Back to Journals » Patient Preference and Adherence » Volume 13
Patient preferences for glucagon-like peptide-1 receptor–agonist treatment attributes
Authors Thieu VT, Robinson S, Kennedy-Martin T, Boye KS, Garcia-Perez LE
Received 17 September 2018
Accepted for publication 1 March 2019
Published 17 April 2019 Volume 2019:13 Pages 561—576
DOI https://doi.org/10.2147/PPA.S187907
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Vivian T Thieu,1 Susan Robinson,2 Tessa Kennedy-Martin,2 Kristina S Boye,1 Luis-Emilio Garcia-Perez1
1Global Patient Outcomes and Real-World Evidence, Eli Lilly and Company, Indianapolis, IN, USA; 2Kennedy-Martin Health Outcomes (KMHO), Brighton, UK
Purpose: The importance of patient-centered care in the management of type 2 diabetes mellitus (T2DM) is widely advocated. Understanding the attributes of T2DM medications important to patients is thus essential for effective management, in order to limit disease progression. This literature review aimed to identify studies comparing patient preferences, based on process and outcome attributes, between GLP1-receptor agonist (RA) profiles and between GLP1 RA and insulin profiles.
Methods: MEDLINE, Embase, PsycINFO, and the Cochrane Library (2005–present) were searched for studies in patients with T2DM or the general population that compared preferences for GLP1 RAs or GLP1 RAs versus insulin using contingent valuation, conjoint analysis (discrete-choice experiments [DCEs], willingness to pay), rating-based approaches of specific attributes, standard gamble, or time trade-off. Studies comparing drug A versus drug B without explicit attribute valuation were excluded.
Results: Ten records met eligibility criteria. Eight studies compared preferences for GLP1 RA–profile attributes, one compared GLP1 RA versus insulin glargine profiles, and one addressed both comparisons. Important attributes driving patient preferences in DCEs were dose frequency, type of device, needle size, change in glycated hemoglobin, and adverse-event profile. Time trade-off evaluations demonstrated that weekly GLP1 RA injection-device attributes (reconstitution, waiting during preparation, needle handling) had a measurable impact on preference. Willingness-to-pay analysis showed that patients were more willing to pay extra for attributes of once-daily liraglutide over twice-weekly exenatide or insulin. Direct preference elicitation in DCEs revealed that patients preferred medication profiles representing GLP1 RAs with less frequent dosing and preferred GLP1 RA profiles over insulin.
Conclusion: Process and outcome attributes are important drivers of patient preference for GLP1 RAs. Findings from patient-preference studies can inform clinical decision-making and help align care with patient values, which has the potential to improve medication adherence and outcomes.
Keywords: T2D mellitus, discrete-choice experiment, GLP1 RA, insulin
Plain-language summary
A wide range of medications is available for type-2 diabetes mellitus (T2DM), each of which is associated with different benefits, risks, and burden to the patient. It is very important that patients are involved in treatment decisions, as this can influence how likely they are to continue taking a medication, which in turn can affect treatment success. GLP1 receptor agonists (RAs) are injectable medications that are effective in lowering blood glucose in patients with T2DM. Understanding which attributes of GLP1 RA treatment are most important to patients with respect to how the medication is taken and outcomes of treatment (eg, how effectively they lower blood glucose and any side effects) may help health care professionals and patients to decide on the best medication for an individual. This review article identifies and describes studies that have used stated-preference research methods to determine patient preferences for treatment attributes associated with GLP1 RAs. No single aspect of GLP1 RA treatment was found to be most important to patients with T2DM. However, patterns emerged that suggested patients favored GLP1 RA profiles that were associated with less frequent dosing (eg, once weekly versus once daily), were more effective at reducing blood glucose, and had fewer side effects, such as nausea, hypoglycemia (low blood sugar), or weight gain. In addition, several studies reported that the more injection inconvenience associated with a GLP1 RA profile (eg, mixing, waiting for the injection to be ready, and needle handling), the lower the patient preference.
Introduction
Type 2 diabetes mellitus (T2DM) is a chronic and progressive disease, which if poorly controlled can result in serious health risks.1 Given its chronic nature and the potential severity of complications, the important role of patient-centeredness in the care of individuals with T2DM is now well accepted.2 Recent consensus recommendations from the American Diabetes Association (ADA) and European Association for the Study of Diabetes (EASD) highlight the importance of providing patient-centered care that respects individual patient preference and barriers, in order to manage T2DM effectively.3 Indeed, these recommendations note that patient preference is a “major factor driving the choice of medication.” Therefore, considering the specific factors that impact choice of treatment is one key aspect of patient-centered care and seeking patient preferences is integral to shared decision-making in T2DM management.3 The National Institute of Health and Care Excellence in the UK also states that in T2DM management, “treatment and care should take into account individual needs and preferences. Patients should have the opportunity to make informed decisions about their care and treatment, in partnership with their healthcare professionals.”4 T2DM is a highly preference-sensitive disease because of the number of available treatment options, each of which is associated with different benefits, risks, and burdens.2
Elicitation of patient preferences provides the bridge toward individualized, patient-centered care, as recommended by professional bodies.3 A better understanding of patient preferences for T2DM therapy and the factors influencing these may potentially result in more appropriate treatment decisions for the individual,3 which in theory could improve patient satisfaction and medication adherence.5,6 For example, it has been demonstrated that patients with T2DM prefer less frequent daily dosing and that this may improve adherence to treatment with oral antihyperglycemic drugs.7 With better adherence may come improved glycemic control and better clinical and economic outcomes.8,9
Formal preference assessments allow the evaluation and quantification of the importance that patients place on specific treatment attributes of existing therapies, as well as assessing preferences for hypothetical products.6 Preference-elicitation methods can be divided into “revealed” or “stated” methods.10 Revealed preferences rely on observed data related to an individual’s actual behavior and indicate patient choices under clinical conditions, but provide little information on why they make these choices.6 Stated preferences are determined from surveys allowing experimental control over choice alternatives, making it possible to estimate the relative importance (RI) of different factors in the study design.6 Methods for stated-preference elicitation include contingent valuation and conjoint analysis. In contingent-valuation approaches, participants are offered a hypothetical treatment with specified features at a stated cost and asked whether they would pay that cost.6 Alternatively, ranking, rating, or choice designs are used in conjoint analysis to quantify preferences for various attributes of an intervention.10 Approaches include discrete-choice experiments (DCEs), in which participants are asked to select their preferred option from different sets of hypothetical medication choices containing various attributes, each described by a number of variations or levels.
Another preference method commonly used is health state-utility assessment, in which choice-based tasks can be completed by study participants to indicate preferences for their current health or descriptions of hypothetical health states.11 These methods include time trade-off (TTO) or standard-gamble evaluations, which yield utility values representing the strength of preferences for the valued health states. Quantification of the impact of treatment attributes on utility can be incorporated into cost-utility assessments that are used to inform decisions on health care-resource allocation.11,12
Patients with T2DM move through a treatment continuum to improve glycemic control as their disease progresses. Use of metformin and lifestyle modification are recommended as first-line therapy, while second-line dual therapy recommends the addition of a second oral agent or injectable agent, primarily a GLP1-receptor agonist (RA), as first injectable therapy.3,13 GLP1 RAs have consistently demonstrated good efficacy and tolerability in patients with T2DM, with minimal risk of hypoglycemia and modest weight loss.14 There are, however, important differences between available GLP1 RAs with respect to key attributes, such as dosing regimen and injection process.12,15 Given the importance of patient-focused care, the aim of this literature review was to identify and describe studies that have compared preferences based on treatment attributes between different GLP1 RA profiles or between GLP1 RA and insulin profiles, in patients with T2DM.
Methods
This literature review aimed to identify and describe studies that compared patient preferences based on treatment attributes between one GLP1 RA profile versus another or between a GLP1 RA profile versus insulin. GLP1 RAs and insulin were included because they represent the only two injectable therapies for T2DM. Attributes that characterize a health care intervention can be categorized as: process attributes, including those related to mode of administration, dose frequency, and waiting times; outcome attributes, including efficacy and adverse events (AEs); and cost attributes.16 Cost attributes of a given medication vary from country to country, and may complicate preference assessment when comparing across regions. This review aimed to evaluate patient preferences independently of varying cost influences, and so cost was not considered a treatment attribute in this search.
The review was conducted according to a robust and reproducible protocol that outlined the review focus with respect to study population, treatment type, and preference study design, and provided details of the search approach and data extraction. Development of this protocol minimized any author bias, ensured transparency and accountability, and increased the chances of correct data extraction.
Study-selection criteria
Studies were included if they were English-language journal articles published from 2005 (launch of the first GLP1 RA to the market) to April 2018 describing primary research in patients with T2DM or the general population that evaluated preferences for process and/or outcome attributes of GLP1 RAs and compared preferences for these attributes among GLP1 RAs (albiglutide, dulaglutide, exenatide, liraglutide, lixisenatide, and semaglutide), between GLP1 RAs and insulin fixed-ratio combinations (IDegLira and iGlarLixi), or between GLP1 RAs and insulin. The review included the stated-preference and utility-assessment methods outlined in a literature review by Stewart et al.11 At least one of contingent valuation, conjoint analysis (including DCE, willingness to pay [WTP], or MaxDiff), rating-based approaches (of specific attributes), standard gamble, or TTO had to have been used in the study.
Studies excluded were those that were not specifically on T2DM or the general population, that elicited preferences of a family member or caregiver, that used revealed preference methods, or that compared drug A versus drug B without explicit valuation of different process or outcome attributes. Reviews, discussion papers, letters, and editorials were also excluded, as were studies published before 2005 or congress abstracts published prior to the most recent meeting. All studies of relevance were included, and exclusions were not made based on any assessment of study quality.
Information sources
MEDLINE, Embase, PsycINFO, Cochrane Controlled Register of Trials, the Health Technology Assessment database, and the NHS Economic Evaluation Database were searched. To identify any relevant studies that perhaps were not yet fully published, abstract books of the following congresses were also searched: International Society for Pharmacoeconomics and Outcomes Research (ISPOR) international meeting 2017, European meeting 2017, Asia–Pacific meeting 2016, Latin-American meeting 2017, International Society for Quality of Life Research 2017, International Diabetes Federation 2017, European Association for the Study of Diabetes 2017, and ADA 2017.
Search strategy
The main structure of the draft strategy comprised a combination of two concepts: GLP1 RA and patient preference. Search concepts were captured using subject headings and text-word searches in title, abstract, keyword-heading word fields, CAS registry/EC number, and name of substance fields. The strategy excluded animal studies, using a standard algorithm. A base-case strategy was developed for MEDLINE and adapted for the other databases (Box S1). The search syntax used for this review was developed to target records most relevant to the research question. Search terms for patient preference were restricted, and focused on identifying records that explicitly referred to the term “preference” or that explicitly referred to the main terms for the specific patient-preference methods of interest. Authors of relevant abstracts identified through hand-searching recent congresses were contacted by email to ascertain whether they had any manuscripts containing these data that were in press. Where this were the case, abstract data were replaced with information from the manuscripts.
Study identification and selection
Study identification was done by two independent researchers: titles and abstracts were screened for relevance to the research questions, and any studies meeting or potentially meeting eligibility criteria were selected for further full-text review. Studies considered ineligible after review of the full text were assigned an exclusion code (Figure 1). Disagreements among the study team on selection of records at any stage in the review process were resolved by discussion until consensus was met.
After selection for inclusion, study characteristics were examined and summarized in data-extraction tables. Variables captured for each record were study-participant characteristics, comparator drugs, preference-assessment method, study design and conduct, attribute selection and levels, RI of attributes and direction of preferences, direct preference for profiles representing comparator agents, subgroup analyses, and other findings of relevance.
Results
Search results
Figure 1 provides an overview of study selection. Database searching identified 588 records after deduplication. Following title/abstract screening, 574 records were excluded. A hand search of recent congresses identified four relevant abstracts, such that 18 records were reviewed for eligibility. Seven records were excluded (Figure 1). As already described, congress-abstract authors were subsequently contacted regarding potential manuscripts, and two were identified. One of these included data from two of the congress abstracts identified by the hand search.17 A total of ten records were thus finally included in the review (eight fully published studies at the time of the search, one fully published postsearch, and one ePub in advance of print; Figure 1).
Of the ten records included, eight studies had compared patient preferences between different GLP1 RA-attribute profiles,12,15,18–23 one had compared a GLP1 RA versus insulin-glargine profile,17 and one compared patient preference among GLP1 RA profiles and between a GLP1 RA versus insulin-glargine profile.24 Results for GLP1 RA versus GLP1 RA-attribute comparisons and GLP1 RA versus insulin glargine-attribute comparisons are summarized separately.
Study characteristics
An overview of study characteristics is provided in Table 1 for studies comparing the attributes of different GLP1 RAs and in Table 2 for those comparing attributes of a GLP1 RA versus insulin glargine. Studies were conducted across numerous countries, including the UK (n=3), US (n=2), Italy, Japan, and Sweden (n=1 each). Two studies were multinational. DCEs were employed for preference elicitation in six studies,15,17–20,23 two studies used TTO,12,21 one reported a DCE with WTP,24 and one study used both TTO and DCE.22 Sample sizes ranged from 182 to 1,482 participants, and all studies included adults with T2DM. Four studies included injectable-naïve patients only,15,17–19, one injectable-experienced patients only,23 and four both injectable-naïve and -experienced.12,20–22 Injection experience was not reported in one study.24
Attributes explored
Across the ten studies, a total of 19 treatment attributes were evaluated: ten process attributes (52.6%) and nine outcome attributes (47.4%). Figure 2 shows the frequency of individual attribute evaluations across studies. The most commonly evaluated process attribute was dose frequency (eight studies), while the most commonly evaluated outcome attribute was change in glycated hemoglobin (HbAlc; efficacy, seven studies) (Figure 2).
  | Figure 2 Frequency of individual treatment-attribute evaluation across ten patient-preference studies identified by literature review. |
Comparison among attributes of GLP1 RAs
Nine studies – six DCEs, two TTO evaluations, and one DCE with WTP analysis – described comparisons among GLP1 RA profiles.
DCE approach: attribute level
An overview of the main findings from the six DCEs is provided in Table 3. Dose frequency had the highest RI of attributes in three of the six studies (Table 4).18–20 In the remaining DCEs, dose frequency was rated an important attribute, but less than change in HbAlc or AE profile (Tables 3 and 4).15,22,23 In all studies, patients preferred GLP1 RA profiles associated with less frequent dosing (ie, once weekly preferred versus once daily, which itself was preferred over twice daily; Table 3). One study demonstrated that all other process attributes became less important to patients when dosing was once weekly compared with once daily.20
  | Table 4 Importance of attributes of GLP1 RA treatments determined in DCEs conducted in patients with T2DM |
Type of delivery device was ranked the second-most important attribute in a UK and a Japanese DCE: injectable-naïve patients with T2DM preferred a single-use pen to a multidose prefilled pen (Table 4).18,19 However, in other studies among injectable-naïve patients, this was not the case, with type of device being rated less important in both a multinational DCE and a US DCE (Table 4).15,20 In studies including injectable-experienced patients, type of device was also less important than many other process and outcome attributes,20,23 although in one study it was suggested by the authors that preference for this attribute may vary depending on the current injectable medication used by the individual.20
Three studies examined needle size as a process attribute, with variable results reported. Needle size was a significant predictor of device choice in both injectable-naïve and injectable-experienced patient subgroups in the US study by Hauber et al,20 with individuals preferring the switch from a longer/thicker to a shorter/thinner needle (P<0.05, Tables 3 and 4). However, needle size was rated as being of little importance among injectable-naïve and injectable-experienced patients in a multinational DCE.15,23
Differences in the RI of GLP1 RA-process attributes of injection preparation and need for titration were noted, depending on a patient’s experience with injections. In injectable-naïve patients included in a multinational DCE, injection preparation was not an important driver of preference,15 while in injectable-experienced patients completing the same survey, it was much more important, with patients preferring a GLP1 RA profile associated with a multidose pen or autoinjector compared with vial and syringe (P<0.001, Tables 3 and 4).23 The opposite was true of the need for titration, which was not a significant predictor of preference in experienced patients, but was more important in the injectable-naïve (P<0.05).15,23 Five studies examined outcome attributes, with change in HbAlc and AE profile generally rated as the most important across studies (Tables 3 and 4).
DCE approach: treatment level
All the DCEs identified included direct preference elicitation for medication profiles representing a GLP1 RA (Figure 3). In injectable-naïve patients with T2DM, preferences were for the medication profile associated with the lowest dose frequency.15,18–20 For example, patients preferred the profile representing dulaglutide once weekly over liraglutide once daily in the UK and Japanese studies by Gelhorn et al18,19 (Figure 3A). Similarly, Qin et al15 and Hauber et al20 demonstrated that injectable-naïve patients preferred the medication profile representing exenatide once weekly over the liraglutide once-daily profile (Figure 3B). This was even the case when liraglutide efficacy was assumed to be greater than exenatide.15 Less frequent dosing was also preferred in a population of patients with mixed injectable experience, with once-daily liraglutide being preferred over exenatide twice daily (Figure 3C).22 However, findings also indicated that current device type may influence preferences for treatment (Figure 3B).20
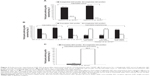  | Figure 3 Preference for hypothetical GLP1 RA drug profiles determined in DCEs among patients with T2DM. (A) Preferences for a dulaglutide QW versus liraglutide QD profile among injectable-naive patients; (B) preferences for a exenatide QW versus liraglutide QD profile among injectable-naive or injectable-experienced patients; (C) preferences for a exenatide BID versus liraglutide QD profile in injectable and naive patients. |
DCE-with-WTP approach
A single study evaluated preference of patients with T2DM for liraglutide once daily compared with other glucose-lowering drugs, including exenatide twice daily and insulin glargine, using a DCE-with-WTP approach (Table 3).24 WTP for liraglutide versus exenatide was reported for seven treatment attributes (Table 3). Across all attributes, patients were prepared to pay an extra €0.81/day for a medication profile approximating liraglutide (1.2 mg once daily) versus exenatide (10 μg twice daily).24 The main component driving the preference for liraglutide was the dosing schedule: once a day and not limited to mealtimes.
TTO approach
Three studies used a TTO approach.12,21,22 In the earliest of these, patients with T2DM were presented with a pair of product profiles representing liraglutide once daily and exenatide twice daily.22 Overall, 96% of respondents preferred the medication profile representing liraglutide. Mean TTO scores for each hypothetical product profile were 0.978 (95% CI 0.964–0.989) for the liraglutide versus 0.94 (95% CI 0.923–0.955) for the exenatide profile, giving a mean difference in TTO score of 0.038 (P<0.05). This difference was driven by the additional utility gained for the liraglutide profile across different attributes: utility differences were 0.016 for change in HbAlc, 0.011 for incidence of nausea, 0.006 for incidence of hypoglycemia, and 0.005 for dosing schedule (frequency and timing regarding mealtimes). Significantly more patients indicated that they would prefer to live fewer years than take the medication profile representing exenatide twice daily compared with liraglutide (22% versus 7%, P<0.05).22
Two similar TTO studies conducted in the UK and Italy involved the development of seven health states with identical descriptions of T2DM, but which were associated with different treatment process.12,21 The first health state described oral-only treatment, and the remaining six described oral treatment plus a weekly injection. Injection health states varied in three aspects – requirement for reconstitution, waiting during preparation, and needle handling – that were selected as likely to distinguish between three once-weekly GLP1 RAs (albiglutide, dulaglutide, and exenatide). Health states with more administration steps were typically ranked less preferably in both UK and Italian study populations.12,21 Utility scores followed the rank order of preference, with greater number of administration steps associated with lower utility (Table 5). For example, lowest utility values were determined for the health state including all injection inconveniences (approximating the profile for the albiglutide device), while the highest were observed for the injection health state with no inconveniences (representing the profile for the dulaglutide device).12,21
Comparison between attributes of GLP1 RA therapy and insulin glargine
Two studies (one DCE and one DCE with WTP) compared patient preferences for treatment features of a GLP1 RA versus those of insulin glargine.17,24
DCE approach
In a DCE evaluating dulaglutide (1.5 mg) compared with insulin glargine (SoloStar) in injectable-naïve patients, attributes with the highest RI values were type of delivery system and frequency of nausea (Table 6).17 No single attribute appeared clearly to drive patient preferences. Direct comparison of medication profiles for dulaglutide and insulin glargine revealed that 75% (n=174) of patients preferred the dulaglutide profile compared with 25% (n=58) who preferred the insulin-glargine profile (P<0.0001).17 Among patients preferring dulaglutide, the two most important medication attributes were type of delivery system (RI 24.5%) and dose frequency (RI 19.2%).
DCE-with-WTP approach
Findings from a DCE-with-WTP study indicated that patients with T2DM were willing to pay more for the liraglutide once-daily profile than for the insulin-glargine profile (Table 6).24 Overall, patients with T2DM were willing to pay an extra €3.36/day for liraglutide (1.2 mg) compared with insulin glargine (average 24 IU once daily).24 The largest component driving WTP compared with insulin glargine was weight loss (€2.35/day).
Discussion
The current review identified several studies that used stated preference or health state-valuation methods to compare patient preference based on process and outcome attributes among GLP1 RAs or a GLP1 RA versus insulin. Across studies, the most important attributes driving patient preferences were dose frequency, delivery device, change in HbAlc, and attributes related to frequency of AEs (Table 4). These are consistent with the GLP1 RA attributes identified as most important in a multinational qualitative study among injectable-naïve and GLP1 RA injectable-experienced patients.25 The current review found that in DCEs, patients preferred medication profiles that involved once-weekly rather than once-daily dosing, were delivered via a single-use pen, and were associated with a greater improvement in glycemic control, less weight gain, and fewer AEs.15,17–20,22–24 In TTO evaluations, GLP1 RA profiles with fewer injection inconveniences were favored.12,21
While the findings of the review help us to understand patient preferences for GLP1 RAs better, they have wider implications and provide information useful in the management of T2DM in general. The consideration of patient preferences is critical for the individualization of treatment goals and strategies, and an understanding of the specific factors that impact choice of treatment such as HbAlc target, impact on weight, medication AE profiles, and regimen complexity, are key components in the delivery of patient-centered care.3
Despite the emergence of general patterns across the studies included, cross-study comparisons are difficult, should be viewed with caution, and results interpreted within the context of each individual analysis.11 Across studies, attribute selection and levels, patient populations, geography, and methods varied considerably. To address such inconsistencies, a checklist for good research practice has been developed by an ISPOR task force that provides guidance on method development for conjoint analysis in health care settings.10
Variability in attribute levels and the way in which specific attributes are described across studies can potentially affect participant responses and data interpretation, thereby introducing bias into the results.16 Patients may place greater importance on those attributes characterized as more severe, more frequent, or that differentiate more clearly between medication profiles.16,17,25 This is demonstrated across some of the studies reviewed herein, eg, where the influence of efficacy to drive patient preference varied was most likely due to the different levels chosen to describe change in HbAlc.15,18,19 Using head-to-head data in DCEs may increase the likelihood that attribute levels reflect genuine observed differences between medications in the population of interest.18,19 It should also be noted that previous injection experience appears to influence patient preferences, and there are indications that preferences for GLP1 RA attributes may vary depending on current treatment.15,23 Injectable-naïve patients may provide the most relevant findings regarding preference, and so could be a better population in which to demonstrate the true value of GLP1 RA attributes, since their opinions have not been influenced by any previous good or bad experiences with injectable medications.15,20
Patient responses may also vary according to how a preference task is administered. Face-to-face interviews12,17–19,21 and online questionnaires15,20,22–24 were employed in included studies. As indicated by the ISPOR taskforce, data quality in conjoint analysis may be improved by interviewer-led administration, because the interviewer can sense when a participant requires more explanation, explain the requirements of the task more fully, and answer questions without influencing the respondent.10
Findings from the included studies are largely consistent with previously published patient-preference evaluations. For example, using a standard-gamble approach, Boye et al26 evaluated the utility and disutility of three injection-related attributes of aspecific T2DM therapies (dose frequency, dose flexibility, and injection-site reaction) and reported that higher utility was associated with health states that included once-weekly dosing, flexible dosing, and no injection-site reactions.26 The most important attribute to patients was weekly dosing (average added utility of 0.023 versus once-daily dosing). Although excluded from our review because it was not an English-language publication, a conjoint analysis by Otto et al27 in German patients with T2DM switching from oral therapy to injectable treatment also demonstrated that a minimum number of injections was the most important attribute compared with low rate of hypoglycemia and weight loss (RI 33.1% versus 15% and 10%, respectively).
Patient preferences and perceptions of treatment-process attributes associated with GLP1 RAs have also been evaluated in real-world settings, and specific instruments have been developed to elicit preferences in this scenario. For example, the ten-item Diabetes Injection Device Experience Questionnaire and the Diabetes Injection Device Preference Questionnaire have been shown to distinguish patient preferences between different GLP1 RA devices with respect to ease of use, satisfaction, and convenience.28
The current review is subject to several limitations. Although conducted according to a robust and reproducible protocol, it must be considered a pragmatic rather than a systematic review, and we cannot rule out that other studies relevant to the research question may have been published. In addition, it can be challenging to capture patient-preference studies robustly in literature searches, since a wide range of definitions and terms for the concept are used by researchers in their abstracts, these terms are used inconsistently, and some studies fail to refer explicitly to the method of preference elicitation in the abstract. An assessment of the quality of studies included was not attempted, as there are no standard methods to assess quality or risk of bias in preference studies.29 A quality checklist specific to conjoint analysis has been developed (PREFS), which focuses on purpose, respondents, explanation, findings, and significance,29 but its application was inappropriate, because the scope of our review went beyond conjoint analysis. However, most DCEs included herein would likely score relatively highly on this quality checklist.
Other limitations arise from the content of the publications that were included in the review. Issues of inconsistent study methods and differing populations, attributes, and attribute levels have been discussed, but it is also unclear how the preferences elicited in these studies actually reflect real-life treatment decisions, because other parameters may have an influence.18,20 In addition, preferences are gained based on a patient’s interpretation of hypothetical medication profiles or health states, rather than on personal experience.21 Generalizability of the study populations to the wider T2DM population may also be limited, since in some cases participants were recruited via advertisements and online. It is important to note that the evidence base is limited: we identified only ten studies meeting our strict eligibility criteria, and not all available GLP1 RAs were covered by the evidence. More studies of robust design are needed to facilitate our understanding of patient preferences for GLP1 RAs, and future studies should seek to extend our knowledge regarding important GLP1 RA attributes to other agents, such as lixisenatide and once-weekly semaglutide.
Conclusion
The findings from this review indicate that both process and outcome attributes are important drivers of patient preference for GLP1 RAs. In general, patients prefer a medication profile that is dosed less often, requires minimal injection preparation, and is associated with improved glycemic control and fewer AEs. No single attribute predicted preference across studies, but it appears that when differences between treatments with respect to efficacy and safety are small, such process attributes as dose frequency and type of device gain value in the eyes of patients. As such, even when clinical trial evidence suggests that a given medication should be beneficial, patient preferences with respect to process or outcome attributes may preclude or limit its use.3 Consideration of patient preference is thus important for informing the process of individualizing treatment goals and strategies in T2DM management, as acknowledged by recent consensus guidelines from the ADA/EASD.3 Results from studies employing preference-based approaches – like those reviewed herein, which include specific medication comparisons – thus have the potential to facilitate clinical decision-making and align patient preferences with patient care, which could result in improved medication adherence and better clinical and economic outcomes.
Abbreviation list
ADA, American Diabetes Association; AE, adverse event; BG, blood glucose; BID, bis in die (twice daily); DCE, discrete-choice experiment; EASD, European Association for the Study of Diabetes; GI, gastrointestinal; RA, receptor agonist; ISPOR, International Society for Pharmacoeconomics and Outcomes Research; MUP, multiuse pen; NICE, National Institute of Health and Care Excellence; NR, not reported; PREFS, Purpose, Respondents, Explanation, Findings, Significance; QD, quaque die (once daily); QW, once weekly; RI, relative importance; SBP, systolic blood pressure; SUP, single-use pen; T2DM, type 2 diabetes mellitus; TTO, time trade-off; WTP, willingness to pay.
Acknowledgments
The authors thank Mick Arber (York Health Economic Consortium [YHEC]) for assistance with the literature search, and Sharon Raynor and Alison Terry for assistance with writing and editing, respectively. This study was funded by Eli Lilly and Company (Indianapolis, IN, USA).
Disclosure
KSB, LEGP, and VTT are full-time employees of Eli Lilly. SR and TKM are employees of KMHO, who received funding from Eli Lilly for time spent conducting this research. SR reports grants from Eli Lilly during the conduct of the study. TKM reports grants from Eli Lilly during the conduct of the study and outside the submitted work, and KSB and LEGP report grants from Eli Lilly outside the submitted work. The authors report no other conflicts of interest in this work.
References
International Diabetes Federation (IDF). Diabetes atlas. 8th ed.; 2017. Available from: http://www.diabetesatlas.org/resources/2017-atlas.html. Accessed May 21, 2018. | ||
Purnell TA, Joy S, Little E, Bridges JF, Maruthur N. Patient preferences for noninsulin diabetes medications: a systematic review. Diabetes Care. 2014;37(7):2055–2062. doi:10.2337/dc13-2527 | ||
Davies MJ, D’Alessio DA, Fradkin J, et al. Management of hyperglycaemia in type 2 diabetes, 2018. A consensus report by the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2018;41(12):2669–2701. doi:10.2337/dci18-0033 | ||
National Institute of Health and Care Excellence. Type 2 diabetes in adults: management. NICE guideline 28; Dec 2015. Available from: https://www.nice.org.uk/guidance/ng28. Accessed May 24, 2018. | ||
Inzucchi SE, Bergenstal RM, Buse JB, et al. American Diabetes Association (ADA); European Association for the Study of Diabetes (EASD). Management of hyperglycemia in type 2 diabetes: a patient-centered approach: position statement of the American Diabetes Association (ADA) and the European Association for the Study of Diabetes (EASD). Diabetes Care. 2012;35(6):1364–1379. | ||
Bridges JF, Onukwugha E, Johnson F, Hauber A. Patient preference methods – A patient centered evaluation paradigm. ISPOR Connections. 2007;13(6):4–7. Available from: https://www.ispor.org/news/articles/Dec07/Bridgesetal2007-Patientpreferencemethods.pdf. Accessed May 24, 2018. | ||
Hauber AB, Han S, Yang J-C, et al. Effect of pill burden on dosing preferences, willingness to pay, and likely adherence among patients with type 2 diabetes. Patient Prefer Adherence. 2013;7:937–949. doi:10.2147/PPA.S43465 | ||
García-Pérez LE, Alvarez M, Dilla T, Gil-Guillén V, Orozco-Beltrán D. Adherence to therapies in patients with type 2 diabetes. Diabetes Ther. 2013;4(2):175–194. doi:10.1007/s13300-013-0034-y | ||
Kennedy-Martin T, Boye KS, Peng X. Cost of medication adherence and persistence in type 2 diabetes mellitus: a literature review. Patient Prefer Adherence. 2017;11:1103–1117. doi:10.2147/PPA.S134792 | ||
Bridges JFP, Hauber AB, Marshall D, et al. Conjoint analysis applications in health – a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–413. doi:10.1016/j.jval.2010.11.013 | ||
Stewart KD, Johnston JA, Matza LS, et al. Preference for pharmaceutical formulation and treatment process attributes. Patient Prefer Adherence. 2016;10:1385–1399. doi:10.2147/PPA.S101821 | ||
Matza LS, Boye KS, Stewart KD, Davies EW, Paczkowski R. Health state utilities associated with attributes of weekly injection devices for treatment of type 2 diabetes. BMC Health Serv Res. 2017;17(1):774. doi:10.1186/s12913-017-2648-7 | ||
American Diabetes Association (ADA). Pharmacologic approaches to glycemic treatment: standard of medical care in diabetes – 2018. Diabetes Care. 2018;41(suppl 1):S73–S85. doi:10.2337/dc18-0733 | ||
Levin PA, Nguyen H, Wittbrodt ET, Kim SC. Glucagon-like peptide-1 receptor agonists: a systematic review of comparative effectiveness research. Diabetes Metab Syndr Obes. 2017;10:123–139. doi:10.2147/DMSO.S130834 | ||
Qin L, Chen S, Flood E, et al. Glucagon-like peptide-1 receptor agonist treatment attributes important to injection-naive patients with type 2 diabetes mellitus: a multinational preference study. Diabetes Ther. 2017;8(2):321–334. doi:10.1007/s13300-017-0230-2 | ||
Bien DR, Danner M, Vennedey V, Civello D, Evers SM, Hiligsman M. Patients’ preferences for outcome, process and cost attributes in cancer treatment: a systematic review of discrete choice experiments. Patient. 2017;10(5):553–565. doi:10.1007/s40271-017-0235-y | ||
Poon JL, Boye KS, Thieu VT, Norrbacka K, Hassan SW, Gelhorn HL. Preferences for attributes of medications among patients with type 2 diabetes: a cross-medication class comparison of injection therapies. Curr Res Diabetes Obesity J. 2018;6(5):555700. EPub ahead of print. | ||
Gelhorn HL, Poon JL, Davies EW, Paczkowski R, Curtis SE, Boye KS. Evaluating preferences for profiles of GLP-1 receptor agonists among injection-naive type 2 diabetes patients in the UK. Patient Prefer Adherence. 2015;9:1611–1622. doi:10.2147/PPA.S82441 | ||
Gelhorn HL, Bacci ED, Poon JL, Boye KS, Suzuki S, Babineaux SM. Evaluating preferences for profiles of glucagon-like peptide-1 receptor agonists among injection-naive type 2 diabetes patients in Japan. Patient Prefer Adherence. 2016;10:1337–1348. doi:10.2147/PPA.S109289 | ||
Hauber AB, Nguyen H, Posner J, Kalsekar I, Ruggles J. A discrete-choice experiment to quantify patient preferences for frequency of glucagon-like peptide-1 receptor agonist injections in the treatment of type 2 diabetes. Curr Med Res Opin. 2016;32(2):251–262. doi:10.1185/03007995.2015.1117433 | ||
Matza LS, Boye KS, Jordan JB, et al. Patient preferences in Italy: health state utilities associated with attributes of weekly injection devices for treatment of type 2 diabetes. Patient Prefer Adherence. 2018;12:971–979. doi:10.2147/PPA.S176067 | ||
Polster M, Zanutto E, McDonald S, Conner C, Hammer M. A comparison of preferences for two GLP-1 products – liraglutide and exenatide – for the treatment of type 2 diabetes. J Med Econ. 2010;13(4):655–661. doi:10.3111/13696998.2010.529377 | ||
Qin L, Chen S, Flood E, et al. Glucagon-like peptide-1 receptor agonist treatment attributes important to injection-experienced patients with type 2 diabetes mellitus: a preference study in Germany and the United Kingdom. Diabetes Ther. 2017;8(2):335–353. doi:10.1007/s13300-017-0237-8 | ||
Jendle J, Torffvit O, Ridderstrale M, Ericsson A, Nilsen B, Bogelund M. Willingness to pay for diabetes drug therapy in type 2 diabetes patients: based on LEAD clinical programme results. J Med Econ. 2012;15(suppl 2):1–5. doi:10.3111/13696998.2012.703633 | ||
Rydén A, Chen S, Flood E, Romero B, Grandy S. Discrete choice experiment attribute selection using a multinational interview study: treatment features important to patients with type 2 diabetes mellitus. Patient. 2017;10(4):475–487. doi:10.1007/s40271-017-0225-0 | ||
Boye KS, Matza LS, Walter KN, Van Brunt K, Palsgrove AC, Tynan A. Utilities and disutilities for attributes of injectable treatments for type 2 diabetes. Eur J Health Econ. 2011;12(3):219–230. doi:10.1007/s10198-010-0224-8 | ||
Otto T, Stralka R, Schimmelpfenning H, Jung H, Bruns K. Umstellung von oralen auf injektable antidiabetika bei fort schreitendem typ-2-diabetes: welche präferenzen haben die patienten? [Treatment options for patients with progressing type 2 diabetes: what are patients’ preferences in Germany when switching from oral to injectable antidiabetic medication]. Gesundh Okön Qual Manag. 2016;21:181–198. German. | ||
Matza LS, Boye KS, Currie BM, et al. Patient perceptions of injection devices used with dulaglutide and liraglutide for treatment of type 2 diabetes. Curr Med Res Opin. 2018;34(8):1457–1464. doi:10.1080/03007995.2018.1465903. | ||
Joy SM, Little E, Mauthur NM, Purnell TS, Bridges JF. Patient preferences for the treatment of type 2 diabetes mellitus: a scoping review. Pharmacoeconomics. 2013;31(10):877–892. doi:10.1007/s40273-013-0089-7. |
Supplementary material
  | Box S1 MEDLINE search strategy |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.






