Back to Journals » Open Access Rheumatology: Research and Reviews » Volume 13
Patterns of Response to Different Treatment Strategies in Seropositive Rheumatoid Arthritis Patients in a Tertiary Hospital in South‐Western Saudi Arabia: A Retrospective Study
Authors AlOmair M , AlMalki H, AlShamrani N, Habtar G, AlAsmari M, Mobasher W, AlQahtani H, Rahman A , Asiri A
Received 12 June 2021
Accepted for publication 3 August 2021
Published 14 August 2021 Volume 2021:13 Pages 239—246
DOI https://doi.org/10.2147/OARRR.S322833
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Chuan-Ju Liu
Mohammed AlOmair,* Hanan AlMalki,* Nouf AlShamrani, Ghadah Habtar, Maram AlAsmari, Wejdan Mobasher, Hanan AlQahtani, Aydah Rahman, Alhussain Asiri
Division of Rheumatology, Department of Medicine, Aseer Central Hospital, Abha, Saudi Arabia
*These authors contributed equally to this work
Correspondence: Mohammed AlOmair
Division of Rheumatology, Department of Medicine, Aseer Central Hospital, P.O. Box 34, Abha, Saudi Arabia
Tel +966 507735466
Fax +966 172243595
Email [email protected]; [email protected]
Purpose: To study the pattern of response to different treatment strategies in seropositive rheumatoid arthritis (RA) patients and to describe our clinical practice in RA management.
Patients and Methods: Over a period of two years from April 2018 to April 2020, we conducted a retrospective analysis of data for 288 consecutive seropositive RA patients attending rheumatology clinics and the daycare unit at Aseer Central Hospital. Data were collected on patient demographics, disease duration, extraarticular manifestations, comorbidities and treatment. Disease activity was assessed using the clinical disease activity index (CDAI).
Results: Out of the total 288 patients, 42% (120) are on csDMRADs, while 54% (162) are on bDMRADs and 4% (6) are on tsDMARDs. Of the patients on csDMARDS, 51%, 43% and 7% of them were on remission, low and moderate disease activity, respectively. However, of the patients on non-csDMARDS, 36.3%, 49.4% and 14.3% of them were on remission, low and moderate disease activity, respectively. Failure of csDMARDs was affected by the presence of high disease activity at baseline, extraarticular lung manifestations and coexistent fibromyalgia, with a significant effect of the latter on remission rate. Among patients on non-csDMARDs, 42 (25%) showed one or more therapy changes. Tumor necrosis factor inhibitors were the predominant first-line agents in biologically naive patients (65%) followed by abatacept (18%). Abatacept was the most frequently prescribed second biologic in 52% of cases followed by tocilizumab in 19%.
Conclusion: The current clinical practice in our hospital is consistent with the latest American College of Rheumatology (ACR)/The European League Against Rheumatism (EULAR) guidelines. Treat-to-target strategy was achieved in the vast majority of our patients, while remission was observed in almost half of the patients.
Keywords: rheumatoid arthritis, DMARDs, biologics, remission, CDAI
Introduction
RA is a chronic systemic autoimmune disease that clinically manifests as symmetrical and erosive polyarthritis affecting between 0.5 and 1% of the adult population.1 The prevalence of rheumatoid arthritis in Saudi Arabia is 2.2 per 1000 cases.2
RA often causes severe joint destruction, functional limitation and disability. Currently, the evidence recommends “treat-to-target” strategy (T2T) with early and intensive treatment intended for remission or low disease activity, which can significantly improve outcomes.3 In case of failure to achieve the therapeutic target within 3 to 6 months, adjustment of therapy is essential.
T2T strategy can be applied by the early use of conventional synthetic disease-modifying antirheumatic drugs (csDMARDs), which are considered the mainstay of treatment for RA. csDMARDs include methotrexate (MTX), hydroxychloroquine (HCQ), sulfasalazine (SZZ) and leflunomide (LEF). MTX is the first treatment choice of RA because it is a well-tolerated drug with excellent efficacy. In patients with a contraindication or intolerance to MTX, SZZ or LEF should be used as an alternative. In cases of failure of the initial regimen and the absence of poor prognosis markers, other csDMARDs should be considered. While in cases of failure of the initial regimen and the presence of poor prognosis markers, biological DMARDs (bDMARDs) or targeted synthetic DMARDs (tsDMARDs) should be added. Poor prognostic factors, which are used to guide the treatment decisions in RA, include high disease activity, presence of rheumatoid factor (RF), anti-citrullinated protein-peptide antibodies (anti-CCP), early structural damage and failure of two or more csDMARDs.4
Tumor necrosis factor inhibitors (TNFi) were the first biologic therapies to be licensed for RA. Recently, a wide range of different drugs with different modes of action of bDMARDs are available, such as abatacept targets T cell co-stimulation, rituximab targets CD20+ B cells and finally tocilizumab targets interleukin 6 receptor.4
Janus kinase (JAK) inhibitors are oral small-molecule inhibitors of the janus kinase family of receptors that affect intracellular signaling pathways such as tofacitinib, which is the first JAK inhibitor approved for RA treatment.4
Reliable measures of disease activity are needed in order to successfully achieve T2T strategy. According to ACR recommendations, the clinical disease activity index (CDAI), the disease activity score with 28‐joint counts (DAS 28) (erythrocyte sedimentation rate or C‐reactive protein), the routine assessment of patient index data with 3 measures (RAPID 3), and the simplified disease activity index (SDAI) are preferable to use for assessment of disease activity in RA.5
The aim of this study is to evaluate the pattern of response to different treatment strategies in seropositive RA patients and to demonstrate the clinical practice in the management of RA in our rheumatology unit in Aseer Central Hospital, a tertiary hospital in South‐Western Saudi Arabia serving a diverse population of about 2 million.
Patients and Methods
A record-based retrospective study was conducted on 942 consecutive patients who met ACR/EULAR 2010 classification criteria for the diagnosis of RA.6 The patients were following up in the outpatient clinics and the daycare unit at Aseer Central Hospital from April 2018 to April 2020. The inclusion criteria included adults older than 18 years with either RF or anti-CCP positivity, apart from patients who started a new drug for less than 6 months, lost follow-up or had incomplete clinical data. The medical records of 288 patients who met the inclusion criteria were reviewed, and data was extracted about patient demographics and characteristics, including disease duration and extraarticular manifestations. History or current evidence of comorbidities was documented including hypertension (HTN), diabetes mellitus (DM), ischemic heart disease, cancers and lymphoma, gastrointestinal diseases, infections (hepatitis and tuberculosis), lung disease, osteoporosis and hypothyroidism. Data on disease activity and the treatment protocol were documented.
CDAI was used to assess disease activity because it has been validated as a comparable assessment with DAS‐28‐CRP and SDAI but has a better performance for assessing disease remission than DAS‐28‐CRP.7 CDAI is defined as the sum of tender and swollen joint counts [28 joints] and global assessments by patient and physician. Additionally, remission was defined as a score of ≤2.8, low activity of ≤10, moderate activity of ≤22, and high activity of >22.5
This study was approved by Aseer General Directorate of Health Affair-Regional Committee for Research Ethics (IRB Registration No: H-06-B-091).
Informed patient consents were not required by the IRB for this retrospective chart review study, as there was no direct implication to the subjects involved. All aspects of the study were conducted in accordance with the Declaration of Helsinki. Patients’ clinical data was used only for research purposes while maintaining confidentiality of patient’s records throughout the study.
The data was collected, coded and entered into the statistical software IBM SPSS version 22 (SPSS, Inc. Chicago, IL). All statistical analyses were done using two-tailed tests. A P-value less than 0.05 was considered to be statistically significant. Descriptive analysis based on frequency and percent distribution was done for all variables including demographic data, extraarticular manifestations, disease activity and DMARDs. Cross tabulation was used to compare treatment regimens by demographic characteristics of the patients, in addition to test distribution of current disease activity in fibromyalgia patients versus non-fibromyalgia patients according to the treatment received. The Pearson chi-square test was used to test for relative significance.
Results
A total of 288 patients were included with a mean age of 41.6 ± 11.7 years old, of which the majority were females (88%). Out of all the patients, HTN was the most commonly observed comorbidity (25%), while DM was diagnosed among 21.5%. More than half of the cases (57%) had the disease for less than 10 years and 96% of the patients had high CDAI at presentation time (Table 1).
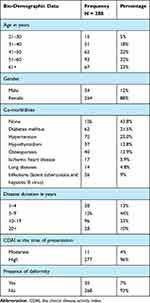 |
Table 1 Demographic Characteristics of Our Patients |
Features of extraarticular manifestations (ExRA) were recorded in 81 (28%) patients. Hematological manifestations were observed in 64 patients (24%) in the form of anemia in 42 patients (15%), thrombocytosis in 11 patients (4%) and leukopenia in 10 patients (3.5%).
Interstitial lung disease (ILD) occurred in 24 patients (8%), while Sjogren syndrome (SS) was found in only 6 patients (2%). Rheumatoid nodule and vasculitic rash were the least frequent features found in RA patients, occurring in 2 patients only (1%).
Regarding the current csDMARD therapy, monotherapy was reported among 92 patients (32%), combination therapy in 191 patients (66%) and 5 patients (2%) stopped csDMARDs. csDMRADs failed among 58% of the patients with an average duration of 4 years. Currently, after failing csDMARDs, bDMRADs were added to 54% (162) of the cases and tsDMARDs in 4% (6). Forty-eight percent of the patients were on steroids at doses of 10 mg (5%), 5 mg (35%) and 2.5 mg (7%) (Table 2).
 |
Table 2 Medications Used to Treat Our Patients |
Disease activity assessment according to CDAI is detailed in Figure 1.
 |
Figure 1 Disease activity according to CDAI. |
Before initiating bDMRAD or tsDMARD therapy, 168 patients were biologically naive. Forty-two of them had switched to a second agent (termed “first-time switchers”), 16 of whom switched again within 1 year of starting therapy. Ten patients had switched to their third agent (termed “second-time switchers”), 7 of whom switched again within 1 year and 2 within 2 years to their fourth agent (termed “third-time switchers”) (Table 3). In the majority of cases, the main cause for switching is lack of efficacy in 90% of the cases, followed by the presence of contraindications and rarely adverse events. Among biologically naive patients, the first choice of biological drug was adalimumab (42%), followed by etanercept (23%) and abatacept (18%). Abatacept was the most frequently prescribed second biological drug in 52% of cases followed by tocilizumab in 19%.
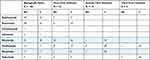 |
Table 3 Choice of Biologic Agents and JAK Inhibitors in Our Patients |
Failure of csDMARDs was significantly higher among patients diagnosed with fibromyalgia syndrome (FMS) (73.2%) than those without FMS (55.9%) (P = 0.037). Also, 59.9% of those with high CDAI failed csDMARD in comparison to 18.2% with a moderate level (P = 0.006). Furthermore, 84% of reported failed cases had lung manifestations, whereas 55.9% did not (P = 0.006) (Table 4).
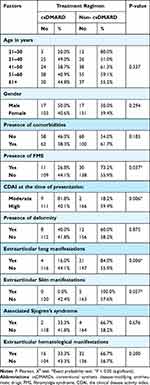 |
Table 4 Comparison Between Treatment Regimens According to Demographic Characteristics of the Patients |
Among patients with FMS, remission was achieved in 6.7% on non-csDMARD therapy and none on csDMARD. However, remission of patients without FMS reached 55% on csDMARD and 42.8% on non-csDMARD (P = 0.001) (Table 5).
 |
Table 5 Current CDAI in Fibromyalgia Patients vs Non-Fibromyalgia Patients According to the Treatment Regimen |
Discussion
This study was carried out in a tertiary hospital in south‐western Saudi Arabia to evaluate different aspects of RA management.
With regard to participants’ demographics, the mean age of cases was 41.6 ± 11.7 years, with predominance of females (88.2%), which is similar to the findings in previously published demographics of RA patients in Saudi Arabia and Arabic population.8–10 Our patients have long-standing diseases ranging from 1 to 40 years.
Moreover, our study’s population had either RF or anti-CCP positivity. As reported in the literature, RF is more commonly associated with the development of extraarticular manifestation.11 Both RF and anti-CCP are prognostic of a more disease severity and greater radiographic joint damage.12,13
In our study, 56% of patients had at least one comorbidity. The most common associated comorbidities were HTN (25%), DM (21.5%), osteoporosis (13.9%) and hypothyroidism (12.8%). Our results are in line with the previously mentioned studies regarding demographics of RA patients in our region.9,10 However, our findings suggest that none of these diseases were associated with the failure of csDMARDs.
The overall prevalence of ExRA was 28% among our patients. Anemia was the most common ExRA in our population, occurring in 15% of patients, while ILD occurred in 8%, followed by SS in 2%. According to published data, the prevalence of ExRA in our cases is higher than that in East Asia and Africa and lower than that in the UK and North America due to the ethnic differences.14
There are several limitations to the current study regarding ExRA. Our study was retrospective; hence, missing data was probably due to the usage of nonelectronic medical records. More than half of the cases (57%) had the disease for less than 10 years, and some of ExRA tend to develop later in the disease course such as amyloidosis and Felty syndrome.
Despite the lack of evidence of the superiority of MTX-based combination regimen over MTX monotherapy, it was the most frequently used regimen. Similar results were detected in a previous study regarding the management strategies adopted by rheumatologists in Saudi Arabia.15 According to a systematic review of 19 trials published in 2009, it showed no statistically significant difference of methotrexate alone or in combination with other non‐biologic DMARDs.16
Our data reveal that more than half of the patients had inadequate response to csDMARDs. Our findings suggest that the presence of high disease activity at baseline, extraarticular lung manifestations and comorbid FMS tend to increase the likelihood of failure of csDMARDs, which is in agreement with Sepriano et al and Provan et al.17,18 However, in the SWEFOT trial, smoking, the female gender, longer symptom duration and younger age are the main predictors of poor response to MTX.19 In the RA BIODAM cohort, high number of comorbidities, smoking and high number of tender joints were significantly associated with failure to implement T2T strategy.17
Saudi Arabia has one of the highest prescription rates of biologics in the Middle East. Moreover, our administration of biological treatment was higher than that in similar studies conducted in our country. That is, in a Saudi private hospital, the use of biologics was at 30%, and in another tertiary care hospital, it was at 26%,10,20 while we administered at about 54%. That is mainly because all our cases are seropositive RA, whereas roughly 40% of the cases in the mentioned studies were seronegative. Also, all the patients in this study were Saudi citizens following up in a governmental hospital, in which there is no limitation in prescribing DMARDs to Saudi RA patients.
The presence of either RF or anti-CCP have been regarded as poor prognostic factors of RA and, consequently, are evidence for intensive treatment consideration, according to the latest recommendation.4
Currently, adalimumab is the most common treatment (32%), followed by abatacept (21%) and etanercept (20%). In biologically naive patients, TNFi were the predominant first-line biologic given to 66%, while abatacept was used in 52% of first-time switchers.
After the first TNFi failure, none of our patients were switched to another TNFi. This approach is in accordance with the updated EULAR recommendations regarding the second-line biologic choice of patients who failed their first TNFi, which suggests that switching to another agent with a different mode of action is associated with significant improvement in clinical effectiveness compared to switching to a second TNFi.3 However, this evidence was based on observational studies and one randomized controlled trial. In a systematic review conducted by Fabrizio and his colleagues, they concluded that the effectiveness of the second TNFi is related to the reasons for stopping the first. Thus, switching to a second TNFi is preferable if the first discontinuation occurs because of secondary no response or presence of adverse event compared with primary no response.21
Our clinical practice correlates with the current evidence that suggests no superiority of certain bDMARDs or tsDMARDs over others.4 Therefore, treatment decisions depend upon the presence of adverse prognostic factors, medical comorbidities, availability and patient preference.
Patients on csDMARDs have higher remission rates compared with patients on non-csDMARDs which can be explained by diminished responsiveness in non-csDMARD group due to previous drug experience,22 which makes their conditions more difficult to treat and prevalent FMS in the latter group.
Possible limitations of our study may be due to the results being extracted from a single center. Also, the prevalence of comorbidities and extraarticular manifestation data could be missing from the patients’ medical records. Additionally, the disease onset to diagnosis was not a factor because the related data was missing in the majority of the cases.
Conclusion
We detected a high failure rate of csDMARDs and thus a higher prescription rate of biologics. T2T strategy was achieved in the vast majority of our patients, while remission was observed in almost half of them. The findings not only provide important information regarding our clinical practice in the management of RA which is consistent with the latest ACR/EULAR guidelines but also contribute to the advancement of RA treatment, especially biologic therapy.
Abbreviations
RA, rheumatoid arthritis; CDAI, the clinical disease activity index; csDMARDs, conventional disease-modifying antirheumatic drugs; bDMARDs, biological disease-modifying antirheumatic drugs, tsDMARDs, targeted synthetic disease-modifying antirheumatic drugs; ACR, American College of Rheumatology; EULAR, European League Against Rheumatism; T2T, treat to target; MTX, methotrexate; HCQ, hydroxychloroquine; SZZ, sulfasalazine; LEF, leflunomide; TNFi, tumour necrosis factor inhibitors; JAK, Janus kinase; DAS 28, disease activity score with 28‐joint counts; ESR, erythrocyte sedimentation rate; CRP, C‐reactive protein; RAPID 3, the routine assessment of patient index data with 3 measures; SDAI, simplified disease activity index; RF, rheumatoid factor; anti-CCP, anti-citrullinated protein antibody; HTN, hypertension; DM, diabetes mellitus; ExRA, extraarticular manifestations; ILD, Interstitial lung disease; SS, Sjogren syndrome; FMS, fibromyalgia syndrome.
Funding
The authors did not receive any financial support for this work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Kvien TK. Epidemiology and burden of illness of rheumatoid arthritis. Pharmacoeconomics. 2004;22(S1):1–12. doi:10.2165/00019053-200422001-00002
2. Al-Dalaan A, Al Ballaa S, Bahabri S, Biyari T, Al Sukait M, Mousa M. The prevalence of rheumatoid arthritis in the Qassim region of Saudi Arabia. Ann Saudi Med. 1998;18(5):396–397. doi:10.5144/0256-4947.1998.396
3. Smolen JS, Breedveld FC, Burmester GR, et al. Treating rheumatoid arthritis to target: 2014 update of the recommendations of an international task force. Ann Rheum Dis. 2016;75(1):3–15. doi:10.1136/annrheumdis-2015-207524
4. Smolen JS, Landewé RBM, Bijlsma JWJ, et al. EULAR recommendations for the management of rheumatoid arthritis with synthetic and biological disease-modifying antirheumatic drugs: 2019 update. Ann Rheum Dis. 2020;79(6):685–699. doi:10.1136/annrheumdis-2019-216655
5. Anderson J, Caplan L, Yazdany J, et al. Rheumatoid arthritis disease activity measures: American College of Rheumatology recommendations for use in clinical practice: ACR RA Disease Activity Measures Recommendations. Arthritis Care Res (Hoboken). 2012;64(5):640–647. doi:10.1002/acr.21649
6. Aletaha D, Neogi T, Silman AJ, et al. 2010 Rheumatoid arthritis classification criteria: an American College of Rheumatology/European League Against Rheumatism collaborative initiative. Arthritis Rheum. 2010;62(9):2569–2581. doi:10.1002/art.27584
7. Dhaon P, Das SK, Srivastava R, Dhakad U. Performances of Clinical Disease Activity Index (CDAI) and Simplified Disease Activity Index (SDAI) appear to be better than the gold standard Disease Assessment Score (DAS-28-CRP) to assess rheumatoid arthritis patients. Int J Rheum Dis. 2018;21(11):1933–1939. doi:10.1111/1756-185X.13110
8. Almoallim HM, Alharbi LA. Rheumatoid arthritis in Saudi Arabia. Saudi Med J. 2014;35(12):1442–1454.
9. Dargham SR, Zahirovic S, Hammoudeh M, et al. Epidemiology and treatment patterns of rheumatoid arthritis in a large cohort of Arab patients. PLoS One. 2018;13(12):e0208240. doi:10.1371/journal.pone.0208240
10. Bawazir YM. Clinicodemographic profiles of rheumatoid arthritis patients from a single center in Saudi Arabia. Open Access Rheumatol. 2020;12:267–275. doi:10.2147/OARRR.S277956
11. Masi AT, Maldonado-Cocco JA, Kaplan SB, Feigenbaum SL, Chandler RW. Prospective study of the early course of rheumatoid arthritis in young adults: comparison of patients with and without rheumatoid factor positivity at entry and identification of variables correlating with outcome. Semin Arthritis Rheum. 1976;5(4):299–326. doi:10.1016/0049-0172(76)90013-5
12. de Vries-bouwstra JK, Goekoop-Ruiterman YPM, Verpoort KN, et al. Progression of joint damage in early rheumatoid arthritis: association with HLA-DRB1, rheumatoid factor, and anti-citrullinated protein antibodies in relation to different treatment strategies. Arthritis Rheum. 2008;58(5):1293–1298. doi:10.1002/art.23439
13. Lindqvist E, Eberhardt K, Bendtzen K, Heinegård D, Saxne T. Prognostic laboratory markers of joint damage in rheumatoid arthritis. Ann Rheum Dis. 2005;64(2):196–201. doi:10.1136/ard.2003.019992
14. Richman NC, Yazdany J, Graf J, Chernitskiy V, Imboden JB. Extraarticular manifestations of rheumatoid arthritis in a multiethnic cohort of predominantly Hispanic and Asian patients. Medicine (Baltimore). 2013;92(2):92–97. doi:10.1097/MD.0b013e318289ce01
15. Omair MA, Omair MA, Halabi H. Survey on management strategies of rheumatoid arthritis in Saudi Arabia: a Saudi Society for Rheumatology Initiative. Int J Rheum Dis. 2017;20(9):1185–1192. doi:10.1111/1756-185X.12735
16. Katchamart W, Trudeau J, Phumethum V, Bombardier C. Efficacy and toxicity of methotrexate (MTX) monotherapy versus MTX combination therapy with non-biological disease-modifying antirheumatic drugs in rheumatoid arthritis: a systematic review and meta-analysis. Ann Rheum Dis. 2009;68(7):1105–1112. doi:10.1136/ard.2008.099861
17. Sepriano A, Ramiro S, FitzGerald O, et al. Adherence to treat-to-target management in rheumatoid arthritis and associated factors: data from the international RA BIODAM cohort. J Rheumatol. 2020;47(6):809–819. doi:10.3899/jrheum.190303
18. Provan SA, Austad C, Halsaa V, Hammer HB, Kvien TK, Uhlig T. Fibromyalgia in patients with rheumatoid arthritis. A 10-year follow-up study, results from the Oslo Rheumatoid Arthritis Register. Clin Exp Rheumatol. 2019;37 Suppl 116(1):58–62.
19. Saevarsdottir S, Wallin H, Seddighzadeh M, et al. Predictors of response to methotrexate in early DMARD naïve rheumatoid arthritis: results from the initial open-label phase of the SWEFOT trial. Ann Rheum Dis. 2011;70(3):469–475. doi:10.1136/ard.2010.139212
20. Almoallim H, Hassan R, Cheikh M, et al. Rheumatoid arthritis Saudi database (RASD): disease characteristics and remission rates in a tertiary care center. Open Access Rheumatol. 2020;12:139–145. doi:10.2147/OARRR.S260426
21. Cantini F, Niccoli L, Nannini C, et al. Second-line biologic therapy optimization in rheumatoid arthritis, psoriatic arthritis, and ankylosing spondylitis. Semin Arthritis Rheum. 2017;47(2):183–192. doi:10.1016/j.semarthrit.2017.03.008
22. Smolen JS, Aletaha D. Rheumatoid arthritis therapy reappraisal: strategies, opportunities and challenges. Nat Rev Rheumatol. 2015;11(5):276–289. doi:10.1038/nrrheum.2015.8
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
