were differentially expressed between the cell lines above mentioned were located and clustered around the PAX8 binding sites. Epigenetic remodeling of PAX8 cistrome may be responsible for gene expression alterations leading HGSC from Fallopian tube; in fact, only in tumor the interaction between PAX8 and YAP1, important regulator of Hippo pathway, was observed. The majority of the PAX8 binding sites were enriched in intergenic regions and super-enhancers (SEs); this might explain the low phenotypic effects observed in normal Fallopian tube following PAX8 knockdown, also consistent with the results in the murine tissue.67 In tumor context, PAX8 cistrome alterations may enrich the binding sites for PAX8 in intragenic regions and/or SEs, to ensure the presence in the PAX8 network of co-regulators and target genes that encompass differentiation, development and tumorigenesis. Reddy et al68 developed the Cancer Core Transcription Factor Specificity (CaCTS) algorithm, to identify tumor-driving master Transcription Factors (MTFs), that is a set of SEs–associated transcription factors whose predicted binding motifs are enriched at SEs, that exhibit tumor type–specific expression. By this approach it has been shown that PAX8, SOX17 and MECOM form a core regulatory circuit driving tumor cell survival in high-grade serous ovarian carcinoma cell lines. Recently, it has been demonstrated that the protein complex PAX8/SOX17 promotes angiogenesis in OC through inhibition of SERPINE1, that is a potent suppressor of VEGF pathway.69 Bleu et al70 have examined in detail the mechanistic interaction between PAX8 and MECOM. They demonstrated that PAX8 and MECOM are part of the same transcriptional complex and determined a PAX8-MECOM gene signature featuring patients of OC with poor prognosis. Studies based on transcriptional signatures allowed to distinguish epithelial subpopulation during Fallopian tube differentiation and to identify an early secretory cluster enriched in HGSC.71 PAX8 role in non-HGSC is similar to that in HGSC. Cheung et al13 indicated PAX8 as a lineage-specific survival gene, essential for ovarian cancer cells’ proliferation. Another study reported that PAX8 knockdown reduces anchorage dependent and independent growth and impairs in vivo tumorigenesis.72 Furthermore, ovarian lineage-specific PAX8 regulon (regulatory network) is involved in decisive events that determine cell fate and phenotype during Müllerian duct organogenesis, and if dysregulated aids ovarian carcinogenesis.73 PAX8 also is involved with RB in a reciprocal relation wherein PAX8 regulates E2F1 promoter and stabilizes RB helping in tumor cell growth.60 All studies above mentioned point to PAX8 as an important regulator of signaling pathways in the context of OC. Its ability to associate with different partners in normal versus tumor cells suggests that the PAX8 protein complexes could be targeted specifically in tumor cells, leaving normal cells and tissues completely unaffected.
PAX8 as a Target for Ovarian Cancer Therapy
HGSC is still one of the most lethal malignancies that affect women worldwide because the majority of the cases are diagnosed after intraperitoneal spread, leading to a poor prognosis. Since PAX8 is expressed in Fallopian tube secretory cells and is retained in HGSC and mediates several tumorigenic effects in serous carcinoma, it can be considered a promising drug target. As already mentioned, the altered PAX8 cistrome in HGSC offers the opportunity to target PAX8 pharmacologically only in the transformed tissue. Recently, it has been demonstrated that PAX8 regulon in OC is susceptible to HDAC inhibitors.73 Briefly, operative PAX8 regulon composed by 9 genes is epigenetically disabled by HDAC inhibitors, resulting in downregulation of PAX8 and its entire responsive network and suppression of ovarian cancer growth. In the same year, Hardy et al74 demonstrated that PAX8 targeting either through CRISPR genomic alteration or by drug treatment with thiostrepton leads to a reduction of tumor growth and metastasis. Nowadays, it is clear that strategies aimed at reducing PAX8 levels or inhibiting binding to crucial interacting proteins may impair tumor progression and modulate the aggressive properties of cancer cells. As already cited, SOX17 functions as driving-tumor MTF together with PAX868; along the same line Lin et al75 showed that both transcription factors display Müllerian lineage-specific co-expression and co-regulate a broad range of downstream genes, including those involved in cell cycle and tissue morphogenesis. Moreover, they uncover that transcriptional CDK inhibitors efficiently reduce SOX17 and PAX8 protein levels and also putative PAX8 regulon genes was appreciably abolished. These orally bioavailable CDK inhibitors are able to efficiently suppress tumorigenesis in vivo, in xenograft models. Another strategy employed to target PAX factors is to inhibit their binding activity. Grimley et al76 identified among 227 potential PAX2 inhibitors a small molecule, termed EG1 that effectively blocked PAX2-5-8 DNA binding activity and decreased the viability of cancer cells. Recently, the same research group used an unbiased, cell-based, high-throughput screen to isolate compounds that inhibit PAX2-5-8 transactivation ability.77 These small molecules, BG, do not interfere with PAX2-5-8 DNA binding activity, whereas exert inhibitory effect on PAX2-5-8 transcription activation and consequently on cancer cells proliferation. Given that EG1 and BG compounds exert their action through two different mechanisms, the authors suggest the possibility that the combining of both molecules may add therapeutic benefit in the future.
Conclusion
Epithelial ovarian cancers, shown to develop from both OSE and FTE, are the most common and aggressive form of OC. About 80% of these are attributed to HGSC, that is the deadliest histological subtype of EOC. This is due to the fact that at an early-stage HGSC is asymptomatic and specific biomarkers still do not exist, consequently the majority of the cases are detected only at an advanced stage often with distant metastases (FIGO stage III–IV). The primary line of therapy includes surgical debulking followed by taxanes and platinum-based chemotherapies. HGSCs are initially responsive to drugs treatment, but usually very soon become chemoresistant and highly metastatic with subsequent patient relapse. For many years, the ovary was considered the site of origin of HGSC development; however, this theory fails to explain the presence of STICs in 60% of the patients with HGSC.78 Non-invasive STICs derived by secretory Fallopian tube epithelial cells that undergo p53 mutations acquire invasive malignant behavior by accumulating genomic instability and being exposed to paracrine factors of the ovarian microenvironment reach the ovary and peritoneum with all the signatures of HGSC.
It is relevant to underline that PAX8 is expressed in normal Fallopian tube secretory cells and is preserved through every stage of transformation until HGSC. Such retention is one of the evidences supporting the tubal origin of HGSC,9 thus investigating PAX8 function in normal Fallopian tube cells could shed light on its role in tumor progression and provide critical clues in the understanding of its exact niche in ovarian cancer development. Unpublished data from our laboratory indicate that PAX8 silencing affects stemness in 3D spheroids of Fallopian tube cell line, suggesting a critical role of PAX8 in the regulation of the secretory cells multipotency.
Given its cell-type and tissue type-specificity, limited expression in healthy adults and the dependency of OC to its expression and activity, PAX8 is a very promising diagnostic and therapeutic target for HGSC. It is known that PAX8 is associated with secretory pathways in thyroid and it is most likely that it has a similar role in the Müllerian tract, as recently described for SERPINE1.79 Hence, it is reasonable to suppose that its putative targets could have an important role as secretory factors and serve both as suitable biomarkers for the early diagnosis of HGSC and as useful therapeutic target against this malignancy. In parallel, the early evidences on PAX8 involvement in the metabolic reprogramming54,61 could indicate new candidates in terms of novel targets and predictive biomarkers. In conclusion, we think that the combined therapeutic approaches of PAX inhibitors with other chemotherapeutic drugs may enhance their efficacy and in particular improve the survival of patients with a worse prognosis.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Saad AF, Hu W, Sood AK. Microenvironment and pathogenesis of epithelial ovarian cancer. Horm Cancer. 2010;1:277–290. doi:10.1007/s12672-010-0054-2
2. Chen VW, Ruiz B, Killeen JL, Cotè TR, Wu XC, Correa CN. Pathology and classification of ovarian tumors. Cancer. 2003;97(10 Suppl):2631–2642. doi:10.1002/cncr.11345
3. Gilks CB. Molecular abnormalities in ovarian cancer subtypes other than high-grade serous carcinoma. J Oncol. 2010;2010:740968. doi:10.1155/2010/740968
4. Press JZ, De Luca A, Boyd N, et al. Ovarian carcinomas with genetic and epigenetic BRCA1 loss have distinct molecular abnormalities. BMC Cancer. 2008;22(8):17. doi:10.1186/1471-2407-8-17
5. Shaw PA, McLaughlin JR, Zweemer RP, et al. Histopathologic features of genetically determined ovarian cancer. Int J Gynecol Pathol. 2002;21:407–411. doi:10.1097/00004347-200210000-00011
6. Nik NN, Vang R, Shih M, Kurman RJ. Origin and pathogenesis of pelvic (ovarian, tubal, and primary peritoneal) serous carcinoma. Annu Rev Pathol. 2014;9:27–45. doi:10.1146/annurev-pathol-020712-163949
7. Kurman RJ, Shihle M. The origin and pathogenesis of epithelial ovarian cancer: a proposed unifying theory. Am J Surg Pathol. 2010;34(3):433–443. doi:10.1097/PAS.0b013e3181cf3d79.
8. Kim J, Coffey DM, Creighton CJ, Yu Z, Hawkins SM, Matzuk MM. High-grade serous ovarian cancer arises from fallopian tube in a mouse model. Proc Natl Acad Sci USA. 2012;109:3921–3926. doi:10.1073/pnas.1117135109
9. Perets R, Wyant GA, Muto KW, et al. Transformation of the fallopian tube secretory epithelium leads to high-grade serous ovarian cancer in Brca;Tp53;Pten models. Cancer Cell. 2013;24(6):751–765. doi:10.1016/j.ccr.2013.10.013.
10. Beirne JP, McArt DG, Roddy A, et al. Defining the molecular evolution of extrauterine high grade serous carcinoma. Gynecol Oncol. 2019;155(2):305–317. doi:10.1016/j.ygyno.2019.08.029
11. Merritt MA, Bentink S, Schwede M, et al. Gene expression signature of normal cell-of-origin predicts ovarian tumor outcomes. PLoS One. 2013;8(11):e80314. doi:10.1371/journal.pone.0080314
12. Xiang L, Rong G, Zhao J, Wang Z, Shi F. Identification of candidate genes associated with tubal origin of high-grade serous ovarian cancer. Oncol Lett. 2018;15(5):7769–7775. doi:10.3892/ol.2018.8346
13. Cheung HW, Cowley GS, Weir BA, et al. Systematic investigation of genetic vulnerabilities across cancer cell lines reveals lineage-specific dependencies in ovarian cancer. Proc Natl Acad Sci U S A. 2011;108:12372–12377. doi:10.1073/pnas.1109363108
14. Bouchard M, Souabni A, Mandler M, Neubuser A, Busslinger M. Nephric lineage specification by Pax2 and Pax8. Genes Dev. 2002;16(22):2958–2970. doi:10.1101/gad.240102
15. Plachov D, Chowdhury K, Walther C, Simon D, Guenet JL, Gruss P. Pax8, a murine paired box gene expressed in the developing excretory system and thyroid gland. Development. 1990;110(2):643–651. doi:10.1242/dev.110.2.643
16. Di Palma T, Filippone MG, Pierantoni GM, Fusco A, Soddu S, Zannini M. Pax8 has a critical role in epithelial cell survival and proliferation. Cell Death Dis. 2013;4(7):e729. doi:10.1038/cddis.2013.262
17. Poleev A, Fickenscher H, Mundlos S, et al. PAX8, a human paired box gene: isolation and expression in developing thyroid, kidney and Wilms’ tumors. Development. 1992;116(3):611–623. doi:10.1242/dev.116.3.611
18. Sangoi AR, Ohgami RS, Pai RK, Beck AH, McKenney JK, Pai RK. PAX8 expression reliably distinguishes pancreatic well-differentiated neuroendocrine tumors from ileal and pulmonary well-differentiated neuroendocrine tumors and pancreatic acinar cell carcinoma. Mod Pathol. 2011;24(3):412–424. doi:10.1038/modpathol.2010.176
19. Chen YJ, Campbell HG, Wiles AK, et al. PAX8 regulates telomerase reverse transcriptase and telomerase RNA component in glioma. Cancer Res. 2008;68(14):5724–5732. doi:10.1158/0008-5472.CAN-08-0058
20. Ozcan A, de la Roza G, Ro JY, Shen SS, Truong LD. PAX2 and PAX8 expression in primary and metastatic renal tumors: a comprehensive comparison. Arch Pathol Lab Med. 2012;136(12):1541–1551. doi:10.5858/arpa.2012-0072-OA
21. Hardy LR, Salvi A, Burdette JE. UnPAXing the divergent roles of PAX2 and PAX8 in high-grade serous ovarian cancer. Cancers. 2018;10(8):262. doi:10.3390/cancers10080262
22. Nonaka D, Chiriboga L, Soslow RA. Expression of pax8 as a useful marker in distinguishing ovarian carcinomas from mammary carcinomas. Am J Surg Pathol. 2008;32(10):1566–1571. doi:10.1097/PAS.0b013e31816d71ad
23. Laury AR, Hornick JL, Perets R, et al. PAX8 reliably distinguishes ovarian serous tumors from malignant mesothelioma. Am J Surg Pathol. 2010;34(5):627–635. doi:10.1097/PAS.0b013e3181da7687
24. Ye J, Hameed O, Findeis-Hosey JJ, et al. Diagnostic utility of PAX8, TTF-1 and napsin A for discriminating metastatic carcinoma from primary adenocarcinoma of the lung. Biotech Histochem. 2012;87(1):30–34. doi:10.3109/10520295.2011.591838
25. Dahl E, Koseki H, Balling R. Pax genes and organogenesis. Bioessays. 1997;19(9):755–765. doi:10.1002/bies.950190905
26. Mansouri A, Hallonet M, Gruss P. Pax genes and their roles in cell differentiation and development. Current Opinion Cell Biol. 1996;8(6):851–857. doi:10.1016/s0955-0674(96)80087-1
27. Poleev A, Wendler F, Fickenscher H, et al. Distinct functional properties of three human paired-box-protein, PAX8, isoforms generated by alternative splicing in thyroid, kidney and Wilms’ tumors. European J of Biochem. 1995;228(3):899–911. doi:10.1111/j.1432-1033.1995.tb20338.x
28. Kozmik Z, Kurzbauer R, Dörfler P, Busslinger M. Alternative splicing of Pax-8 gene transcripts is developmentally regulated and generates isoforms with different transactivation properties. Mol Cell Biol. 1993;13(10):6024–6035. doi:10.1128/mcb.13.10.6024-6035.1993
29. Czerny T, Schaffner G, Busslinger M. DNA sequence recognition by Pax proteins: bipartite structure of the paired domain and its binding site. Genes Dev. 1993;7(10):2048–2061. doi:10.1101/gad.7.10.2048
30. De Felice M, Di Lauro R. Thyroid development and its disorders: genetics and molecular mechanisms. Endocrine Rev. 2004;25(5):722–746. doi:10.1210/er.2003-0028
31. De Felice M, Di Lauro R. Minireview: intrinsic and extrinsic factors in thyroid gland development: an update. Endocrinology. 2011;152(8):2948–2956. doi:10.1210/en.2011-0204
32. Pasca Di Magliano M, Di Lauro R, Zannini M. Pax8 has a key role in thyroid cell differentiation. Proc Natl Acad Sci U S A. 2000;97(24):13144–13149. doi:10.1073/pnas.240336397
33. Mansouri A, Chowdhury K, Gruss P. Follicular cells of the thyroid gland require Pax8 gene function. Nat Genet. 1998;19(1):87–90. doi:10.1038/ng0598-87
34. Mittag J, Winterhager E, Bauer K, Grümmer R. Congenital hypothyroid female pax8-deficient mice are infertile despite thyroid hormone replacement therapy. Endocrinology. 2007;148(2):719–725. doi:10.1210/en.2006-1054
35. Wistuba J, Mittag J, Luetjens CM, et al. Male congenital hypothyroid Pax8-/- mice are infertile despite adequate treatment with thyroid hormone. J Endocrinol. 2007;192(1):99–109. doi:10.1677/JOE-06-0054
36. Narlis M, Grote D, Gaitan Y, Boualia SK, Bouchard M. Pax2 and pax8 regulate branching morphogenesis and nephron differentiation in the developing kidney. J Am Soc Nephrol. 2007;18(4):1121–1129. doi:10.1681/ASN.2006070739
37. Muratovska A, Zhou C, He S, Goodyer P, Eccles MR. Paired-box genes are frequently expressed in cancer and often required for cancer cell survival. Oncogene. 2003;22(39):7989–7997. doi:10.1038/sj.onc.1206766
38. Credendino SC, Bellone ML, Lewin N, et al. A ceRNA circuitry involving the long noncoding RNA Klhl14-AS, Pax8, and Bcl2 drives thyroid carcinogenesis. Cancer Res. 2019;79(22):5746–5757. doi:10.1158/0008-5472.CAN-19-0039
39. Kang HC, Ohmori M, Harii N, Endo T, Onaya T. Pax-8 is essential for regulation of the thyroglobulin gene by transforming growth factor-β1. Endocrinology. 2001;142(1):267–275. doi:10.1210/endo.142.1.7918
40. Eberhardt NL, Grebe SK, McIver B, Reddi HV. The role of the PAX8/PPARgamma fusion oncogene in the pathogenesis of follicular thyroid cancer. Mol Cell Endocrinol. 2010;321(1):50–56. doi:10.1016/j.mce.2009.10.013
41. Marques AR, Espadinha C, Catarino AL, et al. Expression of PAX8-PPAR gamma 1 rearrangements in both follicular thyroid carcinomas and adenomas. J Clin Endocrinol Metab. 2002;87(8):3947–3952. doi:10.1210/jcem.87.8.8756
42. Raman P, Koenig RJ. Pax-8-PPAR-γ fusion protein in thyroid carcinoma. Nat Rev Endocrinol. 2014;10(10):616–623. doi:10.1038/nrendo.2014.115
43. Placzkowski KA, Reddi HV, Grebe SK, Eberhardt NL, McIver B. The role of the PAX8/PPARgamma fusion oncogene in thyroid cancer. PPAR Res. 2008;2008:672829. doi:10.1155/2008/672829
44. Kroll TG, Sarraf P, Pecciarini L, et al. PAX8-PPARgamma1 fusion oncogene in human thyroid carcinoma. Science. 2000;289(5483):1357–1360. doi:10.1126/science.289.5483.1357
45. Nonaka D, Tang Y, Chiriboga L, Rivera M, Ghossein R. Diagnostic utility of thyroid transcription factors Pax8 and TTF-2 (FoxE1) in thyroid epithelial neoplasms. Mod Pathol. 2008;21(2):192–200. doi:10.1038/modpathol.3801002
46. Puglisi F, Cesselli D, Damante G, Pellizzari L, Beltrami CA, Di Loreto C. Expression of Pax-8, p53 and bcl-2 in human benign and malignant thyroid diseases. Anticancer Res. 2000;20(1A):311–316.
47. Bishop JA, Sharma R, Westra WH. PAX8 immunostaining of anaplastic thyroid carcinoma: a reliable means of discerning thyroid origin for undifferentiated tumors of the head and neck. Hum Pathol. 2011;42:1873–1877. doi:10.1016/j.humpath.2011.02.004
48. Marotta P, Amendola E, Scarfò M, et al. The paired box transcription factor Pax8 is essential for function and survival of adult thyroid cells. Mol Cell Endocrinol. 2014;396(1–2):26–36. doi:10.1016/j.mce.2014.08.004
49. Rosignolo F, Sponziello M, Durante C, et al. Expression of PAX8 target genes in papillary thyroid carcinoma. PLoS One. 2016;11(6):e0156658. doi:10.1371/journal.pone.0156658
50. Tacha D, Zhou D, Cheng L. Expression of PAX8 in Normal and Neoplastic Tissues A Comprehensive Immunohistochemical Study. Appl Immunohistochem Mol Morphol. 2011;19(4):293–299. doi:10.1097/PAI.0b013e3182025f66
51. Yemelyanova A, Gown AM, Wu LS, Holmes BJ, Ronnett BM, Vang R. PAX8 expression in uterine adenocarcinomas and mesonephric proliferations. Int J Gynecol Pathol. 2014;33(5):492–499. doi:10.1097/PGP.0b013e3182a54afa
52. Bowen NJ, Logani S, Dickerson EB, et al. Emerging roles for PAX8 in ovarian cancer and endosalpingeal development. Gynecol Oncol. 2007;104(2):331–337. doi:10.1016/j.ygyno.2006.08.052
53. Chai HJ, Ren Q, Fan Q, et al. PAX8 is a potential marker for the diagnosis of primary epithelial ovarian cancer. Oncol Lett. 2017;14(5):5871–5875. doi:10.3892/ol.2017.6949
54. Bleu M, Gaulis S, Lopes R, et al. PAX8 activates metabolic genes via enhancer elements in renal cell carcinoma. Nat Commun. 2019;10(1):3739. doi:10.1038/s41467-019-11672-1
55. Barr ML, Jilaveanu LB, Camp RL, Adeniran AJ, Kluger HM, Shuch B. PAX-8 expression in renal tumours and distant sites: a useful marker of primary and metastatic renal cell carcinoma? J Clin Pathol. 2015;68(1):12–17. doi:10.1136/jclinpath-2014-202259
56. Ozcan A, Shen SS, Hamilton C, et al. PAX 8 expression in non-neoplastic tissues, primary tumors, and metastatic tumors: a comprehensive immunohistochemical study. Mod Pathol. 2011;24(6):751–764. doi:10.1038/modpathol.2011.3
57. Laury AR, Perets R, Piao H, et al. A comprehensive analysis of PAX8 expression in human epithelial tumors. Am J Surg Pathol. 2011;35(6):816–826. doi:10.1097/PAS.0b013e318216c112
58. Khizer K, Padda J, Khedr A, et al. Paired-box gene 8 (PAX8) and its association with epithelial carcinomas. Cureus. 2021;13(8):e17208. doi:10.7759/cureus.17208
59. Hewitt SM, Hamada S, Monarres A, Kottical LV, Saunders GF, McDonnell TJ. Transcriptional activation of the bcl-2 apoptosis suppressor gene by the paired box transcription factor PAX8. Anticancer Res. 1997;17(5A):3211–3215.
60. Li CG, Nyman JE, Braithwaite AW, Eccles MR. PAX8 promotes tumor cell growth by transcriptionally regulating E2F1 and stabilizing RB protein. Oncogene. 2011;30(48):4824–4834. doi:10.1038/onc.2011.190
61. Fares B, Berger L, Bangiev-Girsh E, et al. PAX8 plays an essential antiapoptotic role in uterine serous papillary cancer. Oncogene. 2021;40(34):5275–5285. doi:10.1038/s41388-021-01925-z
62. Di Palma T, Lucci V, de Cristofaro T, Filippone MG, Zannini M. A role for PAX8 in the tumorigenic phenotype of ovarian cancer cells. BMC Cancer. 2014;14:292. doi:10.1186/1471-2407-14-292
63. Soriano AA, de Cristofaro T, Di Palma T, et al. PAX8 expression in high-grade serous ovarian cancer positively regulates attachment to ECM via Integrin β3. Cancer Cell Int. 2019;19:303. doi:10.1186/s12935-019-1022-8
64. Ghannam-Shahbari D, Jacob E, Kakun RR, et al. PAX8 activates a p53-p21-dependent pro-proliferative effect in high grade serous ovarian carcinoma. Oncogene. 2018;37(17):2213–2224. doi:10.1038/s41388-017-0040-z
65. de Cristofaro T, Di Palma T, Soriano AA, et al. Candidate genes and pathways downstream of PAX8 involved in ovarian high-grade serous carcinoma. Oncotarget. 2016;7(27):41929–41947. doi:10.18632/oncotarget.9740
66. Elias KM, Emori MM, Westerling T, et al. Epigenetic remodeling regulates transcriptional changes between ovarian cancer and benign precursors. JCI Insight. 2016;1(13):e87988. doi:10.1172/jci.insight.87988
67. Rodgers LH. Loss of PAX8 in high-grade serous ovarian cancer reduces cell survival despite unique modes of action in the fallopian tube and ovarian surface epithelium. Oncotarget. 2016;7(22):32785–32795. doi:10.18632/oncotarget.9051
68. Reddy J, Fonseca MAS, Corona RI, et al. Predicting master transcription factors from pan-cancer expression data. Sci Adv. 2021;7(48):eabf6123. doi:10.1126/sciadv.abf6123
69. Chaves-Moreira D, Mitchell MA, Arruza C, et al. The transcription factor PAX8 promotes angiogenesis in ovarian cancer through interaction with SOX17. Sci Signal. 2022;15(728):eabm249. doi:10.1126/scisignal.abm2496
70. Bleu M, Mermet-Meillon F, Apfel V, et al. PAX8 and MECOM are interaction partners driving ovarian cancer. Nat Commun. 2021;12(1):2442. doi:10.1038/s41467-021-22708-w
71. Dinh HQ, Lin X, Abbasi F, et al. Single-cell transcriptomics identifies gene expression networks driving differentiation and tumorigenesis in the human fallopian tube. Cell Rep. 2021;35(2):108978. doi:10.1016/j.celrep.2021.108978
72. Adler EK, Corona RI, Lee JM, et al. The PAX8 cistrome in epithelial ovarian cancer. Oncotarget. 2017;8(65):108316–108332. doi:10.18632/oncotarget.22718
73. Shi K, Yin X, Cai MC, et al. PAX8 regulon in human ovarian cancer links lineage dependency with epigenetic vulnerability to HDAC inhibitors. Elife. 2019;8:e44306. doi:10.7554/eLife.44306
74. Hardy LR, Pergande MR, Esparza K, et al. Proteomic analysis reveals a role for PAX8 in peritoneal colonization of high grade serous ovarian cancer that can be targeted with micelle encapsulated thiostrepton. Oncogene. 2019;38(32):6003–6016. doi:10.1038/s41388-019-0842-2
75. Lin L, Shi K, Zhou S, et al. SOX17 and PAX8 constitute an actionable lineage-survival transcriptional complex in ovarian cancer. Oncogene. 2022;41(12):1767–1779. doi:10.1038/s41388-022-02210-3
76. Grimley E, Liao C, Ranghini EJ, Nikolovska-Coleska Z, Dressler GR. Inhibition of Pax2 Transcription Activation with a Small Molecule that Targets the DNA Binding Domain. ACS Chem Biol. 2017;12(3):724–734. doi:10.1021/acschembio.6b00782
77. Bradford STJ, Grimley E, Laszczyk AM, Lee PH, Patel SR, Dressler GR. Identification of Pax protein inhibitors that suppress target gene expression and cancer cell proliferation. Cell Chem Biol. 2022;29(3):412–422.e4. doi:10.1016/j.chembiol.2021.11.003
78. Chen F, Gaitskell K, Garcia MJ, Albukhari A, Tsaltas J, Ahmed AA. Serous tubal intraepithelial carcinomas associated with high-grade serous ovarian carcinomas: a systematic review. BJOG. 2017;124(6):872–878. doi:10.1111/1471-0528.14543
79. Chaves-Moreira D, Mitchell MA, Arruza C, et al. The transcription factor PAX8 promotes angiogenesis in ovarian cancer through interaction with SOX17. Sci Signal. 2022;15(728):eabm2496. doi:10.1126/scisignal.abm2496
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.