Back to Journals » Vascular Health and Risk Management » Volume 18
Patient Selection for Renal Denervation in Hypertensive Patients: What Makes a Good Candidate?
Authors Li S, Phillips JK
Received 20 February 2022
Accepted for publication 22 April 2022
Published 13 May 2022 Volume 2022:18 Pages 375—386
DOI https://doi.org/10.2147/VHRM.S270182
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Professor Mirna N Chahine
Sheran Li,1,2 Jacqueline K Phillips1
1Macquarie Medical School, Faculty of Medicine, Health and Human Sciences, Macquarie University, Sydney, New South Wales, Australia; 2Department of Emergency Medicine, Sun Yat-sen Memorial Hospital of Sun Yat-sen University, Guangzhou, People’s Republic of China
Correspondence: Sheran Li, Department of Emergency Medicine, Sun Yat-sen Memorial Hospital of Sun Yat-sen University, 107 West Yanjiang Road, Yuexiu District, Guangzhou, Guangdong Province, 510120, People’s Republic of China, Tel +86 20 81332723, Fax +86 20 8133 2650, Email [email protected]
Abstract: Renal denervation (RDN) as a therapeutic intervention in patients with hypertension has been intensively studied for over a decade, yet a critical question remains unanswered: what kind of patients are the ideal target population for RDN to achieve its maximum clinical benefit? We herein provide a review of current literature to answer questions related to patient selection to identify populations that will benefit most from RDN, drawing first from human studies but also important clues derived from preclinical animal models. Different aspects that may influence the selection of patients such as the cause of hypertension, the severity of hypertension, concurrent pharmaceutical treatment, renal function, and renal artery anatomy are discussed. Based on current evidence, patients who have severe primary hypertension, regardless of medication or degree of renal dysfunction, who have an accessible accessory renal artery, can achieve a desirable response if a thorough ablation is achieved. In preclinical models, as in humans, RDN shows variable impact, with evidence indicating it does not work in specific conditions such as reduced renal mass, salt-sensitive hypertension, and autosomal recessive polycystic kidney disease. The thresholds, however, for indicators are such that it is still not possible to reliably predict which patients could benefit from the technique. Confirmation of predictive factors and identification of biomarkers are needed before RDN can be integrated in clinical practice on clear and reliable grounds.
Keywords: renal denervation, hypertension, accessory renal artery, renal function, preclinical hypertensive models
Introduction
Renal denervation (RDN) is a minimally invasive surgical procedure that serves to reduce high blood pressure by interrupting the renal nerves located in the renal adventitia using various forms of energy such as radiofrequency and ultrasound1 and in preclinical studies neurotoxic chemicals are also used. Since the publication of the first proof-of-concept study of catheter-based RDN in humans,2 the outcomes of numerous clinical trials have been published. While many show a blood-pressure lowering effect in carefully selected patient cohorts, others fail to prove the effectiveness of the procedure,3,4 most notably the SYMPLICITY HTN-3, being the first prospective, single-blind, randomised, sham-controlled trial, failed to meet its primary efficacy endpoint, being unable to demonstrate a significant difference in blood pressure reduction between RDN and sham control group.5 Moreover, in trials documenting efficacy of the procedure, great variability in response exists.6 This reflects a critical question that needs to be answered for the clinical application of RDN, i.e., what kind of patients are the ideal target population for RDN to achieve its maximum clinical benefit. We herein provide a review of current literature to answer questions related to patient selection to identify populations that will benefit most from RDN, drawing first from human studies but also important clues derived from preclinical animal models.
Human Patients
Does the Cause of Hypertension Matter?
Hypertension can present as essential or primary hypertension or as secondary hypertension caused by factors such as kidney disease, obstructive sleep apnea (OSA), renal artery stenosis and others.7 In the first tranche of clinical RDN studies, patients with secondary hypertension were typically excluded from the trials5,6,8 possibly because a curative intervention may be present if the cause of secondary hypertension is identified early. This leaves very much open the question as to whether or not patients with secondary hypertension are suitable for RDN. Interesting findings are, however, starting to be published looking at discrete patient cohorts. In a recent study of patients with resistant hypertension as a result of primary aldosteronism caused by a unilateral aldosterone-producing adenoma, laparoscopic-based RDN plus adrenalectomy resulted in a greater reduction in blood pressure at 36-months follow-up compared to adrenalectomy alone.9 The additional benefit of RDN in these patients is significant as around 50% still need antihypertensive treatment after adrenalectomy,10 though a caveat is that despite the promising results of RDN in the above-mentioned study, the study enrolled only a relatively small number of patients from a single center and did not include a sham procedure and is therefore prone to observational biases.
Obstructive sleep apnoea is characterised by sympathetic overactivity and hypertension11 suggesting likely efficacy of RDN, as increased sympathetic drive and specifically sympathetic cross-talk between the kidneys and the brain is a primary mechanism hypothesised to be targeted by the procedure.5 And indeed a randomised control trial has shown RDN can reduce blood pressure in hypertensive patients with OSA at 3- and 6-month follow-up,12 suggesting these patients are another cohort that might be suitable candidates for RDN.
There are however patient groups where the cause of hypertension appears to preclude them from being likely candidates for RDN. For instance, patients with hypertension secondary to renal artery stenosis are often excluded from clinical trials in part due to the likelihood of worsening of renal artery stenosis following the procedure.13
A major association exists between structural or functional changes of the kidney and the development of hypertension, and conversely, long-term hypertension can in turn cause chronic kidney disease (CKD) and kidney failure.14 In patients with both hypertension and kidney disease, it sometimes remains difficult to differentiate the exact cause of hypertension in these patients. Despite this, available evidence suggests that RDN is safe and effective to reduce high blood pressure in patients with impaired renal function.15 A detailed discussion on this topic is presented in the following section of “Does renal function matter?”.
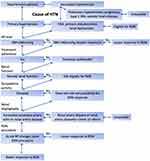
In conclusion, although patients with primary hypertension are the primary cohort considered as suitable candidates for RDN, new evidence supports the use of RDN in patients with primary aldosteronism or renal dysfunction (Figure 1). Large multicenter double-blinded sham-controlled clinical trials are warranted to test the effectiveness of RDN in these patient groups.
 |
Figure 1 Considerations in patient selection to predict a better response to renal denervation (RDN)Dear author, please confirm the new copyright note and reference are correct. Abbreviation: BP, blood pressure. Note: Adapted from iStock by Getty Images. Vector kidneys illustration stock illustration; 2015 [updated February 25, 2015]. Available from: https://www.istockphoto.com/vector/vector-kidneys-illustration-gm464688692-58892822 Accessed May 10, 2022.106 |
Does the Severity of Hypertension Matter?
Clinically, hypertension is stratified into three different grades based on the blood pressure level, being Grade 1 with systolic blood pressure (SBP) 140–159 mmHg and/or diastolic blood pressure (DBP) 90–99 mmHg, Grade 2 with SBP 160–179 mmHg and/or DBP 100–109 mmHg and Grade 3 with SBP ≥180 mmHg and/or DBP ≥110 mmHg.16 In many clinical trials of RDN, the blood pressure response in participants with different grades of hypertension has not been adequately reported.5,6,8 Instead, a mean baseline blood pressure level and the final mean blood pressure change after RDN of all participants were reported. For instance, in the DENERHTN trial, a mean reduction of 15.8 mm Hg (95% CI: −19.7 to −11.9) from a baseline level of 155.5 ± 16.4 mmHg in daytime ambulatory SBP at 6 months was reported in the RDN group, whereas a mean reduction of 9.9 mmHg (95% CI: −13.6 to −6.2) from a baseline level of 151.0 ± 16.0 mmHg was observed in the sham group, receiving standardised stepped-care antihypertensive treatment alone.17 Such grouped data mean it is not evident if patients with different degrees of hypertension had a different response to the RDN procedure. Where stratification of pre-RDN blood pressure level is available, the data does suggest that baseline levels of SBP are a predictor of blood pressure response to RDN, with more severe hypertension associated with more pronounced BP-lowering efficacy response to RDN.18–21 For instance, in the work of Rohla et al,20 the change of SBP at 6 months following RDN are 7.06, 0.23, −8.64, −13.56 mmHg respectively, for patients with lowest to highest 4 quartiles of baseline 24 h SBP. In further support of this notion, in a trial of patients with mildly elevated blood pressure (24-hour daytime ambulatory SBP 135–149 mmHg, DBP 90–94 mmHg), RDN failed to produce a significantly different reduction in blood pressure compared to sham groups.22 One explanation for this association is that severe hypertension is aligned with heightened sympathetic tone and is therefore more successfully brought down by RDN.23 Consistent with this hypothesis is that nighttime SBP and variability has predictive value to blood pressure response to denervation,24,25 due to the role of the sympathetic nervous system in maintaining high blood pressure during the nighttime period.26,27 In summary, therefore, pre-RDN blood pressure level may be a reliable indicator for a successful RDN (Figure 1). However, position statements on renal denervation from medical societies including the European Society for Hypertension (ESH),1 Joint UK societies,28 and the SEH-LELHA29 (Spanish Society of Hypertension-Spanish League for Combating High Blood Pressure) do not provide any guidance on the best pre-RDN blood pressure level for patient selection, highlighting the knowledge gap in this area.
Does Pharmaceutical Treatment Matter?
In the early clinical studies, patients underwent RDN while on multiple antihypertensive drugs,5,30 and indeed one of the prerequisites for trial inclusion was resistant hypertension, which by definition was a blood pressure that remains above goal despite concurrent use of at least three antihypertensive agents of different classes taken at maximally tolerated doses, one of which being a diuretic.5 This brought with it confounding factors, as without an objective assessment of drug adherence, it is difficult to differentiate drug versus device effects and determine the presence of additive device effects.31 Further, one of the suspected reasons for lack of significant difference between the RDN and sham groups seen in the SYMPLICITY HTN-3 trial was increased drug adherence in the sham controls, resulting in a comparable blood pressure reduction.5 In the Dutch SYMPATHY trial, in which objective assessment of drug adherence was performed, the change in daytime ambulatory SBP after 6 months was 2.0 mm Hg less in the RDN group compared to the medication-only group.32 However, in those with stable drug adherence during follow-up, the change in daytime ambulatory SBP was 3.3 mmHg more in the RDN group compared to the control group.32 This suggests that imbalanced drug adherence can be a confounding factor when determining the effect of RDN.
In the SPYRAL HTN-ON MED trial, when the prevalence of both numbers of drugs and adherence was similar in both RDN and sham groups, RDN significantly reduced blood pressure compared with sham controls.33 In the DENERHTN trial, where non-adherence to drug treatment was similarly high in the RDN and control group, RDN plus standardised stepped-care antihypertensive treatment resulted in a greater decrease in blood pressure than standardised stepped-care antihypertensive treatment alone.34 Interestingly, an absence of drugs in the SPYRAL HTN-OFF MED trial,6,35 and the RADIANCE-HTN SOLO trial,36 which entered patients who were either not taking medication or had undertaken a 3 to 4-week discontinuation period, RDN significantly reduced office and 24h blood pressure compared with sham controls, substantiating the procedure while avoiding variable patient adherence to treatment. Both studies have inherent limitations, of note being undertaken using well-defined populations with only Grade 1 or 2 hypertension (SBP <180 mmHg), and whether the results will extend to patients with resistant hypertension or secondary hypertension is not yet known, noting further that in clinical practice, the effect of and adherence to pharmaceutical treatment is much more unpredictable. Nevertheless, renal denervation is being supported for patients with persistent BP > 140/90 mmHg despite drug treatment by the ESH,1 the Asia Renal Denervation Consortium37 and SEH-LELHA.29
Does Renal Function Matter?
As RDN requires contrast media to visualise the renal artery, in earlier clinical studies, patients with moderate-to-severe renal dysfunction [estimated glomerular filtration rate (eGFR) <45 mL/min/1.73m2] were excluded from trials due to safety concerns.5,30 In 2012, Hering et al38 reported in a pilot study examining 15 patients with hypertension and renal dysfunction (eGFR between 15 and 45 mL/min/1.73m2) that RDN caused a marked ~30 mmHg reduction in SBP at 1, 3, 6, and 12 months of follow-up without deterioration of renal function. Several case reports and a case series study have subsequently served to establish efficacy and safety of RDN in patients with end-stage renal disease (eGFR<15 mL/min/1.73m2).39–41 Ott et al42 found that RDN not only decreased blood pressure but also ameliorated the decline of renal function in patients with mild-to-moderate renal dysfunction. A study published in 2017 showed that the eGFR decline rate in patients with CKD was slowed down by RDN as the eGFR showed an annual decline by 3.5 mL/min/1.73m2 from 60 months before RDN but, did not change during the 24 months after RDN.15 Results from the Global SYMPLICITY Registry indicate that after adjusting for baseline factors, RDN produced a similar reduction in 24-hour ambulatory SBP and DBP at 36-months follow-up in patients with and without renal dysfunction.43 A recent meta-analysis of RDN studies in patients with renal dysfunction showed that individuals treated with RDN experienced significantly greater 24h ambulatory blood pressure reductions and that RDN did not increase the risk of rapid deterioration of renal function and other major adverse events.44 However, none of these clinical studies were randomised sham-controlled trials, and therefore the strength of the evidence is mitigated. Whether these observed blood pressure lowering and renoprotective effects of RDN can be replicated in large randomised clinical trials needs further investigation. Nevertheless, these preliminary studies do show that RDN can reduce blood pressure in patients with renal dysfunction. Therefore, if clinically necessary, patients with reduced renal function should be considered for the procedure (Figure 1).
Does the Anatomy of the Renal Artery Matter?
The anatomy of the renal artery is a critical aspect when considering RDN in patients with renal artery diameter, renal artery length, the presence of renal artery disease, or accessory renal arteries defining inclusion criteria for clinical trials.5 Typically, patients with an artery length of <20 mm, a diameter of <4 mm and presence of structural abnormalities are thought not suitable because these factors would preclude a proper ablation of renal nerves, therefore undermining the therapeutic effects of renal denervation.
The presence of accessory renal arteries is also a feature of patient selection. In normal subjects, the average occurrence of accessory renal arteries is about 30%.45–50 Importantly, renal accessory arteries are over-represented in hypertensive patients compared with healthy controls.51,52 The presence of accessory renal arteries means accompanying additional renal nerves53 and while the distance from the arterial lumen to the nerves in accessory renal arteries has been shown to be similar to that seen in main renal arteries,53 suggesting that RDN as applied to main renal arteries should work in accessory arteries as well, the anatomy of accessory renal arteries means that they are often too small to allow access for the RDN device and are therefore a potential source of residual sympathetic tone.54 There is some debate over this question. For example, in the study by Ewen et al,48 the blood pressure response to RDN was not significantly different in patients with or without accessory renal arteries, noting that in this study, only about 10% of accessory renal arteries were treated during the RDN procedure. In contrast, other researchers have reported that the presence and non-treatment of accessory renal arteries is associated with a lower response rate to RDN.51,55 This is supported by the work of de Jong et al54 who demonstrated in anesthetised patients with resistant hypertension that stimulation of renal nerves in the untreated accessory renal artery before and after main artery RDN could produce a similar blood pressure increase. Accessory renal arteries may therefore partly account for the unsatisfying response in some patients.54 Therefore, recent clinical trials of RDN6,33 target not only main renal arteries but also accessory renal arteries to achieve the best outcome for patients. Some guidelines, however, do note that arteries with abnormal renal anatomy (for example, diameters <3mm or >8mm) or with significant disease or flow-limiting obstructions should be avoided, as should patients with previous renal angioplasty indwelling ureteral stents and aortic grafts.29,56 The presence of accessory renal arteries is therefore a potential confounder of the therapeutic effect of RDN but not necessarily a factor that would exclude patient selection (Figure 1).
Do Patients Have to Be Sympathetically Overactive?
The rationale to use RDN to treat hypertension is that excessive sympathetic activity to and driven by the kidney is involved in the development of hypertension.57 Therefore, it is natural to speculate that patients with a higher sympathetic activity might benefit more from the RDN procedure, comparable in principle to the use of carotid baroreflex activation therapy to drive renal sympathoinhibition and reduced blood pressure.58 In support of this notion, Mahfoud et al59 showed that a higher baseline plasma renin activity, which can in part represent sympathetic activity, is associated with a greater reduction in office and 24-hour BP after RDN. It needs to be noted that in practice it is difficult to accurately identify patients with increased sympathetic nerve activity (SNA) and more specifically, those with increased renal SNA. Renal noradrenaline spillover rate is the most direct marker for renal SNA but is an invasive procedure that requires infusions of radiolabelled noradrenaline and venous sampling from centrally placed catheters, making it less accessible in clinical practice.60 In a study of 10 patients, Krum et al2 reported a significant reduction in renal noradrenaline spillover (47%) and a mean 6-month office blood pressure reduction of 22/12 mm Hg after RDN procedure. While an indicator of a successful RDN procedure, what is not reported in the study is if the baseline renal noradrenaline spillover rate in these ten patients was elevated compared to normal controls in the same cohort. Nevertheless, the baseline level of sympathetic activity may not be as important as initially assumed in determining the response to RDN. In a recent study comparing the effect of RDN and drug adjustment in hypertensive patients,61 no significant difference was noted in baseline plasma catecholamines, which was used an indirect or surrogate marker for sympathetic activity, between the two groups. Nevertheless, a significantly lower level of plasma catecholamines during stress tests after RDN was noted in two RDN responders. The lack of difference in baseline plasma catecholamines between the two groups was in part attributed to the use of sympatholytic drugs in the patients, reflecting the current approach to treatment of hypertensive patients. In addition, in animal models of sympathetically mediated hypertension such as those induced with chronic angiotensin II (AngII) exposure, increased blood pressure chronically activates the baroreflex pathway, which in turn inhibits sympathetic tone, especially the renal SNA, serving as a compensatory mechanism to attenuate the severity of the hypertension.62 However, the baroreflex-mediated sympathetic inhibition can be attenuated or lost due to baroreflex dysfunction with the progression of hypertension, such as in obesity hypertension, resulting in a net increase in sympathetic tone.62 Therefore, the level of sympathetic activity is neither an ideal criterion to determine the appropriateness for RDN, as both sympathetic activation and inhibition can be present in hypertensive patients, nor will it necessarily therefore be a good predictor for RDN response.
Noting the limitations of assessment of sympathetic tone before RDN as a predictor of response, immediate physiological responses to RDN have been proposed as better markers of sympathetic tone and potential predictors for RDN response. For instance, Xu et al63 suggest that an increase in blood pressure during radiofrequency energy delivery, and indeed the degree of positive change, could predict the long-term procedural success of RDN. de Jong et al64 also discovered that the acute difference in renal nerve stimulation-induced blood pressure changes before versus after RDN could be positively correlated with changes in 24h ambulatory blood pressure monitoring 3 to 6 months after RDN, both for SBP and DBP, such that the greater the acute difference, the greater the long-term reduction in blood pressure. Therefore, current evidence would support hypertensive patients with “normal” sympathetic activity, whether it is assessed by direct or non-direct methods, as still being potential good candidates for RDN (Figure 1).
Miscellaneous Factors
In addition to the considerations mentioned above, growing evidence is pointing to other factors which may predict an individual’s response to RDN. Several studies found that female patients appear to have more pronounced ambulatory blood pressure reductions after RDN.21,65,66 The exact reason for this observation is unclear but may be associated with differences in neurally mediated hypertension pathways and/or antihypertensive treatment adherence between males and females.65 There is also evidence to suggest that arterial stiffness may be a predictor of response to RDN, with patients with less arterial stiffness showing a significantly better response to denervation than those with increased stiffness, as determined using techniques such as ambulatory arterial stiffness index and pulse wave velocity.67,68 Consistent with this are studies that show that patients with isolated systolic hypertension, which is characterised by increased arterial stiffness, have a lesser response to RDN than patients with combined systolic and diastolic hypertension.18,69 A mechanism proposed to underlie this reduced response is that patients with stiffer arteries have greater structural changes and reduced neurogenic drive as a mediator of their hypertension.62 This does not however preclude patients with increased arterial stiffness from potentially benefiting from RDN. A study of patients with resistant hypertension demonstrated an improvement in arterial stiffness, as evidenced by a reduction in pulse wave velocity, after RDN that could not be explained by the blood pressure drop alone, with the authors suggesting that RDN resulted in an improvement in arterial mechanical properties as a result of reducing sympathetic drive to the vasculature.70 Marked endothelial dysfunction and a higher vasomotor tone are also indicators that have been proposed to identify likely responders to RDN.71 A recent study revealed that a baseline heart rate of over 70 beats/min was associated with a greater reduction in mean office, 24-hour, daytime, and nighttime SBP for RDN at 3 months follow-up, suggesting a potential predictive role of heart rate.72 However, many of these observations need to be confirmed in further research for accurate identification of patients likely to respond to RDN with a fall in blood pressure that is clinically significant in magnitude and maintained over time.
Another group of patients that should be considered potential candidates for renal denervation are those with hypertension-mediated organ damage (HMOD), due to the likelihood of their receiving significant clinical benefit from the intervention,29 with meta-analysis supporting an independent effect of renal denervation on HMOD.73
While considering which groups of hypertensive patients are likely good candidates for renal denervation, it is important to note those who are not or for whom there is insufficient data to make an informed clinical decision. For example, patients in whom a blood pressure reduction is considered hazardous such as those with haemodynamically significant valvular heart disease are contraindicated for renal denervation,29 while patients who have primary pulmonary hypertension, are pregnant, nursing or planning to become pregnant or patients with type 1 diabetes mellitus are typically excluded from clinical trials74 and so are not currently deemed suitable candidates for the procedure.
Preclinical Animal Models
The preclinical studies preceding the initial safety and proof of principle RDN radiofrequency ablation study in humans were undertaken in pigs and demonstrated that the procedure significantly reduced renal noradrenaline content, and importantly, did not induce significant vascular or renal injury.2 Prior to this, RDN had been used in numerous small and large animal models to understand the role played by the renal nerves in controlling blood pressure in both health and disease. In contrast to human studies, preclinical studies provide greater control over experimental variables such as age, sex, severity of disease and confounding factors such as pharmaceutical interventions.
In normotensive animals, studies in Lewis75 and Sprague-Dawley76,77 rats have shown that renal denervation produced a reduction of 6~10 mmHg in blood pressure, suggesting a tonic role of the renal nerves in blood pressure maintenance.
The spontaneously hypertensive rat (SHR) model mimics human essential hypertension and has been extensively studied for the effect of RDN on blood pressure as measured by tail-cuff or radiotelemetry in both the developmental and established phase of hypertension in several studies.78–80 It appears that bilateral RDN at 7, 8 or 13 weeks of age i.e., during the developmental phase of hypertension, delayed the onset but did not alter the final level of hypertension, whereas bilateral RDN at 18 weeks of age i.e., during the established phase of hypertension, had no effect on blood pressure.78–80 These studies support an important role of renal nerves in the development but not maintenance of hypertension in this model. From a translational perspective, this suggests that a better clinical response may be anticipated if RDN were performed in patients with essential hypertension early in their disease phase, before secondary vascular, cardiac and renal damage occurs.
Renal denervation also attenuates the development of experimental hypertension in an Ang II hypertension model,81 the Schlager mouse model (a neurogenic hypertension model),82,83 polycystic ovary syndrome (PCOS) rats,84 and an obesity-induced hypertension model.85 In addition, in the 2-kidney-1-clip (2K1C) Goldblatt hypertensive rat, RDN of the clipped kidney resulted in a reduction in SBP and the depressor effect was associated with a decrease in SNA, specifically to the lumbar and renal vascular beds,86,87 while RDN of the unclipped kidney produced no such effect. This work suggests the involvement of renal sensory nerve abnormality in the development of hypertension in this model.88 Selective renal sensory denervation by dorsal root rhizotomy also results in a slight but significant reduction in blood pressure in the SHR compared to sham controls.89 In the deoxycorticosterone acetate (DOCA) salt rat, where hypertension is induced by a combination of administration of DOCA, reduced kidney mass and increased salt intake, total RDN and selective renal sensory denervation attenuated hypertension to a similar degree.90 It needs to be noted that when a lower dose of DOCA is administered, and a nephrectomy is not performed, RDN failed to attenuate hypertension in this model.91 Similarly, although RDN typically reduced blood pressure in the AngII-salt model,92 it had no effect on the AngII-salt model when lower than typical doses of AngII are administered.77 The common factors that make RDN work in these various types of experimental hypertension models are not overtly clear, but in the end, may be attributable to the involvement of activated renal sympathetic or sensory nerves in the underlying pathogenesis. An important consideration in many of these animal studies is that despite that RDN attenuating hypertension, it does not lead to a complete reversal of hypertension, which suggests the participation of other factors in the development and/or maintenance of hypertension.
There are also clear examples of preclinical animal models where RDN is not effective. In the reduced renal mass, salt-sensitive hypertension model, renal denervation did not chronically lower arterial pressure, which was proposed to be due to the presence of sympathoinhibition as a result of sustained baroreflex activation during established hypertension, making it not a good candidate for RDN.93 Renal denervation in a canine model of aldosterone-induced hypertension has been shown to have no effect,94 noting that this is in contrast to the studies noted prior in humans in which hypertension secondary to aldosterone-producing adenoma did show a long-term blood pressure response to renal denervation.9
Chronic kidney disease models have also been investigated to assess the effect of RDN on hypertension secondary to CKD as well as the progression of renal disease. For instance, in the 5/6 nephrectomy model, which mimics secondary hypertension after acute renal failure, both renal sensory nerve ablation by bilateral dorsal rhizotomy and total RDN could prevent the development of hypertension, possibly through reducing SNA to splanchnic and lumbar beds.95–97 Furthermore, RDN improved GFR, lowered proteinuria, and ameliorated the development of glomerular and tubular-interstitial damage in both the 5/6 nephrectomy98 and uninephrectomised Dahl-salt sensitive models.99 Benefits in blood pressure reduction and/or renal protection after renal denervation were also observed in the uninephrectomised SHR model,100 the fetal uninephrectomised CKD sheep model,101 the CKD rabbit model induced by glomerular layer lesioning and uninephrectomy,102 uninephrectomised rats fed with high salt diet103 and the Han: SPRD-Cy/+ model of autosomal dominant polycystic kidney disease,104 though no response, either blood pressure or renoprotective, was seen in the Lewis polycystic kidney disease (LPK) rat model, which resembles autosomal recessive polycystic kidney disease.75 In this case, the CKD model arose from an early onset genetic cause, and the degree of hypertension and renal dysfunction was marked from an early age.75 From a translational perspective, the application of RDN in patients such as those with childhood onset genetic kidney disease should be carefully considered to avoid unnecessary procedures. A lack of renoprotective effect of RDN was also reported in a porcine chronic renal insufficiency model induced by selective renal artery embolisation.105
Conclusion
After over a decade of intensive research in RDN following the development of novel percutaneous approaches using radiofrequency and ultrasound, our understanding and experience with the procedure have allowed us to answer several basic questions related to its clinical use. Nevertheless, being able to predict which patients will respond well to the procedure, with a clinically meaningful long-lasting blood pressure reduction is still not possible. Based on current evidence, patients who have severe primary or resistant hypertension, regardless of medication or degree of renal dysfunction, certain patients with secondary hypertension and patients with an accessible accessory renal artery, independent upon the establishment or otherwise of increased sympathetic activity, can achieve a desirable response if a thorough ablation is achieved (Figure 2). However, the thresholds for these criteria are vague, such that it is still not possible to reliably predict which patients could benefit from the technique. Confirmation of predictive factors and identification of biomarkers are needed before RDN can be integrated in clinical practice on clear and reliable grounds.
Acknowledgments
The authors acknowledge the support of the Hillcrest Foundation Australia for research undertaken in their laboratory examining renal denervation in preclinical animal models.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Schmieder RE, Mahfoud F, Mancia G, et al. European Society of Hypertension position paper on renal denervation 2021. J Hypertens. 2021;39(9):1733–1741. doi:10.1097/HJH.0000000000002933
2. Krum H, Schlaich M, Whitbourn R, et al. Catheter-based renal sympathetic denervation for resistant hypertension: a multicentre safety and proof-of-principle cohort study. Lancet. 2009;373(9671):1275–1281. doi:10.1016/S0140-6736(09)60566-3
3. Ahmad Y, Francis DP, Bhatt DL, Howard JP. Renal denervation for hypertension: a systematic review and meta-analysis of randomized, blinded, placebo-controlled trials. JACC Cardiovasc Interv. 2021;14(23):2614–2624. doi:10.1016/j.jcin.2021.09.020
4. Ogoyama Y, Tada K, Abe M, et al. Effects of renal denervation on blood pressures in patients with hypertension: a systematic review and meta-analysis of randomized sham-controlled trials. Hypertens Res. 2021;45:210–220.
5. Bhatt DL, Kandzari DE, O’Neill WW, et al. A controlled trial of renal denervation for resistant hypertension. N Engl J Med. 2014;370(15):1393–1401. doi:10.1056/NEJMoa1402670
6. Townsend RR, Mahfoud F, Kandzari DE, et al. Catheter-based renal denervation in patients with uncontrolled hypertension in the absence of antihypertensive medications (SPYRAL HTN-OFF MED): a randomised, sham-controlled, proof-of-concept trial. Lancet. 2017;390(10108):2160–2170. doi:10.1016/S0140-6736(17)32281-X
7. Charles L, Triscott J, Dobbs B. Secondary hypertension: discovering the underlying cause. Am Fam Physician. 2017;96(7):453–461.
8. Tsioufis C, Ziakas A, Dimitriadis K, et al. Blood pressure response to catheter-based renal sympathetic denervation in severe resistant hypertension: data from the Greek Renal Denervation Registry. Clin Res Cardiol. 2017;106(5):322–330. doi:10.1007/s00392-016-1056-z
9. Liu Y, Zhu B, Zhu L, et al. Thirty-six-month results of laparoscopic-based renal denervation plus unilateral laparoscopic adrenalectomy for the treatment of patients with resistant hypertension caused by unilateral aldosterone-producing adenoma. J Clin Hypertens. 2021;23(5):946–953. doi:10.1111/jch.14223
10. Sawka AM, Young WF, Thompson GB, et al. Primary aldosteronism: factors associated with normalization of blood pressure after surgery. Ann Intern Med. 2001;135(4):258–261. doi:10.7326/0003-4819-135-4-200108210-00010
11. Chahal CA, Somers VK. Secondary hypertension: obstructive sleep apnea. J Am Soc Hypertens. 2015;9(3):
12. Warchol-Celinska E, Prejbisz A, Kadziela J, et al. Renal denervation in resistant hypertension and obstructive sleep apnea: randomized proof-of-concept Phase II trial. Hypertension. 2018;72(2):381–390. doi:10.1161/HYPERTENSIONAHA.118.11180
13. Kaltenbach B, Id D, Franke JC, et al. Renal artery stenosis after renal sympathetic denervation. J Am Coll Cardiol. 2012;60(25):2694–2695. doi:10.1016/j.jacc.2012.09.027
14. Pugh D, Gallacher PJ, Dhaun N. Management of hypertension in chronic kidney disease. Drugs. 2019;79(4):365–379. doi:10.1007/s40265-019-1064-1
15. Hering D, Marusic P, Duval J, et al. Effect of renal denervation on kidney function in patients with chronic kidney disease. Int J Cardiol. 2017;232:93–97. doi:10.1016/j.ijcard.2017.01.047
16. Williams B, Mancia G, Spiering W, et al. 2018 ESC/ESH guidelines for the management of arterial hypertension: the task force for the management of arterial hypertension of the European Society of Cardiology (ESC) and the European Society of Hypertension (ESH). Eur Heart J. 2018;39(33):3021–3104. doi:10.1093/eurheartj/ehy339
17. Azizi M, Sapoval M, Gosse P, et al. Optimum and stepped care standardised antihypertensive treatment with or without renal denervation for resistant hypertension (DENERHTN): a multicentre, open-label, randomised controlled trial. Lancet. 2015;385(9981):1957–1965. doi:10.1016/S0140-6736(14)61942-5
18. Ewen S, Ukena C, Linz D, et al. Reduced effect of percutaneous renal denervation on blood pressure in patients with isolated systolic hypertension. Hypertension. 2015;65(1):193–199. doi:10.1161/HYPERTENSIONAHA.114.04336
19. Kandzari DE, Bhatt DL, Brar S, et al. Predictors of blood pressure response in the SYMPLICITY HTN-3 trial. Eur Heart J. 2015;36(4):219–227. doi:10.1093/eurheartj/ehu441
20. Rohla M, Nahler A, Lambert T, et al. Predictors of response to renal denervation for resistant arterial hypertension: a single center experience. J Hypertens. 2016;34(1):123–129. doi:10.1097/HJH.0000000000000764
21. Saxena M, Schmieder RE, Kirtane AJ, et al. Predictors of blood pressure response to ultrasound renal denervation in the RADIANCE-HTN SOLO study. J Hum Hypertens. 2021. doi:10.1038/s41371-021-00547-y
22. Desch S, Okon T, Heinemann D, et al. Randomized sham-controlled trial of renal sympathetic denervation in mild resistant hypertension. Hypertension. 2015;65(6):1202–1208. doi:10.1161/HYPERTENSIONAHA.115.05283
23. Hering D, Lambert EA, Marusic P, et al. Substantial reduction in single sympathetic nerve firing after renal denervation in patients with resistant hypertension. Hypertension. 2013;61(2):457–464. doi:10.1161/HYPERTENSIONAHA.111.00194
24. Gosse P, Cremer A, Pereira H, et al. Twenty-four-hour blood pressure monitoring to predict and assess impact of renal denervation: the DENERHTN Study (Renal Denervation for Hypertension). Hypertension. 2017;69(3):494–500. doi:10.1161/HYPERTENSIONAHA.116.08448
25. Gosse P, Cremer A, Kirtane AJ, et al. Ambulatory blood pressure monitoring to predict response to renal denervation: a post hoc analysis of the RADIANCE-HTN SOLO Study. Hypertension. 2021;77(2):529–536. doi:10.1161/HYPERTENSIONAHA.120.16292
26. Jeong JH, Fonkoue IT, Quyyumi AA, DaCosta D, Park J. Nocturnal blood pressure is associated with sympathetic nerve activity in patients with chronic kidney disease. Physiol Rep. 2020;8(20):e14602. doi:10.14814/phy2.14602
27. Kario K, Wang T-D. Perspectives of renal denervation from hypertension to heart failure in Asia. Hypertens Res. 2022;45(2):193–197. doi:10.1038/s41440-021-00751-w
28. Lobo MD, Sharp ASP, Kapil V, et al. Joint UK societies’ 2019 consensus statement on renal denervation. Heart. 2019;105(19):1456–1463. doi:10.1136/heartjnl-2019-315098
29. Rodríguez-Leor O, Jaén-águila F, Segura J, et al. Renal denervation for the management of hypertension. Joint position statement from the SEH-LELHA and the ACI-SEC. REC Interv Cardiol. 2022;4:39–46.
30. Esler MD, Krum H, Sobotka PA, Schlaich MP, Schmieder RE, Bohm M. Renal sympathetic denervation in patients with treatment-resistant hypertension (the Symplicity HTN-2 trial): a randomised controlled trial. Lancet. 2010;376(9756):1903–1909.
31. Kandzari DE, Mahfoud F, Bhatt DL, et al. Confounding factors in renal denervation trials: revisiting old and identifying new challenges in trial design of device therapies for hypertension. Hypertension. 2020;76(5):1410–1417. doi:10.1161/HYPERTENSIONAHA.120.15745
32. de Jager RL, de Beus E, Beeftink MM, et al. Impact of medication adherence on the effect of renal denervation: the SYMPATHY trial. Hypertension. 2017;69(4):678–684. doi:10.1161/HYPERTENSIONAHA.116.08818
33. Kandzari DE, Bohm M, Mahfoud F, et al. Effect of renal denervation on blood pressure in the presence of antihypertensive drugs: 6-month efficacy and safety results from the SPYRAL HTN-ON MED proof-of-concept randomised trial. Lancet. 2018;391(10137):2346–2355. doi:10.1016/S0140-6736(18)30951-6
34. Azizi M, Pereira H, Hamdidouche I, et al. Adherence to antihypertensive treatment and the blood pressure-lowering effects of renal denervation in the Renal Denervation for Hypertension (DENERHTN) trial. Circulation. 2016;134(12):847–857. doi:10.1161/CIRCULATIONAHA.116.022922
35. Bohm M, Kario K, Kandzari DE, et al. Efficacy of catheter-based renal denervation in the absence of antihypertensive medications (SPYRAL HTN-OFF MED Pivotal): a multicentre, randomised, sham-controlled trial. Lancet. 2020;395(10234):1444–1451. doi:10.1016/S0140-6736(20)30554-7
36. Azizi M, Schmieder RE, Mahfoud F, et al. Endovascular ultrasound renal denervation to treat hypertension (RADIANCE-HTN SOLO): a multicentre, international, single-blind, randomised, sham-controlled trial. Lancet. 2018;391(10137):2335–2345. doi:10.1016/S0140-6736(18)31082-1
37. Kario K, Kim BK, Aoki J, et al. Renal denervation in Asia: consensus statement of the Asia Renal Denervation Consortium. Hypertension. 2020;75(3):590–602. doi:10.1161/HYPERTENSIONAHA.119.13671
38. Hering D, Mahfoud F, Walton AS, et al. Renal denervation in moderate to severe CKD. J Am Soc Nephrol. 2012;23(7):1250–1257. doi:10.1681/ASN.2011111062
39. Di Daniele N, De Francesco M, Violo L, Spinelli A, Simonetti G. Renal sympathetic nerve ablation for the treatment of difficult-to-control or refractory hypertension in a haemodialysis patient. Nephrol Dial Transplant. 2012;27(4):1689–1690. doi:10.1093/ndt/gfs044
40. Ott C, Schmid A, Ditting T, et al. Renal denervation in a hypertensive patient with end-stage renal disease and small arteries: a direction for future research. J Clin Hypertens. 2012;14(11):799–801. doi:10.1111/jch.12017
41. Schlaich MP, Bart B, Hering D, et al. Feasibility of catheter-based renal nerve ablation and effects on sympathetic nerve activity and blood pressure in patients with end-stage renal disease. Int J Cardiol. 2013;168(3):2214–2220. doi:10.1016/j.ijcard.2013.01.218
42. Ott C, Mahfoud F, Schmid A, et al. Renal denervation preserves renal function in patients with chronic kidney disease and resistant hypertension. J Hypertens. 2015;33(6):1261–1266. doi:10.1097/HJH.0000000000000556
43. Ott C, Mahfoud F, Mancia G, et al. Renal denervation in patients with versus without chronic kidney disease: results from the global SYMPLICITY Registry with follow-up data of 3 years. Nephrol Dial Transplant. 2021;37:304–310.
44. Xia M, Liu T, Chen D, Huang Y. Efficacy and safety of renal denervation for hypertension in patients with chronic kidney disease: a meta-analysis. Int J Hyperthermia. 2021;38(1):732–742. doi:10.1080/02656736.2021.1916100
45. Maleki H, Shahriar R, Kazemi R, Khodadadi F. Frequencies of accessory renal arteries in 129 Iranian patients. Am J Clin Exp Urol. 2020;8(1):38–42.
46. Tao XF, Zhu JQ, Wu YW, et al. Dual-energy computed tomography angiography for evaluating the renal vascular variants. Chin Med J. 2013;126(4):650–654.
47. Ramadan SU, Yigit H, Gokharman D, et al. Can renal dimensions and the main renal artery diameter indicate the presence of an accessory renal artery? A 64-slice CT study. Diagn Interv Radiol. 2011;17(3):266–271. doi:10.4261/1305-3825.DIR.3507-10.0
48. Ewen S, Ukena C, Luscher TF, et al. Anatomical and procedural determinants of catheter-based renal denervation. Cardiovasc Revasc Med. 2016;17(7):474–479. doi:10.1016/j.carrev.2016.08.004
49. Gulas E, Wysiadecki G, Szymanski J, et al. Morphological and clinical aspects of the occurrence of accessory (multiple) renal arteries. Arch Med Sci. 2018;14(2):442–453. doi:10.5114/aoms.2015.55203
50. Song WH, Baik J, Choi E-K, et al. Quantitative analysis of renal arterial variations affecting the eligibility of catheter-based renal denervation using multi-detector computed tomography angiography. Sci Rep. 2020;10(1):19720. doi:10.1038/s41598-020-76812-w
51. Vonachen P, Hamann JJ, Houghland T, et al. Accessory renal arteries: prevalence in resistant hypertension and an important role in nonresponse to radiofrequency renal denervation. Cardiovasc Revasc Med. 2016;17(7):470–473. doi:10.1016/j.carrev.2016.07.009
52. Sanghvi K, Wang Y, Daemen J, et al. Renal artery variations in patients with mild-to-moderate hypertension from the RADIANCE-HTN SOLO trial. Cardiovasc Revasc Med. 2021. doi:10.1016/j.carrev.2021.09.008
53. Sato Y, Kawakami R, Jinnouchi H, et al. Comprehensive assessment of human accessory renal artery periarterial renal sympathetic nerve distribution. JACC Cardiovasc Interv. 2021;14(3):304–315. doi:10.1016/j.jcin.2020.09.043
54. de Jong MR, Hoogerwaard AF, Gal P, et al. Persistent increase in blood pressure after renal nerve stimulation in accessory renal arteries after sympathetic renal denervation. Hypertension. 2016;67(6):1211–1217. doi:10.1161/HYPERTENSIONAHA.115.06604
55. Id D, Kaltenbach B, Bertog SC, et al. Does the presence of accessory renal arteries affect the efficacy of renal denervation? JACC Cardiovasc Interv. 2013;6(10):1085–1091. doi:10.1016/j.jcin.2013.06.007
56. Mahfoud F, Lüscher TF, Andersson B, et al. Expert consensus document from the European Society of Cardiology on catheter-based renal denervation†. Eur Heart J. 2013;34(28):2149–2157. doi:10.1093/eurheartj/eht154
57. Grassi G, Pisano A, Bolignano D, et al. Sympathetic nerve traffic activation in essential hypertension and its correlates: systematic reviews and meta-analyses. Hypertension. 2018;72(2):483–491. doi:10.1161/HYPERTENSIONAHA.118.11038
58. Lohmeier TE, Iliescu R. Lowering of blood pressure by chronic suppression of central sympathetic outflow: insight from prolonged baroreflex activation. J Appl Physiol. 2012;113(10):1652–1658. doi:10.1152/japplphysiol.00552.2012
59. Mahfoud F, Townsend RR, Kandzari DE, et al. Changes in plasma renin activity after renal artery sympathetic denervation. J Am Coll Cardiol. 2021;77(23):2909–2919. doi:10.1016/j.jacc.2021.04.044
60. Esler M, Jennings G, Korner P, et al. Assessment of human sympathetic nervous system activity from measurements of norepinephrine turnover. Hypertension. 1988;11(1):3–20. doi:10.1161/01.HYP.11.1.3
61. Undrum Bergland O, Larstorp ACK, Lund Soraas C, et al. Changes in sympathetic nervous system activity after renal denervation: results from the randomised Oslo RDN study. Blood Press. 2021;30(3):154–164. doi:10.1080/08037051.2020.1868286
62. Lohmeier TE, Iliescu R. The baroreflex as a long-term controller of arterial pressure. Physiology. 2015;30(2):148–158. doi:10.1152/physiol.00035.2014
63. Xu Y, Xiao P, Fan J, et al. Blood pressure elevation response to radiofrequency energy delivery: one novel predictive marker to long-term success of renal denervation. J Hypertens. 2018;36(12):2460–2470. doi:10.1097/HJH.0000000000001839
64. de Jong MR, Adiyaman A, Gal P, et al. Renal nerve stimulation-induced blood pressure changes predict ambulatory blood pressure response after renal denervation. Hypertension. 2016;68(3):707–714. doi:10.1161/HYPERTENSIONAHA.116.07492
65. Zweiker D, Lambert T, Steinwender C, et al. Blood pressure changes after renal denervation are more pronounced in women and nondiabetic patients: findings from the Austrian Transcatheter Renal Denervation Registry. J Hypertens. 2019;37(11):2290–2297. doi:10.1097/HJH.0000000000002190
66. Persu A, Azizi M, Jin Y, et al. Hyperresponders vs. nonresponder patients after renal denervation: do they differ? J Hypertens. 2014;32(12):
67. Sata Y, Hering D, Head GA, et al. Ambulatory arterial stiffness index as a predictor of blood pressure response to renal denervation. J Hypertens. 2018;36(6):1414–1422. doi:10.1097/HJH.0000000000001682
68. Okon T, Rohnert K, Stiermaier T, et al. Invasive aortic pulse wave velocity as a marker for arterial stiffness predicts outcome of renal sympathetic denervation. EuroIntervention. 2016;12(5):e684–692. doi:10.4244/EIJV12I5A110
69. Mahfoud F, Bakris G, Bhatt DL, et al. Reduced blood pressure-lowering effect of catheter-based renal denervation in patients with isolated systolic hypertension: data from SYMPLICITY HTN-3 and the Global SYMPLICITY Registry. Eur Heart J. 2017;38(2):93–100. doi:10.1093/eurheartj/ehw325
70. Baroni M, Nava S, Giupponi L, et al. Effects of renal sympathetic denervation on arterial stiffness and blood pressure control in resistant hypertensive patients: a Single Centre Prospective Study. High Blood Press Cardiovasc Prev. 2015;22(4):411–416. doi:10.1007/s40292-015-0121-4
71. Steinmetz M, Nelles D, Weisser-Thomas J, Schaefer C, Nickenig G, Werner N. Flow-mediated dilation, nitroglycerin-mediated dilation and their ratio predict successful renal denervation in mild resistant hypertension. Clin Res Cardiol. 2018;107(7):611–615. doi:10.1007/s00392-018-1236-0
72. Böhm M, Tsioufis K, Kandzari david E, et al. Effect of heart rate on the outcome of renal denervation in patients with uncontrolled hypertension. J Am Coll Cardiol. 2021;78(10):1028–1038. doi:10.1016/j.jacc.2021.06.044
73. Kordalis A, Tsiachris D, Pietri P, Tsioufis C, Stefanadis C. Regression of organ damage following renal denervation in resistant hypertension: a meta-analysis. J Hypertens. 2018;36(8):1614–1621. doi:10.1097/HJH.0000000000001798
74. Pisano A, Iannone LF, Leo A, Russo E, Coppolino G, Bolignano D. Renal denervation for resistant hypertension. Cochrane Database Syst Rev. 2021;11:CD011499. doi:10.1002/14651858.CD011499.pub3
75. Li S, Hildreth CM, Rahman AA, et al. Renal denervation does not affect hypertension or the renin-angiotensin system in a rodent model of juvenile-onset polycystic kidney disease: clinical implications. Sci Rep. 2021;11(1):14286. doi:10.1038/s41598-021-93575-0
76. Jacob F, Ariza P, Osborn JW. Renal denervation chronically lowers arterial pressure independent of dietary sodium intake in normal rats. Am J Physiol Heart Circ Physiol. 2003;284(6):H2302–H2310. doi:10.1152/ajpheart.01029.2002
77. King AJ, Osborn JW, Fink GD. Splanchnic circulation is a critical neural target in angiotensin II salt hypertension in rats. Hypertension. 2007;50(3):547–556. doi:10.1161/HYPERTENSIONAHA.107.090696
78. Wang M, Han W, Zhang M, et al. Long-term renal sympathetic denervation ameliorates renal fibrosis and delays the onset of hypertension in spontaneously hypertensive rats. Am J Transl Res. 2018;10(12):4042–4053.
79. Kline RL, Kelton PM, Mercer PF. Effect of renal denervation on the development of hypertension in spontaneously hypertensive rats. Can J Physiol Pharmacol. 1978;56(5):818–822. doi:10.1139/y78-128
80. Winternitz SR, Katholi RE, Oparil S. Role of the renal sympathetic nerves in the development and maintenance of hypertension in the spontaneously hypertensive rat. J Clin Invest. 1980;66(5):971–978. doi:10.1172/JCI109966
81. Iliescu R, Yanes LL, Bell W, Dwyer T, Baltatu OC, Reckelhoff JF. Role of the renal nerves in blood pressure in male and female SHR. Am J Physiol Regul Integr Comp Physiol. 2006;290(2):R341–R344. doi:10.1152/ajpregu.00035.2005
82. Gueguen C, Jackson KL, Marques FZ, et al. Renal nerves contribute to hypertension in Schlager BPH/2J mice. Hypertens Res. 2019;42(3):306–318. doi:10.1038/s41440-018-0147-9
83. Asirvatham-Jeyaraj N, Gauthier MM, Banek CT, et al. Renal denervation and celiac ganglionectomy decrease mean arterial pressure similarly in genetically hypertensive schlager (BPH/2J) mice. Hypertension. 2021;77(2):519–528. doi:10.1161/HYPERTENSIONAHA.119.14069
84. Maranon R, Lima R, Spradley FT, et al. Roles for the sympathetic nervous system, renal nerves, and CNS melanocortin-4 receptor in the elevated blood pressure in hyperandrogenemic female rats. Am J Physiol Regul Integr Comp Physiol. 2015;308(8):R708–R713. doi:10.1152/ajpregu.00411.2014
85. Zhang Z, Yang K, Zeng L, et al. Renal simplicity denervation reduces blood pressure and renal injuries in an obesity-induced hypertension dog model. Clin Exp Pharmacol Physiol. 2017;44(12):1213–1223. doi:10.1111/1440-1681.12661
86. Nishi EE, Lopes NR, Gomes GN, et al. Renal denervation reduces sympathetic overactivation, brain oxidative stress, and renal injury in rats with renovascular hypertension independent of its effects on reducing blood pressure. Hypertens Res. 2019;42(5):628–640. doi:10.1038/s41440-018-0171-9
87. Lincevicius GS, Shimoura CG, Nishi EE, et al. Differential effects of renal denervation on arterial baroreceptor function in Goldblatt hypertension model. Auton Neurosci. 2017;208:43–50. doi:10.1016/j.autneu.2017.06.002
88. Katholi RE, Whitlow PL, Winternitz SR, Oparil S. Importance of the renal nerves in established two-kidney, one clip Goldblatt hypertension. Hypertension. 1982;4(3 Pt 2):166–174.
89. Janssen BJ, van Essen H, Vervoort-Peters LH, Struyker-Boudier HA, Smits JF. Role of afferent renal nerves in spontaneous hypertension in rats. Hypertension. 1989;13(4):327–333. doi:10.1161/01.HYP.13.4.327
90. Banek CT, Knuepfer MM, Foss JD, et al. Resting afferent renal nerve discharge and renal inflammation: elucidating the role of afferent and efferent renal nerves in deoxycorticosterone acetate salt hypertension. Hypertension. 2016;68(6):1415–1423. doi:10.1161/HYPERTENSIONAHA.116.07850
91. Kandlikar SS, Fink GD. Mild DOCA-salt hypertension: sympathetic system and role of renal nerves. Am J Physiol Heart Circ Physiol. 2011;300(5):H1781–H1787. doi:10.1152/ajpheart.00972.2010
92. Hendel MD, Collister JP. Renal denervation attenuates long-term hypertensive effects of angiotensin II in the rat. Clin Exp Pharmacol Physiol. 2006;33(12):1225–1230. doi:10.1111/j.1440-1681.2006.04514.x
93. Tudorancea I, Lohmeier TE, Alexander BT, Pieptu D, Serban DN, Iliescu R. Reduced renal mass, salt-sensitive hypertension is resistant to renal denervation. Front Physiol. 2018;9. doi:10.3389/fphys.2018.00455
94. Lohmeier TE, Liu B, Hildebrandt DA, Cates AW, Georgakopoulos D, Irwin ED. Global- and renal-specific sympathoinhibition in aldosterone hypertension. Hypertension. 2015;65(6):1223–1230. doi:10.1161/HYPERTENSIONAHA.115.05155
95. Veiga GL, Nishi EE, Estrela HF, et al. Total renal denervation reduces sympathoexcitation to different target organs in a model of chronic kidney disease. Auton Neurosci. 2017;204:81–87. doi:10.1016/j.autneu.2016.11.006
96. Chen HH, Cheng PW, Ho WY, et al. Renal denervation improves the baroreflex and GABA system in chronic kidney disease-induced hypertension. Sci Rep. 2016;6(1):38447. doi:10.1038/srep38447
97. Campese VM, Kogosov E. Renal afferent denervation prevents hypertension in rats with chronic renal failure. Hypertension. 1995;25(4 Pt 2):878–882. doi:10.1161/01.HYP.25.4.878
98. Hamar P, Kokeny G, Liptak P, et al. The combination of ACE inhibition plus sympathetic denervation is superior to ACE inhibitor monotherapy in the rat renal ablation model. Nephron Exp Nephrol. 2007;105(4):e124–e136. doi:10.1159/000100494
99. Nagasu H, Satoh M, Kuwabara A, et al. Renal denervation reduces glomerular injury by suppressing NAD(P)H oxidase activity in Dahl salt-sensitive rats. Nephrol Dial Transplant. 2010;25(9):2889–2898. doi:10.1093/ndt/gfq139
100. Nishimura M, Takahashi H, Yoshimura M. Upregulation of the brain renin-angiotensin system in rats with chronic renal failure. Acta Physiol. 2007;189(4):369–377. doi:10.1111/j.1748-1716.2006.01663.x
101. Singh RR, McArdle ZM, Iudica M, et al. Sustained decrease in blood pressure and reduced anatomical and functional reinnervation of renal nerves in hypertensive sheep 30 months after catheter-based renal denervation. Hypertension. 2019;73(3):718–727. doi:10.1161/HYPERTENSIONAHA.118.12250
102. Sata Y, Burke SL, Gueguen C, et al. Contribution of the renal nerves to hypertension in a rabbit model of chronic kidney disease. Hypertension. 2020;76(5):1470–1479. doi:10.1161/HYPERTENSIONAHA.120.15769
103. Peleli M, Flacker P, Zhuge Z, et al. Renal denervation attenuates hypertension and renal dysfunction in a model of cardiovascular and renal disease, which is associated with reduced NADPH and xanthine oxidase activity. Redox Biol. 2017;13:522–527. doi:10.1016/j.redox.2017.06.013
104. Gattone VH
105. Lubanda JC, Chochola M, Mlcek M, et al. The effect of renal denervation in an experimental model of chronic renal insufficiency, The REmnant kidney Denervation In Pigs study (REDIP study). J Transl Med. 2017;15(1):215. doi:10.1186/s12967-017-1319-0
106. iStock by Getty Images [homepage on the Internet]. Vector kidneys illustration stock illustration; 2015 [updated February 25, 2015]. Available from: https://www.istockphoto.com/vector/vector-kidneys-illustration-gm464688692-58892822. Accessed May 10, 2022.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.