Back to Journals » Therapeutics and Clinical Risk Management » Volume 11
Patterns of treatment and costs of intermediate and advanced hepatocellular carcinoma management in four Italian centers
Authors Colombo G, Cammà C, Attili A, Ganga R, Gaeta G, Brancaccio G, Franzini JM, Volpe M, Turchetti G
Received 8 May 2015
Accepted for publication 17 July 2015
Published 19 October 2015 Volume 2015:11 Pages 1603—1612
DOI https://doi.org/10.2147/TCRM.S88208
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Giorgio Lorenzo Colombo,1 Calogero Cammà,2 Adolfo Francesco Attili,3 Roberto Ganga,4 Giovanni Battista Gaeta,5 Giuseppina Brancaccio,5 Jean Marie Franzini,6 Marco Volpe,6 Giuseppe Turchetti7
1Department of Drug Sciences, University of Pavia, Pavia, Italy; 2Section of Gastroenterology, Di.Bi.M.I.S., University of Palermo, Palermo, Italy; 3Department of Clinical Medicine, University of Rome (La Sapienza) Rome, Italy; 4Clinical Medicine Division, Ospedale Brotzu, Cagliari, Italy; 5Viral Hepatitis Unit, Second University, Naples, Italy; 6Business Integration Partners S.p.A., Milan, Italy; 7Scuola Superiore Sant’Anna, Pisa, Italy
Background: Hepatocellular carcinoma (HCC) is a severe health condition associated with high hospitalizations and mortality rates, which also imposes a relevant economic burden.
Purpose: The aim of the present survey is to investigate treatment strategies and related costs for HCC in the intermediate and advanced stages of the disease.
Patients and methods: The survey was conducted in four Italian centers through structured interviews with physicians. Information regarding the stage of disease, treatments performed, and related health care resource consumption was included in the questionnaire. Direct health care cost per patient associated with the most relevant treatments such as sorafenib, transarterial chemoembolization (TACE), and transarterial radioembolization (TARE) was evaluated.
Results: Between 2013 and 2014, 285 patients with HCC were treated in the four participating centers; of these, 80 were in intermediate stage HCC (Barcelona Clinic Liver Cancer Classification [BCLC] B), and 57 were in the advanced stage of the disease (BCLC C). In intermediate stage HCC, the most frequent first-line treatment was TACE (63%) followed by sorafenib (15%), radiofrequency ablation (14%), and TARE (1.3%). In the advanced stage of HCC, the most frequently used first-line therapy was sorafenib (56%), followed by best supportive care (21%), TACE (18%), and TARE (3.5%). The total costs of treatment per patient amounted to €12,214.54 with sorafenib, €13,418.49 with TACE, and €26,106.08 with TARE. Both in the intermediate and in the advanced stage of the disease, variability in treatment patterns among centers was observed.
Conclusion: The present analysis raises for the first time the awareness of the overall costs incurred by the Italian National Healthcare System for different treatments used in intermediate and advanced HCC. Further investigations would be important to better understand the effective health care resource usage.
Keywords: disease costs, drugs cost, transarterial embolization, sorafenib
Introduction
Hepatocellular carcinoma (HCC) is the fifth most common malignancy worldwide, which causes approximately 500,000 deaths every year,1 and it is characterized by biological and clinical heterogeneity and different prognoses.2,3 It is also the second most common cause of death from cancer worldwide and the second most rapidly increasing type of cancer in the United States.4–6 The incidence of HCC increases progressively with advancing age in all populations, reaching its peak at the age of 70.7 Moreover, HCC contributes substantially to health care spending.
In Italy, liver cancer is one of the first five causes of death in males (7%);8 12,500 new cases of liver tumors were expected in 2014, accounting for 3% of the overall incidence of all tumors, with a male:female ratio of 2:1. The incidence of HCC has decreased in both sexes, since the mid 90s.8 Approximately 21,000 patients with a previous diagnosis of HCC (1% of all patients with a cancer) lived in Italy in 2011.8
The most important risk factors for HCC are cirrhosis due to chronic hepatitis B and chronic hepatitis C, alcoholic cirrhosis, or nonalcoholic steatohepatitis, high body mass index, and diabetes mellitus.9–11
Worldwide, the most important risk factor for liver cancer is chronic hepatitis B or hepatitis C virus. People infected with both viruses have a high risk of developing chronic hepatitis, cirrhosis, and liver cancer. The risk of developing liver cancer is higher if patients are also heavy drinkers.12 The vast majority of patients developing liver cancer already have some evidence of cirrhosis. Obesity increases the risk of developing liver cancer, probably because it can result in fatty liver disease and cirrhosis. Type 2 diabetes is associated with an increased risk of liver cancer, usually in patients who also have other risk factors (eg, heavy alcohol use, chronic viral hepatitis).12 This risk may be increased as type 2 diabetes patients tend to be overweight or obese, which can cause liver problems.12 Although HCC patients can benefit from radical resection, transplantation, or radiofrequency ablation thanks to preliminary screening and diagnosis, tumors in some patients (those with background cirrhosis in particular) still progress rapidly because of local spreading or metastases.
The Barcelona Clinic Liver Cancer Classification (BCLC) correlates stages of the disease with treatment modalities, and identifies five stages of HCC (0, A, B, C, and D) according to preestablished prognostic variables and treatment modalities for each stage of the disease, thus providing both prognostic prediction and treatment allocation recommendations.13 The American Association for the Study of Liver Disease (AASLD) guidelines 2010,14 the European Association for the Study of the Liver (EASL) guidelines 2012,15 and the Italian Association for the Study of the Liver (AISF 2012)16 are the most updated guidelines for the treatment of HCC and include staging and prognostic–therapeutic stratification (EASL–BCLC guidelines) (Figure 1).
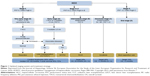  | Figure 1 Updated staging system and treatment strategy. |
In the intermediate stage (BCLC B), patients with asymptomatic tumors (without an invasive pattern) survive 16 months on average;2,17,18 chemoembolization prolongs the survival of these patients up to 19–20 months, according to randomized controlled trial and meta-analysis of pooled data.17
HCC exhibits intense neoangiogenic activity during its progression. The rationale for transarterial chemoembolization (TACE) is that the intra-arterial infusion of a cytotoxic agent followed by embolization of the tumor-feeding blood vessels will result in a strong cytotoxic and ischemic effect. This procedure combines transcatheter delivery of chemotherapy emulsioned with lipiodol, followed by vascular stagnation achieved with embolic agents. Chemoembolization achieves partial responses in 15%–55% of patients and significantly delays tumor progression and macrovascular invasion. Overall, the median survival for intermediate HCC cases is expected to be approximately 16 months, whereas after chemoembolization, the median survival is ~20 months.15,19,20
Patients in the advanced stage of the disease (BCLC stage C) (cancer-related symptoms and/or macrovascular invasion and/or extrahepatic spread) have poorer prognosis, and the expected median survival without treatment is ~6 months,18,21 or 25% at first year,19 which is extended to 11 months by treatment with sorafenib.20
Sorafenib, a multikinase inhibitor, is the standard systemic therapy suggested by guidelines for HCC BCLC stage C or for intermediate stage after failure of or ineligibility for locoregional therapies (treatment-stage migration strategy).13
Efficacy and safety of sorafenib were evaluated in several clinical studies;22 the Phase III SHARP study23 demonstrated that sorafenib significantly increased overall survival by 44% vs placebo (10.7 vs 7.9 months, respectively; P<0.001) and almost doubled median time to progression (5.5 vs 2.8 months, respectively; hazard ratio [HR]: 0.58, P<0.001).
These results were confirmed by a Phase III study conducted in the Asia–Pacific region;24 in this trial, sorafenib significantly increased the overall survival by 47% vs placebo (6.5 vs 4.2 months, respectively; HR: 0.68; P=0.014) and doubled the median time to progression vs placebo (2.8 vs 1.4 months, respectively; HR: 0.57, P=0.0005).
In both Phase III studies, the safety profiles of sorafenib were predictable and manageable, and the benefits of sorafenib were consistent across subgroups by etiology, tumor burden, tumor stage, and prior therapy. Based on these results, international and national guidelines recommended sorafenib as the treatment of choice for advanced HCC (level of evidence IA15,25/positively strong). A few studies26–31 reported the economic burden of HCC worldwide, and while sorafenib cost-effectiveness analyses have been published,32,33 to our knowledge no data exist on the cost-effectiveness of TACE for HCC. The objective of the present analysis is to investigate treatment pathways and related health care costs for HCC BCLC stage B and stage C patients.
Material and methods
The aim of the present survey is to investigate the current patterns of treatment, health care resource consumption, and costs related to the treatment of HCC in the intermediate and advanced stages of the disease, through an analysis of the therapeutic pathways followed in four Italian centers. The survey was conducted through structured interviews involving gastroenterology and interventional radiology units. Ethical approval for this study was obtained from Policlinico Umberto I Roma, AOU Seconda Università di Napoli, Policlinico Paolo Giaccone Palermo, and AO Brotzù Cagliari. Informed consent was obtained from all patients.
The data collected in the project remained the property of each facility provider and were managed in aggregate and anonymous form, without access to individual patient data.
The following information was collected:
- Number of patients with HCC by stage
- Distribution of patients by treatment options (TACE, transarterial radioembolization [TARE] systemic treatment) and line of treatment (first- and second-line treatment)
- Treatments administered (frequency, average number of locoregional therapies, and duration of treatment for systemic therapies)
- Diagnostic procedures and laboratory tests associated with each treatment
- Specialist visits and follow-up associated with each treatment (percentage of patients undergoing examinations/visits and frequency)
- Consumption of medical and nonmedical supplies associated with treatments (type of consumables, quantity, and unitary costs)
- Cost of medical, nursing, and technical staff involved in patient management during the treatment.
Unit costs considered in this analysis and related sources are reported in Table 1.
  | Table 1 Resources consumption and costs |
As for drugs, the cost per milligram was estimated as the average cost of different packs at the maximum price reimbursed by the Italian National Health Service.
In order to estimate the average cost per month of therapy, an 80% adherence to treatment was considered for sorafenib, along with published data on oral antineoplastic treatments.34 The reference year of the present survey was 2013.
Results
The present survey involved 137 patients with intermediate and advanced stage HCC, BCLC stage B, and BCLC stage C among a cohort of 285 patients (mean age: 66 years, 74% males) treated in four Italian centers specialized in the treatment of patients with HCC (Policlinico Umberto I Roma, AOU Seconda Università di Napoli, Policlinico Paolo Giaccone Palermo, AO Brotzù Cagliari). In the overall population, 20% of patients were in the very early stage of the disease (HCC BCLC stage 0), 17% in the early stage (HCC BCLC stage A), 28% in the intermediate stage (HCC BCLC stage B), 20% in the advanced stage (HCC BCLC stage C), and the remaining 15% in the terminal stage (HCC BCLC stage D) (Figure 2).
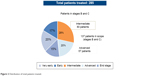  | Figure 2 Distribution of total patients treated. |
First- and second-line treatments for patients in the intermediate and advanced stages of the disease are reported in Figures 3 and 4.
Among the 80 patients in the intermediate stage of the disease treated with first-line therapies, 63% (minimum 50% to maximum 80%) were treated with TACE, 15% with sorafenib (range, 10%–17%), 14% (range, 5%–30%) with radiofrequency ablation, and the remaining 10% with other therapies (best supportive care, TARE, and liver resection).
Of these, 55% (n=44) were treated with second-line or subsequent therapies. The most frequent second-line or subsequent therapies were TACE – 39% (range, 5%−60%) and sorafenib – 36% (range, 9%–40%).
Among the 57 patients in the advanced stage of the disease treated with first-line therapies, sorafenib was the most frequently used first-line treatment: 56% (range, 12%–79%). Best supportive care was used in 21% of patients (range, 15%–24%) as the first-line option.
Of these, 54.4% (n=31) were then treated with second-line or subsequent therapies. TACE was the most frequent second-line option in the advanced stage – 45% (range, 0%–80%), followed by sorafenib – 29% (range, 0%–60%), and best supportive care – 26% (range, 0%–30%).
The mean duration of treatment with sorafenib in 137 patients with intermediate and advanced stage HCC was 6.1 months in all treatment lines combined.
The average number of TACE was 2.5 procedures/patient, and patients treated with TARE received an average of 1.5 procedures/patient.
The health care resources used in association with each type of therapy are reported in Table 2.
  | Table 2 Resources according to type of treatment |
Mean resource consumption related to the different treatments, and related costs of consumables and professional resources are reported in Tables 3 and 4.
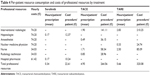  | Table 4 Per-patient resource consumption and costs of professional resources by treatment |
Regarding the systemic treatment, the average cost per tablet (200 mg) of sorafenib to public health care structures (including the two 5% transient price reductions, as per Italian law at the time of the analysis)35,36 accounted for €28.70.
Considering the overall mean daily dosage (612 mg) and an estimated 80% of adherence to therapy,34 the average per-month cost of treatment with sorafenib amounted to €1,986.36.
On the basis of the unitary cost per procedure/month of treatment and the duration of each therapy, the calculated average total costs of the different treatments are reported in Table 5.
Discussion
The results of the present analysis, aimed at evaluating the patterns of treatment and the costs related to HCC treatments, show a high variability among centers concerning procedures used in both the intermediate and advanced stages, first-line and subsequent lines. TACE was the treatment of choice in intermediate HCC, as suggested by the guidelines, but ~38% of patients were treated with alternative therapies. Sorafenib was the most frequently used first-line therapy in advanced HCC as recommended by international and Italian guidelines, although ~45% of these patients received other first-line treatments. A potential role of the combined approach of these therapies in intermediate HCC patients, as suggested by recent publications,37 is still experimental and needs further confirmatory trials. The cost of HCC treatment amounted to ~€12,200/patient for sorafenib, €13,400/patient for TACE, and €26,100/patient for TARE.
The unitary costs of the treatments that were used in the calculations originate from tariffs and average acquisition costs at the national level (determined on a benchmark of 23 tenders). This methodology allows a comparison among treatment strategies based on the same evaluation criteria. From the perspective of health care cost of treatment, some facts should be taken into consideration. Treatment guidelines recommend TACE for intermediate HCC. The costs sustained by the centers for this procedure are, however, only partially covered by their diagnosis-related group’s (DRG)38 tariff (€5,304 vs a reimbursement tariff of €4,085; DRG 203: malignancy of hepatobiliary system or pancreas).32 Regarding TARE, which is not recommended by treatment guidelines either in intermediate stage or in advanced stage HCC, the costs sustained by the centers are relevant (~€17,400/patient/procedure), while at the national level, the DRG’s reimbursement tariff for TARE is DRG 409 (radiotherapy),38 reimbursed at €1,471. Among the centers involved in the present study, only one performs this kind of procedure.
Sorafenib, recommended by national and international guidelines as the treatment of choice for advanced HCC, is dispensed in Italy by hospital pharmacies and registered in the “file F” that is used for specific categories of drugs distributed by NHS health care structures not covered by DRGs or tariffs (sorafenib cost is directly reimbursed to hospitals by local health care units or regions). We note, however, that this evaluation is only a cost analysis and does not take into account unplanned hospitalization, cost of side effects, and their treatment. The inclusion of these “shadow costs” could change the final cost of HCC treatment. Furthermore, we did not find any cases of HCC surgical treatments. Recent literature,39 for example, showed that surgical therapies could also give the best results in patients with intermediate or advanced HCC. A limitation of the present study is that the centers that participated in this analysis may not accurately mirror the situation of the entire Italian territory, although they are centers experienced in the treatment of HCC, selected among hospitals with a wide volume of activity in this field. To our knowledge, this is the first study that evaluates the costs of treatment patterns for intermediate/advanced HCC patients in a real-life setting in Italy.
Conclusion
Sorafenib is recommended by international and Italian guidelines (level of evidence IA/strong positive) as the treatment of choice for advanced HCC. It was the most frequently used treatment in this setting; the associated cost of treatment was €12,200/patient. TACE was the most widely used treatment in intermediate HCC, and its associated costs of €13,400/patient are only partially covered by the DRG’s reimbursement tariffs. TARE, which is not indicated as a treatment choice in HCC management guidelines, was performed in one of the participating centers with an associated average cost of €26,100/patient that is not covered by the national reference DRG. The present study highlights the need for additional analyses of the national health care system resources that are being used routinely for the treatment of HCC and the associated outcomes, and can be the basis for future research and analysis of the cost-effectiveness of TACE, alone or combined with sorafenib, for the treatment of HCC.
Acknowledgment
The survey was supported by an unrestricted grant of Bayer Healthcare S.p.A.
Disclosure
The authors report no conflicts of interest in this work.
References
El-Serag HB. Hepatocellular carcinoma: an epidemiologic view. J Clin Gastroenterol. 2002;35(5 Suppl 2):S72–S78. | ||
Cabibbo G, Enea M, Attanasio M, Bruix J, Craxì A, Cammà C. A meta-analysis of survival rates of untreated patients in randomized clinical trials of hepatocellular carcinoma. Hepatology. 2010;51:1274–1283. | ||
Villa E, Critelli R, Lei B, Marzocchi G, et al. Neoangiogenesis-related genes are hallmarks of fast-growing hepatocellular carcinomas and worst survival. Results from a prospective study. Gut. Epub February 9, 2015. doi:10.1136/gutjnl-2014-308483. | ||
International Agency for Research on Cancer. World Health Organization. GLOBOCAN 2012: estimated cancer incidence, mortality and prevalence worldwide. Available from: http://globocan.iarc.fr/Pages/fact_sheets_cancer.aspx. Accessed January 29, 2015. | ||
Everhart JE, Ruhl CE. Burden of digestive diseases in the United States part II: lower gastrointestinal diseases. Gastroenterology. 2009;136(3):741–754. | ||
Ferlay J, Shin HR, Bray F, Forman D, Mathers C, Parkin DM. Estimates of worldwide burden of cancer in 2008: GLOBOCAN 2008. Int J Cancer. 2010;127(12):2893–2917. | ||
El-Serag HB, Mason AC. Rising incidence of hepatocellular carcinoma in the United States. N Engl J Med. 1999;340:745–750. | ||
I numeri del cancro in Italia 2014. AIOM AIRTUM. [Cancer numbers in Italy in 2014. AIOM AIRTUM]. Available from: http://www.registri-tumori.it/PDF/AIOM2014/I_numeri_del_cancro_2014.pdf. Accessed January 21, 2015. Italian. | ||
Schwartz M, Roayaie S, Konstadoulakis M. Strategies for the management of hepatocellular carcinoma. Nat Clin Pract Oncol. 2007;4(7): 424–432. | ||
Yang JD, Roberts LR. Hepatocellular carcinoma: a global view. Nat Rev Gastroenterol Hepatol. 2010;7(8):448–458. | ||
Kudo M, Izumi N, Kokudo N, et al. Management of hepatocellular carcinoma in Japan: consensus-based clinical practice guidelines proposed by the Japan Society of Hepatology (JSH) 2010 updated version. Dig Dis. 2011;29:339–364. | ||
American Cancer Society. Liver Cancer. Available from: http://www.cancer.org/cancer/livercancer/detailedguide/liver-cancer-risk-factors. Accessed February 3, 2015. | ||
Llovet JM, Brú C, Bruix J. Prognosis of hepatocellular carcinoma: the BCLC staging classification. Semin Liver Dis. 1999;19:329–338. | ||
Bruix J, Sherman M. AASLD Practice Guideline 2010. Management of hepatocellular carcinoma: an update. Hepatology. 2011;53: 1020–1022. | ||
European Association for the Study of the Liver; European Organisation for Research and Treatment of Cancer. EASL–EORTC clinical practice guidelines: management of hepatocellular carcinoma. J Hepatol. 2012;56:908–943. | ||
Italian Association for the Study of the Liver (AISF); AISF Expert Panel; AISF Coordinating Committee, Bolondi L, Cillo U, Colombo M, et al. Position paper of the Italian Association for the Study of the Liver (AISF): the multidisciplinary clinical approach to hepatocellular carcinoma. Dig Liver Dis. 2013;45(9):712–723. doi:10.1016/j.dld.2013.01.012. | ||
Llovet JM, Bruix J. Systematic review of randomized trials for unresectable hepatocellular carcinoma: chemoembolization improves survival. Hepatology. 2003;37:429–442. | ||
Llovet JM, Bruix J. Molecular targeted therapies in hepatocellular carcinoma. Hepatology. 2008;48:1312–1327. | ||
Cammà C, Schepis F, Orlando A, et al. Transarterial chemoembolization for unresectable hepatocellular carcinoma: meta-analysis of randomized controlled trials. Radiology. 2002;224:47–54. | ||
Cabibbo G, Genco C, Di Marco V, et al. Predicting survival in patients with hepatocellular carcinoma treated by transarterial chemoembolisation. Aliment Pharmacol Ther. 2011;34:196–204. | ||
Llovet JM, Bustamante J, Castells A, et al. Natural history of untreated nonsurgical hepatocellular carcinoma: rationale for the design and evaluation of therapeutic trials. Hepatology. 1999;29:62–67. | ||
Bolondi L, Craxi A, Trevisani F, et al. Refining sorafenib therapy: lessons from clinical practice. Future Oncol. 2015;11(3):449–465. | ||
Llovet JM, Ricci S, Mazzaferro V, et al. Sorafenib in advanced hepatocellular carcinoma. N Engl J Med. 2008;359(4):378–390. doi:10.1056/NEJMoa0708857. | ||
Cheng AL, Kang YK, Chen Z, et al. Efficacy and safety of sorafenib in patients in the Asia-Pacific region with advanced hepatocellular carcinoma: a phase III randomised, double-blind, placebo-controlled trial. Lancet Oncol. 2009;10(1):25–34. doi:10.1016/S1470-2045 (08) 70285-7. | ||
AIOM Guidelines, 2014. Available from: http://www.aiom.it. Accessed February 24, 2015. | ||
Thein HH, Isaranuwatchai W, Campitelli MA, et al. Health care costs associated with hepatocellular carcinoma: a population-based study. Hepatology. 2013;58(4):1375–1384. doi:10.1002/hep.26231. | ||
Mishra A, Otgonsuren M, Venkatesan C, et al. The inpatient economic and mortality impact of hepatocellular carcinoma from 2005 to 2009: analysis of the US nationwide inpatient sample. Liver Int. 2013;33(8):1281–1286. doi:10.1111/liv.12201. | ||
Zhu L, Li J, Dong X, et al. Hospital costs and length of hospital stay for hepatectomy in patients with hepatocellular carcinoma: results of a prospective case series. Hepatogastroenterology. 2011; 58(112):2052–2057. doi:10.5754/hge10149. | ||
Gondek K, Lang K, Danchenko N, et al. Economic costs of hepatocellular carcinoma in the United States. J Clin Oncol. 2008;26(Suppl 15):6555. ASCO Annual Meeting Proceedings (Post-Meeting Edition). | ||
Lang HC, Wu JC, Yen SH, et al. The lifetime cost of hepatocellular carcinoma. Appl Health Econ Health Policy. 2008;6(1):55–65. | ||
Marinho RT, Giria J, Moura MG. Rising costs and hospital admissions for hepatocellular carcinoma in Portugal (1993–2005). World J Gastroenterol. 2007;13(10):1522–1527. | ||
Iavarone M, Cabibbo G, Piscaglia F, et al; on behalf of the SOFIA (SOraFenib Italian Assessment) study group. Field-practice study of sorafenib therapy for hepatocellular carcinoma: a prospective multicenter study in Italy. Hepatology. 2011;54:2055–2063. | ||
Cammá C, Cabibbo G, Petta S, et al; on behalf of the WEF and the SOFIA study groups. Cost-effectiveness of sorafenib treatment in field practice for patients with hepatocellular carcinoma. Hepatology. 2013;57:1046–1054. | ||
Spoelstra SL, Given CW. Assessment and measurement of adherence to oral antineoplastic agents. Semin Oncol Nurs. 2011;27(2): 116–132. | ||
Determinazione AIFA del 3 luglio 2006, Gazzetta Ufficiale n. 156 del 7 luglio 2006. [AIFA Determination of 3 July 2006. Official Gazette no 156 of 7 July 2006]. Available from: http://www.medicoeleggi.com/argomenti00/italia2006/19145.htm. Accessed January 28, 2015. Italian. | ||
Determinazione AIFA del 27 settembre 2006, Gazzetta n. 227 del 29 settembre 2006. [AIFA Determination 26 September 2006. Official Gazette no 156 of 29 September 2006]. Available from: http://www.medicoeleggi.com/argomenti00/italia2006/19186.htm. Accessed January 28, 2015. Italian. | ||
Cabibbo G, Maida M, Cammà C, Craxì A. Is the efficacy of sorafenib treatment in patients with hepatocellular carcinoma affected by age? Expert Rev Anticancer Ther. 2013;13(12):1355–1361. | ||
DECRETO 18 ottobre 2012. Remunerazione prestazioni di assistenza ospedaliera per acuti, assistenza ospedaliera di riabilitazione e di lungodegenza post acuzie e di assistenza specialistica ambulatoriale. (13A00528) (GU Serie Generale n.23 del 28-1-2013 – Suppl. Ordinario n. 8. [Decree 18 October 2012. Remuneration performance of hospital assistance for acutes, hospital assistance to rehabilitation and long-term assistance after acute and specialist outpatient assistance]. Available from: http://www.gazzettaufficiale.it/eli/id/2013/01/28/13A00528/sg. Accessed January 29, 2015. Italian. | ||
Zhang ZM, Guo JX, Zhang ZC, et al. Therapeutic options for intermediate-advanced hepatocellular carcinoma. World J Gastroenterol. 2011;17(13):1685–1689. | ||
Conferenza Stato-Regioni 21/11/2014 – Rapporto STEM: “Prima analisi dei costi per il personale del Servizio Sanitario Nazionale Anni 2010/2012.” [State-Regions Conference 11/21/14. STEM Report: “First cost analysis for National Health Service personnel in 2010/2012”]. Available from: http://www.regioni.it/riforme/2014/11/21/conferenza-stato-regioni-21112014-rapporto-stem-prima-analisi-dei-costi-per-il-personale-del-servizio-sanitario-nazionale-anni-20102012-376317/. Accessed January 23, 2015. Italian. | ||
Italian Medicines Agency. Reimbursement list. Class H list. Available from: http://www.agenziafarmaco.gov.it/sites/default/files/111.84470.1172743348645ce4c.pdf. Accessed January 23, 2015. | ||
Setacci F, de Donato G, Chisci E, et al. Economic impact of endarterectomy vs carotid artery stenting: a one year, single center study. EuroIntervention. 2007;3(3):340–344. |
 © 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2015 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




