Back to Journals » Patient Preference and Adherence » Volume 13
Patient satisfaction and acceptability of an on-demand and on-prophylaxis device for factor VIII delivery in patients with hemophilia A
Authors Di Minno G, Santagostino E, Morfini M , Ettorre C, Cultrera D, Baldacci E , Russo E , Gallucci C
Received 24 May 2018
Accepted for publication 9 October 2018
Published 31 January 2019 Volume 2019:13 Pages 233—240
DOI https://doi.org/10.2147/PPA.S175254
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Johnny Chen
Giovanni Di Minno,1 Elena Santagostino,2 Massimo Morfini,3 Cosimo Ettorre,4 Dorina Cultrera,5 Erminia Baldacci,6 Eleonora Russo,7 Cristiano Gallucci7
On behalf of the Italian FusENGO B1831081 Study Group
1Department of Clinical Medicine and Surgery, Azienda Universitaria Policlinico Federico II, Naples, Italy; 2Hemophilia and Thrombosis Center, IRCCS Fondazione Ca’ Granda, Ospedale Maggiore Policlinico, Milan, Italy; 3Scientific Committee, Italian Association of Haemophilia Centres (AICE), Florence, Italy; 4Haemophilia and Thrombosis Center, Policlinico Giovanni XXIII, Bari, Italy; 5Hematology Unit, Regional Center for Hemophilia, Italy Ospedaliera-Universitaria “Policlinico – Vittorio Emanuele”, Catania, Italy; 6Hemophilia, Thrombosis, and Hematology Center, Dipartimento Biotecnologie cellulari ed Ematologia, Università la Sapienza, Rome, Italy; 7Medical Department, Pfizer srl Rome, Italy
Background: FuseNGO is a relatively new device consisting of a prefilled dual-chamber syringe (DCS) that was recently introduced for the reconstitution of recombinant factor VIII. Herein, the DCS device was assessed using five questionnaires with the primary aim of evaluating patient perceptions and preferences.
Methods: An observational, non-interventional, longitudinal study on 86 patients with a confirmed diagnosis of hemophilia A was carried out at 21 sites in Italy. Each patient underwent a baseline visit and final study visit within 3–6 months. Patients were administered five questionnaires: HemoPREF; Treatment Satisfaction Questionnaire for Medication (TSQM); VeritasPRO; Hemophilia Well-being Index (HWBI); Work Productivity and Activity Impairment Questionnaire (WPAI) + Classroom Impairment Questions (CIQ): Hemophilia Specific (HS).
Results: Compared to baseline, scores for HemoPREF were higher at follow-up; significant increases in the percentage of positive responses were seen for all questions regarding the ease of use (P<0.05). The mean time needed for the reconstruction of the device at baseline was 11 minutes (range 1–30 minutes), which decreased to 6 minutes (range 30 seconds to 25 minutes) at follow-up. All scores in the TSQM indicated good satisfaction with the device. Patients reported an adherence of >70% in the VeritasPRO questionnaire, and the majority of patients reported in the HWBI that hemophilia A did not affect their lives in a significant way. The perceived level of overall impairment was 30% as reported in the WPAI + CIQ: HS, indicating little impairment. There were no safety concerns.
Conclusion: Considering patient-reported outcomes, the DCS device was associated with easier preparation, storage, disposal of equipment, and overall use. Of particular note, preparation times were reduced by around 50%. The majority of patients were satisfied with the device and overall adherence scores were high. Considering these results, the device has the potential to increase adherence to therapy and, possibly, reduce healthcare costs.
Keywords: factor VIII delivery, device, hemophilia A, patient satisfaction, adherence
Introduction
Hemophilia A poses a significant lifetime burden on the affected patients not only in terms of quality of life and social consequences but also due to increased utilization of healthcare resources.1 Recurrent bleeding into joints is one of the most severe consequences of hemophilia as it reduces movement and causes both chronic pain and stiffness.2 Survey data have shown that in adult patients the quality of life is worse than that in child sufferers, and >75% of adult patients indicate physical problems.3 Moreover, 43% of adult patients refer to problems of anxiety.3 The same survey, carried out in Italy, reported that the estimated mean annual total cost per patient in 2012 was €117,732, with drugs representing 92% of total costs.3 In the US, among younger adults (19–44 years), hemophilia-related non-pharmacy costs were lower for patients receiving prophylaxis ($22,028 vs $56,311, respectively).4
The use of prophylaxis in severe hemophilia patients is associated with significant reduction in emergency department visits and bleeding episodes compared with those who were treated episodically.5 Accordingly, the standard management of hemophilia A involves prophylaxis with infusion of coagulation factor VIII (FVIII).6 Indeed, one of the primary goals of prophylactic therapy is to prevent bleeding episodes and the subsequent development of chronic arthropathy.2 As with all chronic conditions, adherence to prophylactic treatment of hemophilia A is often problematic.7 Recent evidence indicates that adherence to prophylaxis for hemophilia A varies from 44% to 87%, even in developed countries.8–12 As such, it is obvious that there is ample room for improving adherence to therapy across different treatment settings.
In recent years, several new treatment strategies have emerged with the overall aim of reducing the burden associated with the administration and improving patient perceptions of the treatment; in turn, such innovations should increase the levels of adherence to therapy. One such approach includes increasing the half-life of FVIII in order to reduce the frequency of administration13 and several devices, to facilitate its reconstitution and administration, have been developed.14 Regarding the use of the devices, for some devices, the patient or caregiver is required to reconstitute the product which is supplied as a lyophilized powder and diluent, and may require multiple steps. This is relevant as some of the barriers to adherence are specifically related to the reconstitution step, perceived pain related to infusion and time needed for preparation.15,16 Thus, a reconstitution device that is quick and easy to use has the potential to increase adherence to therapy, and as a consequence, may also improve the overall quality of life of patients while reducing the treatment-associated burden and associated costs of treatment. Importantly, another way of improving patient adherence involves switching from on-demand to prophylactic treatment, which has been identified as a likely driver in improving health-related quality of life in patients with hemophilia A.17,18 Pharmacokinetic-tailored prophylaxis thus offers an alternative to standard prophylaxis for the prevention of bleeding, with similar efficacy and safety as a standard protocol for management of bleeding.18
Among various devices, the FuseNGO is a relatively new device consisting of a prefilled dual-chamber syringe (DCS; FuseNGo®; Pfizer Ltd., Sandwich, Kent, UK) recently introduced for the reconstitution of recombinant FVIII. The DCS integrates both lyophilized powder and diluent in a single syringe. The DCS was developed with the specific aim of rendering the treatment less burdensome, and therefore, increase adherence to the prescribed treatment regimen. The device has been previously evaluated in two patient-based surveys, the first in an ad hoc survey of 299 patients in five European countries14 and the second using the specifically developed HemoPREF questionnaire for patient-related outcomes.19
In the present investigation, we have extended these previous studies of the DCS device by using five questionnaires, with the primary aim of evaluating patient perceptions and preferences of the delivery system for FVIII reconstitution. Secondary objectives included analysis of the quality of life, adherence to therapy, work productivity, activities of daily living, and global impressions of severity recorded by patients following the use of the DCS delivery system compared to reconstitution devices routinely used by the same patients.
Materials and methods
Study design
This was an observational, non-interventional, longitudinal study of patients with hemophilia A, carried out at 21 sites in Italy. Patients enrolled in the study were required to complete at least ten exposure days with the DCS device. No medical interventions or invasive procedures outside of the standard care for these patients were required by the study protocol.
After enrollment, each patient underwent two visits. A baseline visit (V1) and a final visit after 3–6 months (V2). The timing of follow-up assessments depended on the local therapeutic plan as decided by their physician.
Five questionnaires were administered to patients. These included:
- HemoPREF: A 14-item instrument measuring the experience of clotting-factor treatment including ease of use, burden, impact of treatment, treatment related risk and influence on others.19
- Treatment Satisfaction Questionnaire for Medication (TSQM): A 11-item measure of treatment satisfaction including side effects, effectiveness, convenience and a global satisfaction item.20
- VeritasPRO: A 24-item measure of treatment adherence, measuring timing of administration, dose, planning, remembering, skipping and communication.21
- Hemophilia Well-being Index (HWBI): An 8-item measure of well-being specific to hemophilia.22
- Work Productivity and Activity Impairment Questionnaire (WPAI) + Classroom Impairment Questions (CIQ): Hemophilia Specific (HS): A 9-item measure of productivity, ability to work, and daily activities.23
The HemoPREF questionnaire was administered at V1 and V2, and the others were administered at the final study visit (V2).
Eligible patients (aged 18–65) were male, had a clinician-confirmed diagnosis of hemophilia A, and were currently under treatment with FVIII, either prophylactically or on-demand. To be included in the study, patients also had to be advised, for any reason, to switch to the new factor VIII delivery device by their physician; alternatively, they could have requested to be treated with the new factor VIII delivery device. Patients also had to demonstrate an understanding of the study and willingness to take part in it. No constraints were placed on the type of delivery device used earlier, in order to be considered for inclusion. All patients who enrolled signed an informed consent form and gave consent for handling of personal data according to local regulations. Study participants were informed that they were free to withdraw from the study at any time. The study was performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments, and received ethical approval from the Ethics Committee of Federico II University in Naples, Italy. Main exclusion criteria included: anticipation that patients would not undergo at least 10 infusions in the 12 month period following inclusion in the study, not previously recommended by their physicians to switch to this FVIII delivery system or did not previously ask their physician to be switched to this FVIII delivery system, not willing to sign an informed consent form, did not demonstrate understanding of the study and willingness to take part in it, affected by concomitant pathologies or concomitant conditions that may impair adherence to study procedures according to clinician’s opinion.
Demographic and clinical data were collected during the baseline visit. The follow-up visit occurred after period of approximately 3–6 months (not exceeding 12 months, depending on their local therapeutic plans) during which all questionnaires were completed. Patients were free to withdraw from the study at any time.
Statistical analyses
All study parameters have been described using descriptive statistics (mean, SE, SD, min, max, 95% CI). Chi-squared test or Fisher’s Exact test have been used for comparing proportions. Cronbach’s Alpha or the Spearman-Brown estimate have been used for internal consistency tests. Parametric tests or non-parametric tests, depending on the type of data, were used for comparing groups or visits. P<0.05 was considered significant. All statistical analyses were carried out using IBM SPSS 22 for Windows. Since this was a non-interventional study, no hypothesis testing was performed and conventional sample sizing was not required. However, 120 subjects were estimated based on quantification of the Ease of Use proportion to indicate preferences using the standard Wald 95% CI with no continuity correction. Unfortunately, the sample size could not be reached during the stipulated timeframe of the study, so the results of the inference analyses should be taken with caution.
Safety analysis
Safety analysis was performed on all subjects receiving at least one dose from the device. Safety outcomes were considered as secondary endpoints.
Results
Patients were recruited at 21 centers in Italy. A total of 86 male patients were enrolled; 84 patients completed the study and were included in the analysis. All patients were Caucasian, except one of Hispanic origin. Patient demographic data are shown in Table 1. The mean age of participants was 37.7 years. Most subjects were employed or self-employed, and the majority had a high school education. The vast majority of subjects were affected with severe hemophilia and most were on a prophylactic treatment regimen.
  | Table 1 Demographic data of subjects (N=84) |
HemoPREF
The HemoPREF questionnaire is divided into five major areas. Patients were asked to provide positive or negative responses to the different questions considering their current device at baseline and after using the DCS device at the follow-up visit. Compared to baseline, higher scores were reported, considering the global score and each of the five areas, at the follow-up visit after using the DCS device (Figure 1). Scores for each of the four questions for the first area on ease of preparation, storage, disposal of needles and syringes, and use of treatment are shown in Figure 2. Significant increases in the percentage of positive responses were seen for all four questions regarding ease of use (P<0.05 for all; Figure 2).
  | Figure 1 Percentage of positive responses on TSQM items related to effectiveness, convenience, and overall satisfaction. |
The mean time needed for reconstruction of the device at baseline was 11 minutes (range 1 minute to 30 minutes). At follow-up, the time of reconstruction was reduced to 6 minutes (range 30 seconds to 25 minutes).
TSQM
At the follow-up visit, all patients were asked to compile the TSQM questionnaire on the satisfaction with the DCS device. All scores were around 70% or higher, indicating good satisfaction. More than 95% of the patients who compiled the items relative to side effects were also very satisfied. Responses related to the effectiveness, convenience and overall satisfaction of the device are shown in Figure 3. The scores reported for these subareas approached or exceed 90% in all cases, and 98.8% of positive responses were seen for both questions related to overall satisfaction (Figure 3).
VeritasPRO
The VeritasPRO questionnaire is composed of 24 items. Since the questionnaire was developed for prophylactic regimens, only patients currently treated in a prophylactic regimen were included in this assessment. The percentage of patients with positive responses (“always” or “often”) exceeded 70% for all questions (not shown). Generally, patients reported an adherence of >70%.
HWBI
At follow-up, patients compiled the 8-item HWBI questionnaire. For all questions except the one on health/well-being (49% of positive responses), 65% or more of patients reported that hemophilia A did not affect their lives in a significant way (positive response as “a little affected” and “not at all affected”) (not shown).
WPAI + CIQ: HS
The 9-item WPAI + CIQ: HS questionnaire measures productivity, ability to work and daily activities. At follow-up, work impairment due to hemophilia was rated at 22% on a scale of 0% (no impairment) to 100% (complete impairment). The perceived level of overall impairment was 30% (not shown). There were no significant differences between patients in a prophylaxis regimen and patients in an on-demand regimen.
Safety
Adverse events reported during the study are listed in Table 2. There were no serious adverse events, and all were mild to moderate in severity. None of the adverse events was considered to be correlated with the study device, and none required a change in treatment.
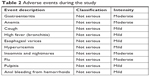  | Table 2 Adverse events during the study |
Discussion
Herein, using patient-reported outcomes, a battery of five questionnaires was used to assess perceptions of a new DCS device. With the HemoPREF questionnaire, the scores querying the ease of preparation, storage, disposal of needles and syringes and use of treatment improved significantly with the new device vs that previously in use. Moreover, the time needed for reconstruction was, on average, reduced by almost one half. The TSQM questionnaire on satisfaction with the device revealed that >90% of patients were pleased with it, considering its effectiveness, convenience, and overall satisfaction. Positive responses were also seen on the VeritasPRO questionnaire, which confirmed that the overall adherence with the device could be considered high. The HWBI questionnaire on general well-being showed that for the majority of patients, hemophilia did not affect their lives in a significant way, while the WPAI + CIQ: HS questionnaire, used to measure productivity, ability to work, and daily activities, showed that patients had a low perceived overall impairment due to hemophilia. Lastly, from a safety point of view, there were no severe adverse events and the few minor/moderate adverse events were not considered to be related to the device. Thus, the device can be considered to be a significant improvement over the one previously in use and was well accepted with an excellent safety profile.
These results confirm those of a previous cross-sectional survey with the same device, which, when compared to the previous one used, was considered to be the one requiring the least equipment and the fewest reconstitution steps.14 In that study, 57% preferred the new device, 26% preferred their current device, and 17% had no preference for either. However, the DCS was rated as easier to use than the device used previously. A recent cross-sectional survey in 74 patients also confirmed that a DCS device was preferred over usual devices, even in practical and direct testing sessions. A DCS device was most likely to be used in prophylactic treatment and was associated with preparation times that were less than one-fourth that of traditional devices.24
In a more recent analysis, the HemoPREF and other questionnaires were used to evaluate the DCS device, which largely confirmed the results seen herein, namely, that patients were satisfied with the device overall and were adherent to treatment with it.19 These findings are relevant as patient perceptions and treatment preferences may act as a barrier to prophylactic treatment adherence in patients with hemophilia A.8,15,16,25 Thus, efforts to improve patient satisfaction with any device are likely to improve adherence to treatment. The HemoPREF specifically assesses key factors such as ease and burden of treatment as well as the risk of treatment and its impact on others and scores using this questionnaire were significantly higher with the DCS device compared to that previously used. Moreover, adherence to treatment also scored high with the new device as measured with the VeritasPRO questionnaire.21
This implies that reducing the burden of treatment and its administration may improve patient perceptions. This concept may apply not only to upgraded reconstitution devices but also to other treatment strategies such as less frequent administration, which warrant further investigation.7,13 In addition, the patient cohort predominantly had a severe level of disease and was mainly on a prophylactic regimen. Both these aspects hinder subgroup analyses due to the small size of the cohort. Notwithstanding all these factors, considering the present and previous studies with the device, assessment of patient-reported outcomes appears to favor the device over that used previously and warrants further investigation in a larger and more diverse patient cohort. It should also be highlighted that there were no safety concerns with the new device.
Patient satisfaction with facilitating devices, such as this DCS device, may contribute to increased adherence to therapy. Importantly, its use was also associated with almost 50% reduction in preparation time, an aspect which should be appreciated by patients. Adherence and satisfaction are especially important in a disease such as hemophilia as it is associated with high burden on the quality of life and substantial economic impact. Based on multiple psychometric questionnaires, the DCS device appears to have a favorable influence on multiple parameters including patient perceptions and preferences for treatment as well as overall satisfaction, adherence, and work productivity.
Lastly, this study has several limitations. Firstly, according to the approved protocol, not all study questionnaires were administered at baseline and thus it was not possible to compare all parameters evaluated with the former and new DCS devices. No attempt was made to compare parameters such as adherence with the prior and new DCS device. Secondly, the follow-up time of 3–6 months may be considered relatively short, as some parameters such as adherence may tend to decrease after a period of initial use. While follow-up times were kept short due to the pilot nature of the study, longer observational times would be warranted to understand more in depth the benefits of the device. Lastly, there is a potential bias due to the non-masked study design as patients were aware that they were using a new device. However, it is unclear what bias this may actually represent in daily use.
Conclusion
Using patient-reported outcomes, the new DCS device for FVIII delivery was found to be associated with easier preparation, storage, disposal of equipment and overall use. Preparation times were reduced by about one-half compared to the previous device. The vast majority of patients were satisfied with the device, and overall adherence to it was high. Its use also reduced the overall burden of hemophilia on productivity, daily activities, and work, and was associated with an excellent safety profile. Given its positive patient-rated evaluation, the device has the potential to increase adherence to therapy and reduce healthcare costs and, thus, warrants further investigation in a broad patient population to assess its benefits in routine settings.
Acknowledgments
This non-interventional clinical trial was sponsored by Pfizer srl. The authors wish to thank 4Pharma CRO srl for their contribution in statistical analysis and Cromnia for ensuring study implementation and monitoring. Medical writing support was provided by P Moore on behalf of Health Publishing & Service srl and was funded by Pfizer srl.
The authors also wish to thank the clinical sites belonging to the Italian FusENGO B1831081 Study Group for their contribution to the study: Divisione di Ematologia Centro Emofilia e Trombosi Policlinico Universitario Palermo (Prof S Siragusa), Azienda Ospedaliera di Padova, Padua (Prof E Zanon), Centro Emofilia Azienda Ospedaliera Careggi, Firenze (Dr S Linari), Servizio Malattie Emorragiche e Trombosi Policlinico Universitario Gemelli Roma (Prof R De Cristofaro), Centro Emofilia, Istituto Clinica Pediatrica, Cagliari (Dr AB Aru), Azienda Ospedaliera “Pugliese-Ciaccio”, Catanzaro (Dr RC Santoro), Centro Emofilia – Servizio Trasfusionale, Ospedale Infantile Regina Margherita, Turin (Dr M Messina), Divisione di Ematologia, Ospedale Molinette, Turin (Dr P Schinco), Centro Emofilia, Istituto Immuno Trasfusionale, Az. Ospedaliera S. Maria della Misericordia, Udine (Dr V De Angelis), Servizio di Immunoematologia e Trasfusione, Azienda Ospedaliera di Verona, Verona (Dr A C Giuffrida), Centro Trasfusionale, Ospedale Civile, Macerata (Dr I Cantori), Centro Emofilia e Trombosi, Ospedale S. Giovanni Bosco, Naples (Dr A Rocino), Centro Emofilia e Trombosi, Servizio Immuno-Trasfusionale, Ospedale SS Annunziata – USL 1, Sassari (Dr L A Mameli), Centro Emostasi e Trombosi, U.O. Medicina Interna I, Arcispedale Santa Maria Nuova, Reggio Emilia (Dr A M Pizzini), Centro Emofilia Perugia. Ospedale Santa Maria della Misericordia. Medicina Interna e Vascolare (Dr P Gresele), and Centro di Riferimento Regionale per le Malattie Emorragiche Istituto G. Gaslini di Genova (Dr A C Molinari).
Disclosure
Eleonora Russo and Cristiano Gallucci are employees of Pfizer srl, Italy. The authors report no other conflicts of interest in this work.
References
Henrard S, Devleesschauwer B, Beutels P, et al. The health and economic burden of haemophilia in Belgium: a rare, expensive and challenging disease. Orphanet J Rare Dis. 2014;9:39. | ||
Thornburg CD. Prophylactic factor infusions for patients with hemophilia: challenges with treatment adherence. J Coag Disord. 2010;2:9–14. | ||
Kodra Y, Cavazza M, Schieppati A, et al. The social burden and quality of life of patients with haemophilia in Italy. Blood Transfus. 2014;12(Suppl 3):s567–s575. | ||
Shrestha A, Eldar-Lissai A, Hou N, Lakdawalla DN, Batt K. Real-world resource use and costs of haemophilia A-related bleeding. Haemophilia. 2017;23(4):e267–e275. | ||
Zhou ZY, Koerper MA, Johnson KA, et al. Burden of illness: direct and indirect costs among persons with hemophilia A in the United States. J Med Econ. 2015;18(6):457–465. | ||
World Federation of Hemophilia. Guidelines for the management of haemophilia. World Federation of Hemophilia; 2012. Available from: www1.wfh.org/publications/files/pdf-1472.pdf. Accessed December 6, 2018. | ||
Khair K, Gibson F, Meerabeau L. The benefits of prophylaxis: views of adolescents with severe haemophilia. Haemophilia. 2012;18(3):e286–e289. | ||
Llewellyn CD, Miners AH, Lee CA, Harrington C, Weinman J. The illness perceptions and treatment beliefs of individuals with severe haemophilia and their role in adherence to home treatment. Psychol Health. 2003;18(2):185–200. | ||
Schrijvers LH, Beijlevelt-van der Zande M, Peters M, et al. Adherence to prophylaxis and bleeding outcome in haemophilia: a multicentre study. Br J Haematol. 2016;174(3):454–460. | ||
Schrijvers LH, Kars MC, Beijlevelt-van der Zande M, Peters M, Schuurmans MJ, Fischer K. Unravelling adherence to prophylaxis in haemophilia: a patients’ perspective. Haemophilia. 2015;21(5):612–621. | ||
Thornburg CD, Carpenter S, Zappa S, Munn J, Leissinger C. Current prescription of prophylactic factor infusions and perceived adherence for children and adolescents with haemophilia: a survey of haemophilia healthcare professionals in the United States. Haemophilia. 2012;18(4):568–574. | ||
Witkop ML, Mclaughlin JM, Anderson TL, Munn JE, Lambing A, Tortella B. Predictors of non-adherence to prescribed prophylactic clotting-factor treatment regimens among adolescent and young adults with a bleeding disorder. Haemophilia. 2016;22(4):e245–e250. | ||
Tiede A. Half-life extended factor VIII for the treatment of hemophilia A. J Thromb Haemost. 2015;13(Suppl 1):S176–S179. | ||
Cimino E, Linari S, Malerba M, Halimeh S, Biondo F, Westfeld M. Patient preference and ease of use for different coagulation factor VIII reconstitution device scenarios: a cross-sectional survey in five European countries. Patient Prefer Adherence. 2014;8:1713–1720. | ||
Remor E. Predictors of treatment difficulties and satisfaction with haemophilia therapy in adult patients. Haemophilia. 2011;17(5):no–905. | ||
Saxena K. Barriers and perceived limitations to early treatment of hemophilia. J Blood Med. 2013;4:49–56. | ||
Santagostino E, Lentz SR, Busk AK, Regnault A, Iorio A. Assessment of the impact of treatment on quality of life of patients with haemophilia A at different ages: insights from two clinical trials on turoctocog alfa. Haemophilia. 2014;20(4):527–534. | ||
Valentino LA, Mamonov V, Hellmann A, et al. A randomized comparison of two prophylaxis regimens and a paired comparison of on-demand and prophylaxis treatments in hemophilia A management. J Thromb Haemost. 2012;10(3):359–367. | ||
Bonanad S, Schulz M, Gordo A, et al. HaemoPREF: Further evaluation of patient perception and preference for treatment in a real world setting. Haemophilia. 2017;23(6):884–893. | ||
Atkinson MJ, Sinha A, Hass SL, et al. Validation of a general measure of treatment satisfaction, the Treatment Satisfaction Questionnaire for Medication (TSQM), using a national panel study of chronic disease. Health Qual Life Outcomes. 2004;2:12. | ||
Duncan N, Kronenberger W, Roberson C, Shapiro A. VERITAS-Pro: a new measure of adherence to prophylactic regimens in haemophilia. Haemophilia. 2010;16(2):247–255. | ||
Remor E. Development and psychometric testing of the Hemophilia Well-being Index. Int J Behav Med. 2013;20(4):609–617. | ||
Reilly Associates [webpage on the Internet]. Health Outcomes Research; 2002. Available from: http://www.reillyassociates.net/WPAI_Scoring.html. Accessed December 6, 2018. | ||
Fernández-Arias I, Kim HK. Factor VIII delivery devices in haemophilia A. Barriers and drivers for treatment adherence. Farm Hosp. 2016;40(n06):579–603. | ||
Hacker MR, Geraghty S, Manco-Johnson M. Barriers to compliance with prophylaxis therapy in haemophilia. Haemophilia. 2001;7(4):392–396. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


