Back to Journals » Patient Preference and Adherence » Volume 11
Pattern of statin use changes following media coverage of its side effects
Authors Kriegbaum M , Liisberg KB, Wallach-Kildemoes H
Received 24 January 2017
Accepted for publication 11 April 2017
Published 10 July 2017 Volume 2017:11 Pages 1151—1157
DOI https://doi.org/10.2147/PPA.S133168
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Margit Kriegbaum,1 Kasper Bering Liisberg,2 Helle Wallach-Kildemoes3
1Department of Public Health, 2Department of Media, Cognition, and Communication, 3Department of Pharmacy, University of Copenhagen, Copenhagen, Denmark
Background: The media plays a role in shaping opinions about medical decisions, for example, whether to initiate or stop treatment. An association between negative media attention and statin discontinuation has been demonstrated, but it may differ depending on the reason for prescription and whether the user is new (incident) or long term (prevalent).
Aim: The aim of this study is to explore whether a Danish newspaper article featuring the side effects of statins affects statin discontinuation in incident versus prevalent users, with the reason for prescription also taken into account.
Methods: The study relies on a quasi-experimental design and uses registry data on statin purchases to explore discontinuation and treatment duration. As a proxy for reason for prescription, data on filled prescriptions and hospital diagnoses from a Danish registry were used. We compared statin discontinuation in all statin users in Denmark in 2007 before the media event (n=343,438) and after it in 2008 (n=404,052).
Results: Compared to 2007, statin discontinuation among prevalent users in 2008 increased by 2.97 percentage points (pp). The change in discontinuation varied with the indication for statin use. Those with myocardial infarction had the smallest increase (1.98 pp) and those with hypercholesterolemia or primary hypertension had the largest increase (3.54 pp). Incident statin users had a higher level of discontinuation and a larger difference in discontinuation between 2007 and 2008. Compared to 2007, more people (5.52 pp) discontinued statin treatment in 2008. Again, those with myocardial infarction had the smallest decrease in statin discontinuation (1.49 pp), while those with a potential atherosclerotic condition (7.05 pp) and hypercholesterolemia or primary hypertension (6.10 pp) had the largest increase.
Conclusion: Statin discontinuation increased in 2008 following a media event, but especially among individuals prescribed statins for primary prevention and among new statin users.
Keywords: statin discontinuation, media attention, quasi-experimental study, primary prevention, secondary prevention
Background
Statins, also known as HMG-CoA reductase inhibitors, were initially introduced on the market as lipid-lowering drugs to reduce mortality after myocardial infarction (MI) in middle-aged men with hypercholesterolemia.1 Since then, recommendations for prescribing statins have gradually expanded,2 first to include patients (independently of sex and age) with different categories of atherosclerotic cardiovascular diseases (CVDs) and diabetes, and then to include individuals assessed as being at high risk of developing CVDs.3,4 Since the introduction of statins, patent expirations have led to declining consumer prices, while policies regulating consumer costs promoted statin use across Europe.5 The widening of prescription criteria, together with a decline in prices, has led to a large increase in the use of statins.6 Today, statins are among the most prescribed medications globally.7,8 In Denmark, simvastatin and pravastatin were approved in the early 1990s. The use of statins, however, was rare until 1994, when international research, international guidelines, national guidelines and reimbursement policies promoted their use.4,9
While statin therapy is, in principle, lifelong, about 50% stop refilling their prescriptions within the first year.10 General practitioners (GPs) are generally hesitant to stop treatment.11 Hence, the decision to discontinue taking statins is often made by the statin user, which is called nonadherence.12,13 Statin users are faced with the challenge of balancing information on the potential benefits and harms of statin treatment. Side effects and fear of side effects play a major role in the decision to discontinue statins.14 The mass media has repeatedly focused on the negative side effects of statins and other medications. This information is likely to influence the choice to discontinue using statins.
In Denmark the tabloid newspaper “BT” has on several occasions reported adverse effects of statins.15 Especially, after an article was published on July 23, 2008, the Danish Medicines Agency registered a marked increase in the reporting of statin-related adverse effects. The media not only plays an agenda-setting role in defining which topics people think and talk about but also lays the frame how to think about a given topic. Recent research on framing highlights that attitudes are shaped by the active processing of information from the media, as well as by discussing this information with, for example, peers.16
A most well-documented example of media influence on health behavior is the association between media coverage of suicides and new suicide cases.17 Few studies investigated the association between media attention and use of medications. A British study found a decline in the use of antidepressants following adverse media publicity.18 A Swedish study investigated the framing of articles on an antiobesity drug and found that positive framing coincided with the launch of the drug.19 Studies investigating the association between media attention and hormone therapy found that medical decisions are influenced by media exposure and framing.20 Recently, some studies focused on the association between media reporting and statin use. Nielsen and Nordestgaard found increased early statin discontinuation in Denmark after exposure to negative media attention on statins. They followed, however, individuals in the early phase of statin use (incident users), where most discontinuation occurs.21 Statin intolerance, which is a major reason for statin discontinuation, often has its onset within the first month of use.22,23 Long-term users (prevalent users) have incorporated statin use into their daily lives and may be less likely to be influenced by negative media events. Furthermore, statin users who have CVD or diabetes have stronger incentives to continue treatment, regardless of negative media coverage of statins. A British study, however, found an equal likelihood of stopping after media attention in primary and secondary care patients,24 while an Australian study found that those who used more cardiovascular drugs were less likely to stop.25 As a result, the extent to which media attention affects incident and prevalent users in different ways, and according to the indication for statin use, is unclear.
The aim of this study is to explore whether a Danish newspaper article featuring the side effects of statins affects statin discontinuation in incident versus prevalent users, with the reason for prescription also taken into account.
Methods
Quasi-experimental study designs take advantage of events such as changes in legislation, policies and prices and examine the effects in either exposed and unexposed, or before and after the societal experiment, without interfering in the experiment.26,27 In this study, we explore the effects of a 2008 media event featuring statin side effects on statin discontinuation by comparing discontinuation before (2007) and after (2008) the event. There is a temporal variation in statin purchases in Denmark, where the number of purchases is 9% lower in July compared to the average in the other 11 months (based on own data). We assume that those with newly diagnosed MI are, however, equally likely to initiate statin treatment during summer, but this subpopulation is a minority of the total population of statin users. This difference is most likely due to the fact that July is the peak of the summer holiday season. To avoid any impact due to season, we compared data from the same months in both 2007 and 2008.
When using a quasi-experimental study design, the goal is not to rule out confounding factors from other factors in conventional statistical analyses. Instead, the plausibility of the design has to be tested by arguments that rule out alternative explanations. First, changes in the pattern of statin use could be influenced by changes in policies or prices. No shifts in policies occurred between 2007 and 2008, and no major changes in consumer prices occurred in the same period; however, the long-term trend shows a decline in prices, which would lead to less statin discontinuation. In addition, the financial crisis may have led to a general decrease in consumption, including a decline in the consumption of medicines. To test this, we compared statin discontinuation with antihypertensive discontinuation in a similar design.
Data
The study was based on administrative registry databases at Statistics Denmark covering the entire Danish population, allowing researchers to combine information at the individual level by means of an encrypted person identifier.28 We retrieved registry information on purchased prescriptions from the Danish National Prescription Registry,29 which contains information on prescription medication purchased at Danish pharmacies from 1995 and onward. Each prescription record includes the prescription date and the Anatomical Therapeutic Chemical classification codes.30,31 Information on hospitalizations (eg, discharge diagnoses) was retrieved from the Danish National Patient Registry and applied for the registry-based indication proxy (see below). Information on age and sex was available from this registry through the encrypted person identifier.
Ethics
Use of Danish registry data does not require permission from ethics committees or the Danish Data Protection Agency. However, all data processing must be done on servers at Statistics Denmark, and no individual level data may be transferred outside this protected environment. Moreover, individual data is de-identified to researchers, and aggregate tables are not allowed to have cell counts of less than five in order to ensure anonymity.
Study cohorts
Two study cohorts were created. The first one comprised all residents of Denmark with at least one statin purchase between January and June 2007. This cohort was further divided into prevalent statin users (first statin purchase before 2007) and incident statin users (new users in 2007). A corresponding cohort with at least one statin purchase between January and June 2008 was also established. We excluded individuals with <10 years residence before 2007/2008 because we wished to track their hospitalizations and medicine use. Furthermore, individuals who died in 2007 were excluded from the 2007 cohort, and those who died in 2008 were excluded from the 2008 cohort. Figure 1 describes the inclusion and exclusion criteria of participants in detail. Finally, the 2007 study cohort consisted of 305,952 prevalent users and 37,486 incident users, while the 2008 cohort comprised 361,701 prevalent users and 42,351 incident users.
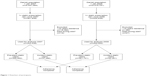  | Figure 1 Flowchart of participants. |
Statin discontinuation
Statin discontinuation, the outcome, was defined as no statin purchases from August to December. Using this simple approach, we assumed that tablets from previous statin purchases had been ingested. This approach shares the assumptions of the refill persistence approach, but avoids overtly strong assumptions about the precision of the method and to circumvent seasonal influence on adherence measures.32
Covariates
Analyses were stratified according to sex, age (≤59, 60+ years), and indication for statin use. In accordance with Wallach-Kildemoes et al,33 a grouped version of the indication hierarchy was used to classify indication for statin use, with a combination of hospital and prescription information for the past 10 years also included. The indications were grouped as 1) primary prevention: hypercholesterolemia (ie, no registry information) or primary hypertension; 2) diabetes; 3) potential atherosclerotic condition (PAC); 4) peripheral arterial disease (PAD), stroke, or ischemic heart disease (IHD) without MI; and 5) acute MI. PAC covers the gray zone conditions not included as indications in statin guidelines. Individuals were classified on 1 July in 2007 and 2008, according to their highest number in the five groups.
Statistical analyses
We calculated discontinuation as the proportion in each year of those who did not have an additional statin purchase from August to December and 95% confidence interval. Differences in discontinuation between 2007 and 2008 were stated in percentage points (pp). The two-sided P-values comparing adherence in 2007 and 2008 were calculated. Separate analyses, stratified according to age, sex, and indication for statin use, were carried out for incident and prevalent users.
Sensitivity analyses
In order to study if any associations were part of a general change in prescribing or purchasing patterns due to for example business cycles, we compared purchases of statins with purchases of antihypertensives. The datasets and analyses for antihypertensives that we constructed were analogous to those for statins. We also analyzed the purchase of statins in 2009 to study if any changes remained over time. SAS statistical software 9.4 was used for all analyses.
Results
Table 1 shows an analysis of discontinuation among prevalent statin users. In the fall of 2007, 7.22% discontinued statins, which increased to 10.25% in 2008, corresponding to an increase of 2.97 pp. The increase was almost identical in men (2.98 pp) and women (2.96 pp), and in statin users aged ≤59 years (3.20 pp) versus those aged ≥60 years (3.38 pp). Changes in statin discontinuation varied with the reason for prescription. Those with hypercholesterolemia or primary hypertension had the largest increase in discontinuation (3.54 pp), whereas those with MI had the smallest increase (1.98 pp). Those with diabetes also had a relatively small increase (2.34 pp). Individuals with PAC had an increase of 3.24 pp and those with PAD, stroke, or IHD had an increase of 2.77 pp.
Table 2 shows the same analysis, but among the incident statin users, who had a higher level of discontinuation and a larger difference in discontinuation between 2007 and 2008. While 16.86% of statin users discontinued purchasing statins in the fall of 2007, the discontinuation increased to 22.38% in 2008, corresponding to an increase of 5.52 pp. The increase was similar in men (5.07 pp) and women (5.66 pp), and in younger (5.01 pp) and older (5.52 pp) statin users. As for the prevalent users, the increase in statin discontinuation varied with the reason for prescription. Those with PAC had the largest increase (7.05 pp), followed by those with hypercholesterolemia or primary hypertension (6.1 pp). Statin users with MI had the smallest increase (1.49 pp), while those with diabetes had a increase of 3.39 pp and those with PAD, stroke, or IHD a increase of 4.04 pp.
Results of sensitivity analyses
The discontinuation of antihypertensives was 5.75 (5.70–5.80) in 2007 and almost identical in 2008 5.65 (5.60–5.70) and, hence, was presumably not influenced by the statin-related media event. Statin discontinuation in 2009 was 9.43 (90.48–90.66) for prevalent statin users and 20.5 (20.06–20.95) for incident statin users, which means a decrease compared to 2008, but still at a higher level compared to 2007. Hence, statin discontinuation did not return to the level before the news event.
Discussion
Both incident and prevalent users were sensitive to media coverage on statin side effects, but incident statin users were more sensitive than prevalent users. Likewise, individuals prescribed statins for primary prevention (ie, without established CVD or diabetes) were more sensitive to the media event than those prescribed statin after MI.
Another Danish study reported that patients newly initiating statin therapy (incident users) were less likely to fill a second prescription, if media stories in the period immediately afterward were dominated more by negative rather than positive statin stories.21 We not only found that incident statin users were more susceptible to media coverage, but also to changes (though small) in discontinuation in prevalent statin users. This indicates that new users who have not yet incorporated statin use in their daily lives are more likely to be influenced by mass media when deciding whether to stop statin treatment.
A British study of primary care patients found that statin users in both primary and secondary prevention were equally more likely to stop statin treatment after a highly negative media coverage period than without such exposure.24 The odds of stopping were almost identical in primary and secondary prevention patients, which is in contrast to this Danish study. This discrepancy can perhaps be explained by the different definitions applied to primary and secondary prevention in the British study compared to ours. While a range of CVDs was included to define secondary prevention in the British study, we subdivided individuals with established CVD into three groups. In our study, statin users with diabetes constituted a separate group, but the British study included statin users with diabetes in its primary prevention group. Similar to our study, there were no sex differences; but unlike our study, older individuals were more likely to stop.24
An Australian study on the short-term effects of a TV program containing criticism about certain aspects of statins showed an increase in the discontinuation of statins in the week of the program (long-term effects not studied), with a larger impact found on individuals who did not take diabetes drugs or any other cardiovascular drugs.34 Their assessment points in the same direction as this study, albeit with different measures of indication for statin use. The studies mentioned differ in general design, in measurement of media attention, and in statin use, which means they are not entirely comparable to this study; nevertheless, they all point to a similar finding: that mass media coverage of statins influences patterns of statin use.
Our study indicates that people are particularly sensitive to the media focus on side effects when their medication is not incorporated as a part of daily life (incident users), as are individuals without CVD or diabetes, that is, people prescribed statin therapy based on a risk assessment. Both groups may be more likely to consider whether the benefits outweigh the harm, concluding that that risk of the medicine being a daily nuisance is too high a price for possible postponement of CVD.
Strengths and limitations
This study was based on registry data on the entire population of statin users, which means that there was no loss to follow-up and that measures were free of any bias stemming from self-reporting. This study also contains detailed information on hospitalizations and use of cardiovascular and diabetes drugs, enabling us to distinguish between subgroups of indication of statin use.
Registry data does not, however, include any specific reason for discontinuation. Analyses are based on purchases, and the extent of the GPs’ role in the amount prescribed is unknown. A study on statin discontinuation among Danish GPs found that they are generally reluctant to stop statin treatment, but that statin cessation may be a joint decision.11 In addition, information on conditions not included in the registry, such as familial hypercholesterolemia, is likely to affect positively decisions on continued statin therapy. By using a quasi-experimental design, this study differs from studies using conventional cohort designs. Instead of controlling for potential confounding factors using regression models, the argument for causal association relies on the assumption that Danish statin users were comparable in 2007 and 2008, apart from the media event. This approach relies on other factors, such as policies and prices being equal. The financial crisis led to a general decrease in consumption and could have also led to a decline in the sale of statins. Since the purchase of antihypertensives was unaffected, we assume that this was not the case. Overall, the use of various methodological approaches is one of the strengths of the emerging field of media influence on medicine use.
Conclusion
Statin discontinuation increased in 2008 following a media event, but especially among individuals prescribed statins for primary prevention. Incident satin users had a larger increase in statin discontinuation compared to prevalent statin users. The results may reflect the fact that medicine users weighed the benefits of medicine therapy compared to its side effects during intense media attention on the side effects of statins. Prevalent statin users seem less prone to consider whether or not therapy is worthwhile.
Disclosure
The authors report no conflicts of interest in this work.
References
Scandinavian Simvastatin Survival Study Group. Randomised trial of cholesterol lowering in 4444 patients with coronary heart disease: the Scandinavian Simvastatin Survival Study (4S). Lancet. 1994;344(8934):1383–1389. | ||
Ong HT. The statin studies: from targeting hypercholesterolaemia to targeting the high-risk patient. QJM. 2005;98(8):599–614. | ||
Perk J, De Backer G, Gohlke H, et al. European Guidelines on cardiovascular disease prevention in clinical practice (version 2012). Eur Heart J. 2012;33(13):1635–1701. | ||
Wallach Kildemoes H, Vass M, Hendriksen C, Andersen M. Statin utilization according to indication and age: a Danish cohort study on changing prescribing and purchasing behaviour. Health Policy. 2012;108(2–3):216–227. | ||
Godman B, Shrank W, Andersen M, et al. Policies to enhance prescribing efficiency in Europe: findings and future implications. Front Pharmacol. 2011;1:141. | ||
Kildemoes HW, Hendriksen C, Andersen M. Drug utilization according to reason for prescribing: a pharmacoepidemiologic method based on an indication hierarchy. 2012;21(10):1027–1035. | ||
Gu Q, Paulose-Ram R, Burt VL, Kit BK. Prescription cholesterol-lowering medication use in adults aged 40 and over: United States, 2003–2012. NCHS Data Brief. 2014;(177):1–8. | ||
Walley T, Folino-Gallo P, Stephens P, Van Ganse E. Trends in prescribing and utilization of statins and other lipid lowering drugs across Europe 1997–2003. Br J Clin Pharmacol. 2005;60(5):543–551. | ||
Pyörälä K, Backer GD, Graham I, Poole-wilson P, Wood D. Prevention of coronary heart disease in clinical practice. Recommendations of the Task Force of the European Society of Cardiology, European Atherosclerosis Society and European Society of Hypertension. Eur Heart J. 1994;15(10):121–161. | ||
Wallach-Kildemoes H, Andersen M, Diderichsen F, Lange T. Adherence to preventive statin therapy according to socioeconomic position. Eur J Clin Pharmacol. 2013;69(8):1553–1563. | ||
Nixon M, Kousgaard MB. Organising medication discontinuation: a qualitative study exploring the views of general practitioners toward discontinuing statins. BMC Health Serv Res. 2016;16:226. | ||
Mantel-Teeuwisse AK, Goettsch WG, Klungel OH, de Boer A, Herings RM. Long term persistence with statin treatment in daily medical practice. Heart. 2004;90(9):1065–1066. | ||
Helin-Salmivaara A, Lavikainen P, Korhonen MJ, et al. Long-term persistence with statin therapy: a nationwide register study in Finland. Clin Ther. 2008;30(Pt 2):2228–2240. | ||
Zhang H, Plutzky J, Skentzos S, et al. Discontinuation of statins in routine care settings: a cohort study. Ann Intern Med. 2013;158(7):526–534. | ||
Danish Health Authorities. Focus Report on Statins–Knowledge about Use and Side Effects of Statins. Copenhagen: Danish Health Authorities; 2012. | ||
Scheufele DA. Framing as a theory of media effects. J Commun. 1999;49(1):103–122. | ||
Ueda M, Mori K, Matsubayashi T. The effects of media reports of suicides by well-known figures between 1989 and 2010 in Japan. 2014;4(2):623–629. | ||
Martin RM, May M, Gunnell D. Did intense adverse media publicity impact on prescribing of paroxetine and the notification of suspected adverse drug reactions? Analysis of routine databases, 2001–2004. Br J Clin Pharmacol. 2006;61(2):224–228. | ||
Brounéus F, Dahlin A, Beermann B. Press coverage and sales of Xenical in Sweden, 1998–2000. Eur J Clin Pharmacol. 2005;61(4):285–289. | ||
Archer DF. Medical decisions regarding hormone therapy for menopausal women are significantly influenced by the media. Pharmacoepidemiol Drug Saf. 2007;16(1):28–31. | ||
Nielsen SF, Nordestgaard BG. Negative statin-related news stories decrease statin persistence and increase myocardial infarction and cardiovascular mortality: a nationwide prospective cohort study. Eur Heart J. 2016:37(11):908–916. | ||
Jacobson TA. Toward “pain-free” stain prescribing: clinical algorithm for diagnosis and management of myalgia. Mayo Clin Proc. 2008;83(6):687–700. | ||
Taylor BA, Thompson PD. Muscle-related side-effects of statins: from mechanisms to evidence-based solutions. Curr Opin Lipidol. 2015;26(3):221–227. | ||
Matthews A, Herrett E, Gasparrini A, et al. Impact of statin related media coverage on use of statins: interrupted time series analysis with UK primary care data. BMJ. 2016;353(9753):1670–1681. | ||
Schaffer AL, Buckley NA, Dobbins TA, Banks E, Pearson SA. The crux of the matter: did the ABCs’ catalyst program change statin use in Australia? Med J Aust. 2015;202(11):591–595. | ||
Craig P, Cooper C, Gunnell D, et al. Using natural experiments to evaluate population health interventions: guidance for producers and users of evidence. Med Res Counc. 2011:1–29. | ||
Sedjo RL, Cox ER. Lowering copayments: impact of simvastatin patent expiration on patient adherence. Am J Manag Care. 2008;14(12):813–818. | ||
Thygesen LC, Daasnes C, Thaulow I, Bronnum-Hansen H. Introduction to Danish (nationwide) registers on health and social issues: structure, access, legislation, and archiving. Scand J Public Health. 2011;39(Suppl 7):12–16. | ||
Lynge E, Sandegaard JL, Rebolj M. The Danish National Patient Register. Scand J Public Health. 2011;39(Suppl 7):30–33. | ||
Gaist D, Sørensen HT, Hallas J. The Danish prescription registries. Dan Med Bull. 1997;44(4):445–448. | ||
Kildemoes HW, Sorensen HT, Hallas J. The Danish National Prescription Registry. Scand J Public Heal. 2011;39(Suppl 7):38–41. | ||
Andrade SE, Kahler KH, Frech F, Chan KA. Methods for evaluation of medication adherence and persistence using automated databases. Pharmacoepidemiol Drug Saf. 2006;15(8):565–574. | ||
Wallach-Kildemoes H, Stovring H, Holme Hansen E, Howse K, Pétursson H. Statin prescribing according to gender, age and indication: what about the benefit-risk balance? J Eval Clin Pract. 2016;22(2):235–246. | ||
Shaffer AL, Buckley NA, Dobbins TA, Banks E, Pearson SA. The crux of the matter: Did the ABC’s Catalyst program change statin use in Australia? Med J Aust. 2015;202(11):591–595. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


