Back to Journals » Journal of Pain Research » Volume 15
Patient Selection for Spinal Cord Stimulation in Treatment of Pain: Sequential Decision-Making Model — A Narrative Review
Authors Goudman L , Rigoard P , Billot M , Duarte RV , Eldabe S, Moens M
Received 16 January 2022
Accepted for publication 5 April 2022
Published 20 April 2022 Volume 2022:15 Pages 1163—1171
DOI https://doi.org/10.2147/JPR.S250455
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Dawood Sayed
Lisa Goudman,1– 5 Philippe Rigoard,6– 8 Maxime Billot,6 Rui V Duarte,9 Sam Eldabe,10 Maarten Moens1– 4,11
1Department of Neurosurgery, Universitair Ziekenhuis Brussel, Jette, 1090, Belgium; 2STIMULUS Consortium (Research and Teaching Neuromodulation VUB/UZ Brussel), Vrije Universiteit Brussel, Brussels, 1090, Belgium; 3Center for Neurosciences (C4N), Vrije Universiteit Brussel, Brussels, 1090, Belgium; 4Pain in Motion (PAIN) Research Group, Department of Physiotherapy, Human Physiology, and Anatomy, Faculty of Physical Education and Physiotherapy, Vrije Universiteit Brussel, Brussels, 1090, Belgium; 5Research Foundation — Flanders (FWO), Brussels, 1090, Belgium; 6PRISMATICS Lab (Predictive Research in Spine/Neuromodulation Management and Thoracic Innovation/Cardiac Surgery), Poitiers University Hospital, Poitiers, 86021, France; 7Department of Spine Surgery and Neuromodulation, Poitiers University Hospital, Poitiers, 86021, France; 8Pprime Institute UPR 3346, CNRS, ISAE-ENSMA, University of Poitiers, Chasseneuil-du-Poitou, 86360, France; 9Liverpool Reviews and Implementation Group, Department of Health Data Science, University of Liverpool, Liverpool, L69 3BX, UK; 10Pain Clinic, James Cook University Hospital, Middlesbrough, TS4 3BW, UK; 11Department of Radiology, Universitair Ziekenhuis Brussel, Jette, 1090, Belgium
Correspondence: Lisa Goudman, Department of Neurosurgery, Universitair Ziekenhuis Brussel, 101 Laarbeeklaan, Jette 1090, Belgium, Tel +32-2-477-5514, Fax +32-2-477-5570, Email [email protected]
Abstract: Despite the well-known efficacy of spinal cord stimulation (SCS) in chronic pain management, patient selection in clinical practice remains challenging. The aim of this review is to provide an overview of the factors that can influence the process of patient selection for SCS treatment. A sequential decision-making model is presented within a tier system that operates in clinical practice. The first level incorporates the underlying disease as a primary indication for SCS, country-related reimbursement rules, and SCS screening–trial criteria in combination with underlying psychological factors as initial selection criteria in evaluating patient eligibility for SCS. The second tier is aligned with the individualized approach within precision pain medicine, whereby individual goals and expectations and the potential need for preoperative optimizations are emphasized. Additionally, this tier relies on results from prediction models to provide an estimate of the efficacy of SCS in the long term. In the third tier, selection bias, MRI compatibility, and ethical beliefs are included, together with recent technological innovations, superiority of specific stimulation paradigms, and new feedback systems that could indirectly influence the decision-making of the physician. Both patients and physicians should be aware of the different aspects that influence patient selection in relation to SCS for pain management to make an independent decision on whether or not to initiate a treatment trajectory with SCS.
Keywords: patient selection, decision-making, neuromodulation, experience-based medicine
Introduction
The application, efficacy, and safety of spinal cord stimulation (SCS) for the management of chronic pain are well known.1–3 During the last decade, refinements and innovations have dominated the landscape of neuromodulation in the form of novel stimulation waveforms,4,5 new anatomical targets,6,7 and novel feedback-loop mechanisms.8,9 Despite the widely accepted effects of SCS for obtaining pain relief,1 increasing quality of life,10 and decreasing pain-medication use,11 clinicians are still faced with a lack of clear criteria for optimal patient selection for this treatment. Patient selection is the process by which a physician decides whether to accept responsibility for the care and treatment of a potential patient, assuming that the physician has the requisite knowledge to assess the anatomical disorder and the technical capacity to render appropriate care if needed.12
The selection of patients in clinical practice differs from patient selection for a clinical trial. That for a clinical trial is a complicated process based on five requirements: firstly, isolating a group for which there is a greater or lesser chance of detecting a possible difference between/among the treatments compared; secondly, establishing a homogeneous group in order to reduce the variability of response, thus making statistical comparisons more sensitive and decreasing the risk of bias due to the constitution of nonhomogeneous groups; thirdly, obtaining representative samples of the condition under study; fourthly, defining the rules corresponding to realistic recruitment, at best transposable to daily practice; and lastly, respecting ethical obligations.13,14 Generalizability of trial results is confined to those patients conforming to the inclusion/exclusion criteria of the study; however, randomized trial samples are highly selected and thus less representative of clinical practice.15,16 Evidence of SCS efficacy in the treatment of neuropathic pain continues to grow as more prospective and randomized trials are performed. Based on the available evidence, SCS for persistent spinal pain syndrome (PSPS type 2) meets best evidence–synthesis criteria for level I–II evidence, based upon clinical efficacy and demonstrated cost-effectiveness.17
Patient selection in day-to-day clinical practice is based on a combination of other variables, such as best research evidence (medical literature), clinical expertise (based on the clinician’s knowledge and experience), and patient values (preferences of the patient), and it remains difficult to compare these with patient-selection processes derived from randomized clinical trials. Results from real-world data generate evidence on the effectiveness and safety of a therapy and may lead to real-world evidence.18 Despite the growing interest in real-world neuromodulation data, real-world evidence concerning patient selection for SCS in treatment for chronic pain is still limited.19–22Within this narrative review, the aim is to provide insights into the indications of SCS for pain in combination with statistical, health-economic, and ethical aspects that may influence the selection of patients for SCS.
Medical Literature
To explore indications for SCS in chronic pain, a RAND–UCLA appropriateness method was applied in 2018–2019, with 18 experts from nine European countries representing anesthesiology, neurosurgery, psychology, physiotherapy, and nursing disciplines.23 Four key indication areas were identified: chronic low-back and leg pain, complex regional pain syndrome, neuropathic pain syndromes, and ischemic pain syndromes. The main indications for SCS treatment are presented in Table 1, together with the relevant clinical variables that result in increased appropriateness for SCS. In addition to these clinical variables, psychological factors were denoted as having an influence on the selection process: lack of engagement, dysfunctional coping, unrealistic expectations, inadequate daily activity levels, problematic social support, secondary gain, psychological distress, and unwillingness to reduce high-dose opioid use. The psychologist panel decided that if psychological characteristics were severe, they may be considered strong contraindications to SCS. A total lack of engagement was also considered a contraindication to SCS.23
 |
Table 1 Appropriateness for SCS based on the RAND–UCLA appropriateness method |
Outcome-Based Patient Selection
Despite the existence of general SCS indications for pain, not every patient will effectively benefit from this therapy form, presumably due to the complexity of chronic pain that expresses itself with a unique combination of input, processing, and output mechanisms in every patient.24 In line with the increased attention on precision medicine, which is built on an understanding of the interrelationships among an individual’s profile, environment, and lifestyle (including phenotypes and biological markers), diagnosis and treatment can also be customized to an individual’s risk profile in the field of pain medicine.25,26
Evaluating each patient individually, identifying their risk profile for disproportionate pain, including the potential development of chronic pain, and optimizing therapeutic strategies to target specific pathological processes underlying chronic pain are key to precision pain medicine.27 No biomarker for the effect of SCS or pain is available yet. Several potential biomarkers for pain have been proposed, such as objective blood biomarkers,28 neuroimaging-based pain biomarkers,29 and heart-rate variability,30,31 but further confirmation for other pain conditions is still needed. However, several studies have tried to develop accurate prediction models to enable clinicians to provide an estimate of SCS efficacy in the long term. Very often, unidimensional pain-intensity reports are utilized as outcome measures.32–34 These are nowadays often replaced by composite measures as outcome variables. Several proposals have been made in relation to SCS among which are a composite score based on measures of pain intensity, pain catastrophizing, health-related quality of life, and physical capability to assess the multidimensional aspects of chronic pain,35 a measure to represent the degree of deviation from being a holistic responder, with a total score ranging from 0 (no pain, no disability, and perfect health status) to 300 (maximal pain, maximal disability, and worst health status),36 and a multidimensional clinical response index ranging from 0 (worst pain-related health status) to 10 (best pain-related health status) constructed from a combination of functional disability score, numeric pain-rating score, depression and anxiety score, and a mapping-intensity score.37 Another composite approach, created to predict patient satisfaction with treatment, consists of a pain-intensity rating in the evening and interaction with walking-tolerance time.38
All composite outcomes have a different interpretation and consist of a broad range of input variables, denoting the lack of comparability among them. Of note, the psychological dimension has been included in some of these measures, and is expected to play a major role.23,39–41 Specifically in the context of precision pain medicine, cognitive behavioral therapy has been suggested in cases of anxiety or depression.27 When patients present with indicators of catastrophizing, cognitive behavioral therapy, acceptance and commitment therapy, or physical therapy are suggested, while cognitive behavioral therapy for insomnia is proposed for sleep disturbances.27 Candidates for implantable pain devices have demonstrated high rates of sleep impairment (73%), depressive symptoms (62%), anxiety symptoms (61%), and pain catastrophizing (37%).42 Perhaps it might be useful to first focus on better preparing patients for SCS and optimising pre-SCS status by tackling these underlying psychological factors before considering SCS implantation. A similar preoperative optimization approach is proposed for medication consumption. SCS is known to decrease opioid use and pain-medication consumption in patients with intractable spine or limb pain.43,44 Nevertheless, a preoperative optimization approach could be conducted in which a detoxification of high opioid consumers is performed first to improve the effect of SCS afterward.45
Instead of focusing only on whether SCS will be effective in the long term, a recent study explored the value of a Bayesian preference-optimization algorithm to assist clinicians in the systematic programming of individualized therapeutic stimulation, based on expressed preferences for stimulation settings.46 The main focus was on optimization of temporal stimulation parameters, namely pulse frequency and pulse width, in combination with optimal spatial configuration, which was determined by clinician tuning and electromyograph measurements. Every month, a preference model was created based on daily preference evaluations with preference predictions also for untested parameters and new settings, with promising results.46 These results on pilot data do not apply to selecting patients suitable for SCS, but on selecting patients for specific stimulation paradigms. As widely acknowledged in this field and despite the inclusion of a temporary SCS-screening trial before IPG implantation, the initial effectiveness of SCS generally declines over time, due to growing tolerance of the central nervous system,47 which eventually causes loss of efficacy. As such, long-term pain coverage with SCS is not observed in up to 30% of patients,48,49 for whom salvage therapy (ie, reprogramming toward another stimulation paradigm and even conversion to alternative SCS devices50,51) could be applied. Despite the promising results of salvage therapy, clinicians are again confronted with selection criteria to determine the exact stimulation parameters to be programmed as salvage therapy (ie, to convert a clinical failure back to SCS success). Possible solutions will arise from SCS devices that can produce multiple SCS-stimulation paradigms, further expanding and fine-tuning optimization algorithms and potentially the development of specific biomarkers for SCS efficacy.
Bias
Temporary SCS-screening trials are widely implemented to determine whether a patient should receive a permanent SCS implant, mainly in terms of evaluating initial patient response to SCS.52 No universally implemented definition of the duration of the trial period or exact criteria that determine success is available. Mostly country-specific definitions are applied in relation to reimbursement criteria. Despite the widely implemented approach of first conducting a temporary SCS-screening trial, the evidence (in terms of clinical value and health-economic considerations) underpinning the value of this remains unclear. The results of a recent RCT on the value of a temporary SCS-screening trial revealed that it might have some diagnostic utility, showing sensitivity of 100% (95% CI 78%–100%) and specificity of 8% (95% CI 1%–25%), although evidence for superior patient outcomes or cost-effectiveness of an SCS-screening trial compared to no trial screening was lacking.52 The fact that permanent SCS implantation is conditional on a positive SCS-screening trial (due to reimbursement criteria) induces a gatekeeping mechanism that is more likely to result in false positives (positive screening trial with failure at follow-up after permanent implant) than in false-negative results (negative screening trial with success at follow-up after permanent implant), with higher sensitivity than specificity as a result of this strategy. Recently, it was proposed that machine algorithms be used to predict response to SCS, based on clinical outcomes from 103 implanted patients.53,54 The authors found that the sensitivity of the screening trial was greater than the machine-learning model (100% vs 83.3%), while specificity was significantly lower for the screening trial than the machine learning trial (33.3% vs 66.7%). Machine-learning and statistical models could be considered in future trials to delineate SCS-screening trial utility.
Patients themselves were also questioned about their preference concerning a temporary SCS screening trial, and clearly favored a one-stage SCS procedure without a screening trial.55 Themes identified included saving time (off work, in hospital, attending appointments), avoiding the worry about having “loose wires” in the two-stage procedure with an SCS-screening trial, having only one period of recovery, and saving national health-service resources.55 As such, patient preferences should be taken into account as well, together with expectations concerning SCS. Qualitative research has already indicated that improved preparation prior to SCS, including information provision (due to the limited understanding of pain and the mechanism of SCS56), cognitive behavioral therapy, and contact with expert patients, may be valuable additions to the current treatment trajectory.57 This clearly indicates that additional information should be provided to patients before considering an SCS trajectory,56 whereby we need to take into account that patients’ views are shaped by multiple influences, including but not limited to past experience, advice of several health-care providers, opinions of friends and family, social media, and consumer advertising.58,59 Patients have expectations concerning physical, social, material, and emotional well-being (ie, quality of life), development and activity, and about the SCS procedure,60 and there is growing evidence that for many different treatments, patients’ expectations are associated with clinical outcomes and thus may be an important predictor of treatment outcomes.61,62
Besides the well-known “patient-selection bias” in clinical research, the same mechanisms can be identified in clinical practice.63 Systematic errors or bias result from methods used by physicians to recruit individuals for treatment. Several types of selection bias may involve day-to-day practice, eg, loss to follow-up bias, volunteer bias, and baseline-score inflation.64,65 The clinical reasoning of physicians is often founded on a mixture of evidence-based medicine and experience-based medicine, whereby long-term experience of previously implanted patients contribute to this process. If losses during follow-up are nonrandom, the loss to follow-up bias will occur in the sense that the true relationship between SCS and chronic pain can be distorted. Hypothetically, it may be possible that physicians see only poor responders during long-term follow-up, since good responders are less likely to revisit the physician. Volunteer bias (or self-selection bias) occurs when patients who volunteer for a study have different relevant clinical characteristics from patients who do not volunteer. Efficacy of SCS is determined based on clinical trials, where a volunteer bias can lead to results that are not representative of daily clinical practice, pointing to the need for real-world data and effectiveness studies.66 Another bias that could potentially occur in clinical practice is baseline-score inflation to fulfill the criteria for a successful screening trial.67
Ethics
Appropriate patient selection for SCS to treat chronic pain is particularly important, and thus an experienced interdisciplinary team has to maintain oversight of ethics and create an environment to protect the patient and implanter to the largest extent possible.68 Due to the lack of international guidelines, an ethical framework to select the right patient for the right SCS paradigm and thus the right device or brand is mandatory to ensure long-term effects, not only in terms of pain reduction but also to account for the limitations intrinsic to SCS devices. As the need for MRI has became more common for diagnosis and disease surveillance, device selection based on MRI compatibility is important.69 About 90% of patients with an implanted SCS device will require an MRI over a 10-year time horizon.70 Counseling of patients regarding the use of MRI-conditional SCS systems should be considered prior to implantation.69,71,72 Chronic pain is well known to interfere with daily activities and level of functioning; however, the choice of SCS device is unequivocally linked to day-to-day life. Ability to operate a vehicle is considered an essential component of an individual’s quality of life and productivity.73 Therefore, it appears reasonable for patients to use a proven pain-relieving modality, such as SCS, to improve their driving ability. Nevertheless, most SCS-device manufacturers recommend that the device be turned off when driving because of the possibility of inadvertent stimulation that may contribute to an accident.73 Besides the limitations and restrictions of the SCS device, patients should also be educated to turn off the device prior to medical and surgical procedures. The US Food and Drug Administration safety manual warns about the use of diathermy, concomitant stimulation devices, lithotripsy, external defibrillation, radiation therapy, ultrasonic scanning, and high-output ultrasound, all of which can lead to permanent implant damage.74
Another consideration for patients and health-care providers is awareness of implanter bias. The interaction between physicians and industry has led to the Advanced Medical Technology Association Code of Ethics to provide medical technology companies guidance on ethical interactions and relationships with health-care professionals.75 In line with this code, a policy of full disclosure for all publications, regardless of relevance, is proposed.76 However, based on the aforementioned guidelines, patients eligible for SCS should also be informed of conflicts of interest of the implanter prior to implantation. Information on financial bias and/or bias by ongoing funded clinical research should be a cornerstone of this process. From a medicolegal point of view, the choice of stimulation paradigm based on superiority in the medical literature differs from clinical practice. Several new stimulation paradigms seem to be superior to standard tonic SCS, suggesting that the use of standard tonic SCS is an inferior and obsolete treatment.9,77–81 However, based on patient preferences, standard tonic SCS is still a widely used paradigm.
Patient-Selection Framework
At the beginning of this review, strict indications for patient selection defined by an international expert panel were proposed. Where indications are fulfilled, a screening trial period will be necessary as per country-specific definitions for a successful trial in order to receive reimbursement for SCS implantation. The downside of this strategy is the potential risk of jeopardizing the evaluation of new potential indications for SCS. Taken together, these criteria (underlying disease, reimbursement rules, and SCS screening–trial criteria with individual psychological factors) could be considered the first selection process in evaluating whether a patient is eligible for SCS, denoted as the first level in the proposed tier system (Figure 1). Nevertheless, in accordance with the individualized approach within precision pain medicine, the goalsetting process and potential need for preoperative optimization incorporate a level of considerations secondary to the more general eligibility criteria for SCS. Within the second tier, the results of current most effective prediction models to provide an estimate of the efficacy of SCS in the longterm (taking into account the potential occurrence of SCS tolerance) are considered as well, especially when no biomarkers are available. The third tier consists of specific patient- and physician-related beliefs, which can be classified as potential bias and ethical considerations. Additionally, recent technological innovations, potential superiority of specific stimulation paradigms, and new feedback systems undoubtedly influence the SCS landscape and thus indirectly the decision-making of the physician. This tier system can be considered a sequential decision-making model operating in daily routine care; however, often only the first and sometimes the second tier are openly discussed with the patient. In light of a free, fully informed decision-making process, patients should be fully informed about each tier in order to make an independent decision on whether or not to be considered adequate candidates for SCS and to initiate a treatment trajectory.
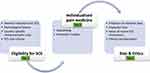 |
Figure 1 The tier system as patient-selection framework in routine daily clinical care. Abbreviation: SCS, spinal cord stimulation. |
Disclosure
Philippe Rigoard reports grants and personal fees from Medtronic, Abbott, and Boston Scientific, outside the submitted work. Rui V Duarte has received consultancy fees from Medtronic, Boston Scientific, Mainstay Medical, and Saluda Medical. Sam Eldabe has received consultancy fees from Medtronic, Mainstay Medical, Boston Scientific, and Abbott. His department received research funding from the National Institute of Health Research, Medtronic, and Nevro. Lisa Goudman is a postdoctoral research fellow funded by the Research Foundation Flanders (FWO), Belgium (project 12ZF622N). Maarten Moens has received speaker fees from Medtronic and Nevro. STIMULUS received independent research grants from Medtronic. The authors report no other conflicts of interest in this work.
References
1. O’Connell NE, Ferraro MC, Gibson W, et al. Implanted spinal neuromodulation interventions for chronic pain in adults. Cochrane Database Syst Rev. 2021;12(12):CD013756.
2. Deer TR, Grider JS, Lamer TJ, et al. A systematic literature review of spine neurostimulation therapies for the treatment of pain. Pain Medicine. 2020;21(7):1421–1432.
3. Duarte RV, McNicol E, Colloca L, Taylor RS, North RB, Eldabe S. Randomized Placebo-/Sham-Controlled Trials of Spinal Cord Stimulation: a Systematic Review and Methodological Appraisal. Neuromodulation. 2020;23(1):10–18.
4. Billot M, Naiditch N, Brandet C, et al. Comparison of conventional, burst and high-frequency spinal cord stimulation on pain relief in refractory failed back surgery syndrome patients: study protocol for a prospective randomized double-blinded cross-over trial (MULTIWAVE study). Trials. 2020;21(1):696.
5. Goudman L, De Smedt A, Eldabe S, et al. High-dose spinal cord stimulation for patients with failed back surgery syndrome: a multicenter effectiveness and prediction study. Pain. 2021;162(2):582–590.
6. Eldabe SS, Taylor RS, Goossens S, et al. A Randomized Controlled Trial of Subcutaneous Nerve Stimulation for Back Pain Due to Failed Back Surgery Syndrome: the SubQStim Study. Neuromodulation. 2019;22(5):519–528.
7. Rigoard P, Ounajim A, Goudman L, et al. The Added Value of Subcutaneous Peripheral Nerve Field Stimulation Combined with SCS, as Salvage Therapy, for Refractory Low Back Pain Component in Persistent Spinal Pain Syndrome Implanted Patients: a Randomized Controlled Study (CUMPNS Study) Based on 3D-Mapping Composite Pain Assessment. Preprints. 2021;2:2021090031.
8. Malinowski MN, Chopra PR, Tieppo Francio V, Budwany R, Deer TR. A narrative review and future considerations of spinal cord stimulation, dorsal root ganglion stimulation and peripheral nerve stimulation. Curr Opin Anaesthesiol. 2021;34(6):774–780.
9. Mekhail N, Levy RM, Deer TR, et al. Long-term safety and efficacy of closed-loop spinal cord stimulation to treat chronic back and leg pain (Evoke): a double-blind, randomised, controlled trial. Lancet Neurol. 2020;19(2):123–134.
10. Kallewaard JW, Paz-Solis JF, De Negri P, et al. Real-World outcomes using a spinal cord stimulation device capable of combination therapy for chronic pain: a European, multicenter experience. J Clin Med. 2021;10(18):455.
11. Goudman L, Duarte RV, De Smedt A, Copley S, Eldabe S, Moens M. Cross-Country differences in pain medication before and after spinal cord stimulation: a pooled analysis of individual patient data from two prospective studies in the United Kingdom and Belgium. Neuromodulation. 2021.
12. McCurry TM, Kasdan ML. Patient selection. Clin Occup Environ Med. 2006;5(2):217–223.
13. Begg CB. Selection of patients for clinical trials. Semin Oncol. 1988;15(5):434–440.
14. Compston A. Selection of patients for clinical trials. Neuroepidemiology. 1987;6(1–2):34–39.
15. Hariton E, Locascio JJ. Randomised controlled trials - The gold standard for effectiveness research: study design: randomised controlled trials. BJOG. 2018;125(13):1716.
16. Kennedy-Martin T, Curtis S, Faries D, Robinson S, Johnston J. A literature review on the representativeness of randomized controlled trial samples and implications for the external validity of trial results. Trials. 2015;16:495.
17. Grider JS, Manchikanti L, Carayannopoulos A, et al. Effectiveness of spinal cord stimulation in chronic spinal pain: a systematic review. Pain Physician. 2016;19(1):E33–54.
18. Pearson SA, Pratt N, de Oliveira Costa J, et al. Generating Real-World Evidence on the Quality Use, Benefits and Safety of Medicines in Australia: history, Challenges and a Roadmap for the Future. Int J Environ Res Public Health. 2021;18(24):456.
19. Luecke T, Kuhlmann H, May M, Petermann M, Libutzki B, Jaehnichen G. Spinal cord stimulation: a real-world data analysis on outcomes and differences between rechargeable and non-rechargeable implantable pulse generators. J Int Med Res. 2021;49(8):3000605211038457.
20. Goudman L, De Smedt A, Putman K, Moens M, Discover C. Long-term quality of life and work status after high-dose spinal cord stimulation in patients with failed back surgery syndrome: a secondary analysis of real-world data. J Neurosurg Spine. 2020;34(3):440–448.
21. Chakravarthy K, Malayil R, Kirketeig T, Deer T. Burst Spinal Cord Stimulation: a Systematic Review and Pooled Analysis of Real-World Evidence and Outcomes Data. Pain Medicine. 2019;20(Suppl 1):S47–S57.
22. DiBenedetto DJ, Wawrzyniak KM, Schatman ME, Kulich RJ, Finkelman M. 10 kHz spinal cord stimulation: a retrospective analysis of real-world data from a community-based, interdisciplinary pain facility. J Pain Res. 2018;11:2929–2941.
23. Thomson S, Huygen F, Prangnell S, et al. Appropriate referral and selection of patients with chronic pain for spinal cord stimulation: European consensus recommendations and e-health tool. European Journal of Pain. 2020;24(6):1169–1181.
24. Gifford L. Pain, the Tissues and the Nervous System: a conceptual model. Physiotherapy. 1998;84:27–36.
25. Perlman RL, Govindaraju DR, Archibald E. Garrod: the father of precision medicine. Genet Med. 2016;18(11):1088–1089.
26. Marson FAL, Bertuzzo CS, Ribeiro JD. Personalized or Precision Medicine? The Example of Cystic Fibrosis. Front Pharmacol. 2017;8:390.
27. Chadwick A, Frazier A, Khan TW, Young E. Understanding the Psychological, Physiological, and Genetic Factors Affecting Precision Pain Medicine: a Narrative Review. J Pain Res. 2021;14:3145–3161.
28. Niculescu AB, Le-niculescu H, Levey DF, et al. Towards precision medicine for pain: diagnostic biomarkers and repurposed drugs. Mol Psychiatry. 2019;24(4):501–522.
29. Mackey S, Greely HT, Martucci KT. Neuroimaging-based pain biomarkers: definitions, clinical and research applications, and evaluation frameworks to achieve personalized pain medicine. PAIN Reports. 2019;4(4):454.
30. Staud R. Heart rate variability as a biomarker of fibromyalgia syndrome. Fut Rheumatol. 2008;3(5):475–483.
31. Evans S, Seidman LC, Tsao JC, Lung KC, Zeltzer LK, Naliboff BD. Heart rate variability as a biomarker for autonomic nervous system response differences between children with chronic pain and healthy control children. J Pain Res. 2013;6:449–457.
32. Goudman L, Van Buyten JP, De Smedt A, et al. Predicting the Response of High Frequency Spinal Cord Stimulation in Patients with Failed Back Surgery Syndrome: a Retrospective Study with Machine Learning Techniques. J Clin Med. 2020;9(12):334.
33. Mekhail N, Costandi S, Saweris Y, Armanyous S, Chauhan G. Impact of biological sex on the outcomes of spinal cord stimulation in patients with chronic pain. Pain Pract. 2021;1:34.
34. Orhurhu V, Chu R, Orhurhu MS, Odonkor CA. Association Between Pain Scores and Successful Spinal Cord Stimulator Implantation. Neuromodulation. 2020;23(5):660–666.
35. Pilitsis JG, Fahey M, Custozzo A, Chakravarthy K, Capobianco R. Composite Score Is a Better Reflection of Patient Response to Chronic Pain Therapy Compared With Pain Intensity Alone. Neuromodulation. 2021;24(1):68–75.
36. Goudman L, Billot M, Duarte RV, Eldabe S, Rigoard P, Moens M. Gradation of Clinical Holistic Response as New Composite Outcome to Evaluate Success in Spinal Cord Stimulation Studies for Pain. Neuromodulation. 2021;2:43.
37. Rigoard P, Ounajim A, Goudman L, et al. A Novel Multi-Dimensional Clinical Response Index Dedicated to Improving Global Assessment of Pain in Patients with Persistent Spinal Pain Syndrome after Spinal Surgery, Based on a Real-Life Prospective Multicentric Study (PREDIBACK) and Machine Learning Techniques. J Clin Med. 2021;10(21):53.
38. Russo M, Verrills P, Santarelli D, et al. Metric for Predicting Patient Satisfaction With Spinal Cord Stimulation. Neuromodulation. 2020;23(5):687–697.
39. Sparkes E, Duarte RV, Mann S, Lawrenc TR, Raphael JH. Analysis of psychological characteristics impacting spinal cord stimulation treatment outcomes: a prospective cohort assessment. Pain Physician. 2015;18(3):E369–E378.
40. Prabhala T, Kumar V, Gruenthal E, et al. Use of a Psychological Evaluation Tool as a Predictor of Spinal Cord Stimulation Outcomes. Neuromodulation. 2019;22(2):194–199.
41. Ounajim A, Billot M, Louis P-Y, et al. Finite Mixture Models Based on Pain Intensity, Functional Disability and Psychological Distress Composite Assessment Allow to Identify Two Distinct Classes of Persistent Spinal Pain Syndrome after Surgery Patients Related to Their Quality of Life. Preprints. 2021;1:2021080527.
42. Gould HM, D’Eon MS, Grinberg AM, Chakravarthy KV, Castellanos J, Rutledge T. Psychosocial characteristics of candidates for implantable pain devices: validation of an assessment model. Pain Manag. 2021;11(2):159–172.
43. Pollard EM, Lamer TJ, Moeschler SM, et al. The effect of spinal cord stimulation on pain medication reduction in intractable spine and limb pain: a systematic review of randomized controlled trials and meta-analysis. J Pain Res. 2019;12:1311–1324.
44. Goudman L, De Smedt A, Forget P, Eldabe S, Moens M. High-Dose Spinal Cord Stimulation Reduces Long-Term Pain Medication Use in Patients With Failed Back Surgery Syndrome Who Obtained at Least 50% Pain Intensity and Medication Reduction During a Trial Period: a Registry-Based Cohort Study. Neuromodulation. 2021;24(3):520–531.
45. Jerjir A, Goudman L, Van Buyten JP, et al. Detoxification of Neuromodulation Eligible Patients by a Standardized Protocol: a Retrospective Pilot Study. Neuromodulation. 2021.
46. Zhao Z, Ahmadi A, Hoover C, et al. Optimization of Spinal Cord Stimulation Using Bayesian Preference Learning and Its Validation. IEEE Trans Neural Syst Rehabil Eng. 2021;29:1987–1997.
47. Waszak PM, Modric M, Paturej A, et al. Spinal Cord Stimulation in Failed Back Surgery Syndrome: review of Clinical Use, Quality of Life and Cost-Effectiveness. Asian Spine J. 2016;10(6):1195–1204.
48. Kapural L, Sayed D, Kim B, Harstroem C, Deering J. Retrospective Assessment of Salvage to 10 kHz Spinal Cord Stimulation (SCS) in Patients Who Failed Traditional SCS Therapy: RESCUE Study. J Pain Res. 2020;13:2861–2867.
49. Ghosh PE, Gill JS, Simopoulos T. The Evolving Role of High-Frequency Spinal Cord Stimulation as Salvage Therapy in Neurostimulation. Pain Pract. 2020;20(7):706–713.
50. Reddy RD, Moheimani R, Yu GG, Chakravarthy KV. A Review of Clinical Data on Salvage Therapy in Spinal Cord Stimulation. Neuromodulation. 2020;23(5):562–571.
51. Rigoard P, Ounajim A, Goudman L, et al. The Challenge of Converting “Failed Spinal Cord Stimulation Syndrome” Back to Clinical Success, Using SCS Reprogramming as Salvage Therapy, Through Neurostimulation Adapters Combined with 3D-Computerized Pain Mapping Assessment. A Real-Life Retrospective Cohort Analysis. Preprints. 2021;1:8743.
52. Eldabe S, Duarte RV, Gulve A, et al. Does a screening trial for spinal cord stimulation in patients with chronic pain of neuropathic origin have clinical utility and cost-effectiveness (TRIAL-STIM)? A randomised controlled trial. Pain. 2020;161(12):2820–2829.
53. Ounajim A, Billot M, Goudman L, et al. Machine learning algorithms provide greater prediction of response to SCS than lead screening trial: a predictive AI-based multicenter study. J Clin Med. 2021.
54. Rigoard P, Billot M, Ingrand P, et al. How Should we Use Multicolumn Spinal Cord Stimulation to Optimize Back Pain Spatial Neural Targeting? A Prospective, Multicenter, Randomized, Double-Blind, Controlled Trial (ESTIMET Study). Neuromodulation. 2021;24(1):86–101.
55. Chadwick R, McNaughton R, Eldabe S, et al. To Trial or Not to Trial Before Spinal Cord Stimulation for Chronic Neuropathic Pain: the Patients’ View From the TRIAL-STIM Randomized Controlled Trial. Neuromodulation. 2021;24(3):459–470.
56. Ryan CG, Eldabe S, Chadwick R, et al. An Exploration of the Experiences and Educational Needs of Patients With Failed Back Surgery Syndrome Receiving Spinal Cord Stimulation. Neuromodulation. 2019;22(3):295–301.
57. Sparkes E, Duarte RV, Raphael JH, Denny E, Ashford RL. Qualitative exploration of psychological factors associated with spinal cord stimulation outcome. Chronic Illn. 2012;8(4):239–251.
58. Kravitz RL, Callahan EJ, Paterniti D, Antonius D, Dunham M, Lewis CE. Prevalence and sources of patients’ unmet expectations for care. Ann Intern Med. 1996;125(9):730–737.
59. Kravitz RL, Marois M, Sim I, et al. Chronic pain treatment preferences change following participation in N-of-1 trials, but not always in the expected direction. J Clin Epidemiol. 2021;139:167–176.
60. Henssen D, Scheepers N, Kurt E, et al. Patients’ Expectations on Spinal Cord Stimulation for Failed Back Surgery Syndrome: a Qualitative Exploration. Pain Pract. 2018;18(4):452–462.
61. Iles RA, Davidson M, Taylor NF, O’Halloran P. Systematic review of the ability of recovery expectations to predict outcomes in non-chronic non-specific low back pain. J Occup Rehabil. 2009;19(1):25–40.
62. Constantino MJ, Arnkoff DB, Glass CR, Ametrano RM, Smith JZ. Expectations. J Clin Psychol. 2011;67(2):184–192.
63. Tripepi G, Jager KJ, Dekker FW, Zoccali C. Selection bias and information bias in clinical research. Nephron Clin Pract. 2010;115(2):c94–99.
64. Biele G, Gustavson K, Czajkowski NO, et al. Bias from self selection and loss to follow-up in prospective cohort studies. Eur J Epidemiol. 2019;34(10):927–938.
65. Howe CJ, Cole SR, Lau B, Napravnik S, Eron JJ. Selection Bias Due to Loss to Follow Up in Cohort Studies. Epidemiology. 2016;27(1):91–97.
66. Blonde L, Khunti K, Harris SB, Meizinger C, Skolnik NS. Interpretation and Impact of Real-World Clinical Data for the Practicing Clinician. Adv Ther. 2018;35(11):1763–1774.
67. Katz N, Dworkin RH, North R, et al. Research design considerations for randomized controlled trials of spinal cord stimulation for pain: initiative on Methods, Measurement, and Pain Assessment in Clinical Trials/Institute of Neuromodulation/International Neuromodulation Society recommendations. Pain. 2021;162(7):1935–1956.
68. Shlobin NA, Rosenow JM. Ethical Considerations in the Implantation of Neuromodulatory Devices. Neuromodulation. 2021;1:34.
69. Sayed D, Chakravarthy K, Amirdelfan K, et al. A Comprehensive Practice Guideline for Magnetic Resonance Imaging Compatibility in Implanted Neuromodulation Devices. Neuromodulation. 2020;23(7):893–911.
70. Desai MJ, Hargens LM, Breitenfeldt MD, et al. The rate of magnetic resonance imaging in patients with spinal cord stimulation. Spine. 2015;40(9):E531–537.
71. Jotwani R, Abd-Elsayed A, Villegas K, et al. Failure of SCS MR-Conditional Modes Due to High Impedance: a Review of Literature and Case Series. Pain Ther. 2021;10(1):729–737.
72. Rubino S, Adepoju A, Kumar V, et al. MRI Conditionality in Patients with Spinal Cord Stimulation Devices. Stereotact Funct Neurosurg. 2016;94(4):254–258.
73. Fan A, Wilson KG, Acharya M, Cranney A, Buenger U, Marshall S. Self-reported issues with driving in patients with chronic pain. PMR. 2012;4(2):87–95.
74. Ghaly RF, Tverdohleb T, Candido KD, Knezevic NN. Do we need to establish guidelines for patients with neuromodulation implantable devices, including spinal cord stimulators undergoing nonspinal surgeries? Surg Neurol Int. 2016;7:18.
75. LaViolette PA. Medical devices and conflict of interest: unique issues and an industry code to address them. Cleve Clin J Med. 2007;74 Suppl 2:S32–S27.
76. Ziai K, Pigazzi A, Smith BR, et al. Association of Compensation From the Surgical and Medical Device Industry to Physicians and Self-declared Conflict of Interest. JAMA Surg. 2018;153(11):997–1002.
77. Kapural L, Yu C, Doust MW, et al. Novel 10-kHz High-frequency Therapy (HF10 Therapy) Is Superior to Traditional Low-frequency Spinal Cord Stimulation for the Treatment of Chronic Back and Leg Pain: the SENZA-RCT Randomized Controlled Trial. Anesthesiology. 2015;123(4):851–860.
78. Veizi E, Hayek SM, North J, et al. Spinal Cord Stimulation (SCS) with Anatomically Guided (3D) Neural Targeting Shows Superior Chronic Axial Low Back Pain Relief Compared to Traditional SCS-LUMINA Study. Pain Med. 2017;18(8):1534–1548.
79. Deer T, Slavin KV, Amirdelfan K, et al. Success Using Neuromodulation With BURST (SUNBURST) Study: results From a Prospective, Randomized Controlled Trial Using a Novel Burst Waveform. Neuromodulation. 2018;21(1):56–66.
80. North RB, Parihar HS, Spencer SD, Spalding AF, Cost-Effectiveness Model SJ. Shows Superiority of Wireless Spinal Cord Stimulation Implantation Without a Separate Trial. Neuromodulation. 2021;24(3):596–603.
81. Fishman M, Cordner H, Justiz R, et al. Twelve-Month results from multicenter, open-label, randomized controlled clinical trial comparing differential target multiplexed spinal cord stimulation and traditional spinal cord stimulation in subjects with chronic intractable back pain and leg pain. Pain Pract. 2021;21(8):912–923.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
