Back to Journals » Patient Preference and Adherence » Volume 16
Patient Preferences for Attributes of Health Canada Approved Weight Loss Medications Among Adults Living with Obesity in Canada: A Qualitative Study
Authors Donnan J , Huang R , Twells L
Received 25 November 2021
Accepted for publication 11 March 2022
Published 5 April 2022 Volume 2022:16 Pages 911—923
DOI https://doi.org/10.2147/PPA.S350411
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Jennifer Donnan,1 Rita Huang,1 Laurie Twells2
1School of Pharmacy, Memorial University, St. John’s, Newfoundland and Labrador, Canada; 2Faculty of Medicine, Memorial University, St. John’s, Newfoundland and Labrador, Canada
Correspondence: Jennifer Donnan, Tel +1 709 864-3587, Email [email protected]
Purpose: Obesity is a complex disease with negative impacts on physical and mental health. The treatment of obesity is an area where shared decision making and patient preferences play an important role. Recommendations surrounding weight loss medications are evolving and only recently, with the publication of the 2020 Canadian Obesity Management Clinical Guidelines, pharmacotherapy has become a recommended alternative for obesity management. Guidelines recommend three medications: orlistat, liraglutide, and naltrexone/bupropion. This study sought to identify medication attributes relevant to patients starting pharmacotherapy for weight management.
Patients and Methods: Semi-structured focus groups and interviews were conducted with Canadian residents who were ≥ 18 years of age and were living with obesity (body mass index [BMI] ≥ 30kg/m2 or ≥ 27kg/m2 with adiposity-related complications). Sessions were conducted virtually, audio recorded, and transcribed. Two team members used a combination of inductive and deductive coding to independently code the data. A final coding template was agreed upon through discussion.
Results: A total of 21 individuals participated (85.7% female, 76.2% ≥ 40 years of age) with the average BMI being 44.3 kg/m2. Participants touched upon many attributes which were categorized into five categories: 1) cost, 2) regimen, 3) side effects, 4) benefits, and 5) non-medication attributes. Cost of medications, lack of coverage by insurance companies, and stigma were identified as major barriers to accessing medications. There was consensus in the desire for a simple regimen, however there was heterogeneity among opinions on tolerability of side effects, desired benefits, and route of administration.
Conclusion: This study identified attributes that influenced patient’s decisions when considering a new anti-obesity medication. Understanding these attributes can assist clinicians in shared decision-making. This study highlighted the stigma that is prevalent among providers and the need for education. Further research should be conducted to understand the tradeoffs patients in our study make between the identified attributes.
Keywords: patient preferences, obesity, weight loss, anti-obesity medications, Canada
Introduction
Obesity is a complex, chronic disease in which abnormal or excess body fat impairs health, increases the risk of long-term medical complications, and reduces lifespan.1 According to Statistics Canada, in 2018, 26.8% of Canadians were living with obesity, a threefold increase since 1985.1,2 Being a complex, chronic disease, obesity must be treated as such. Along with other therapies, medications play an important role in obesity management.3 The landscape surrounding medications and their recommendations is rapidly changing in Canada. In February 2015, the Canadian Task Force for Preventative Health recommended that clinicians do not routinely offer available pharmacological agents to patients4 and only discussed two options – orlistat (Xenical) which was approved by Health Canada in 1999 for weight loss5 and metformin (Glucophage) which was used off-label.4 Since then, two new options for weight management have been approved by Health Canada, liraglutide (Saxenda) in 20156 and Contrave, an extended-release naltrexone and bupropion combination in 2018.7
In 2020, Obesity Canada published the new Obesity Management Clinical Guidelines, the first update since 2006. These guidelines highlight the rapidly evolving nature of obesity management and recognize pharmacotherapy as one of the pillars of recommended treatments in patients with a body mass index (BMI) ≥30kg/m2 or ≥27kg/m2 with adiposity related complications. Based on an extensive review of the literature, the guidelines recommend all three Health Canada approved medications as weight management options.3
Currently, these medications are not covered by any provincial plan and less than 20% of Canadians with private insurance have coverage.8 The lack of coverage and rapidly evolving landscape has resulted in a lack of clinician awareness about available options. Therapy for obesity is individualized and must not only meet the clinical needs of patients, but also their personal preferences and risk tolerance levels. That is to say that shared decision making needs to account for how patients make choices about starting medication therapy. According to the multi-attribute utility theory,9 individuals make choices by weighing the pros and cons of the characteristics that make up that choice (eg cost, risk of side effects, impact of side effects, chance of benefit, etc.). Typically, there is not one option that is superior on all characteristics, but individuals will choose the one that maximizes their overall utility (the benefit received from the choice) by making trade-offs between the pros and cons. As such, when it comes it supporting patients in their choices to start a new medication, clinicians must understand how patients weigh the pros and cons of the available alternatives.
The three approved medications each have different mechanisms of action,10 therefore come with different risk-benefit profiles. (Table 1) For effective shared decision making, it is important to get an understanding about patient preferences towards the different treatment options, however research in this area is lacking. Studies have shown that patient’s preferences can differ from those of clinicians. McAlister et al highlighted that patients had a lower risk threshold for side effects and required a greater beneficial effect than did physicians before acceptance of antihypertensives.11 While financial coverage for anti-obesity medications is limited, clinicians cannot assume that this precludes patients from wanting to consider them.
 |
Table 1 Comparison of Health Canada Approved Weight Loss Medications |
Research is lacking on patient preferences for the three approved medications, however, there is growing knowledge in other areas of weight management. Preferences have been studied in patients living with obesity and considering bariatric surgery. A discrete choice experiment (DCE) identified costs of surgery, expected weight loss, and resolution of medical conditions as the most important decisions for bariatric surgery.12 Data on patient preferences has been used to develop tools to support shared decision making. Ho et al used data from a DCE on preferences for weight loss devices, resulting in a minimal clinical benefit – maximal acceptable risk calculator that is currently used in practice.13
The purpose of this study is to identify what factors influence patient preferences when considering a new medication for weight management. The current study is the first of a two-stage study. This first stage involved conducting focus groups and interviews with patients living with obesity in Canada. The second stage will quantify patient preference weights for the attributes identified using a DCE.
Methods
Study Design
Qualitative data collection was used to identify key attributes. Specifically, focus groups and interviews were conducted to understand patients’ thoughts regarding anti-obesity medications approved in Canada. Eligible participants were English speaking Canadian residents ≥18 years, who had self-reported obesity, defined as a BMI≥30kg/m2 or ≥27kg/m2 with adiposity related complications. All sessions were conducted virtually via Zoom. Given the general lack of knowledge of anti-obesity medications, we recognized that it would be difficult for participants to discuss specific factors that would influence their choices. We therefore developed a 20-minute educational presentation on obesity and treatment strategies as outlined in the updated Canadian Guidelines, with a focus on approved medications. (Appendix A) We made sure to present each medication option in an unbiased manner to not influence participant opinions.
The study team consisted of a licensed pharmacist practicing in academia with expertise in patient preference research (JD), a Doctor of Pharmacy student in her final year of training (RH), and a professor of clinical epidemiology specializing in obesity (LT). Each of these researchers approached the study from a pragmatic perspective, exploring a practical understanding and to identify solutions to a real-world issue. Participants were introduced to the team members at their session and their research interests and experiences were disclosed.
Recruitment
A convenience sample of individuals were recruited using social media (Facebook, Twitter, Reddit) and obesity related discussion forums, using recruitment materials that highlighted the purpose of the study. Participants were not known to the team. Interested participants were contacted by telephone to collect verbal consent and demographic information, and to register for a session. Where possible participants were scheduled into a focus group session, however if there were scheduling difficulties or privacy concerns, an interview was offered. Each participant was offered a $20 gift card in appreciation.
Data Collection
Semi-structured focus groups and interviews were conducted virtually using Zoom between May and June 2021. The first half of the session, participants listened to the presentation. This was followed by an open discussion following a semi-structured discussion guide. (Appendix B) Each session lasted 30–40 minutes. The discussion started with a broad question asking: If you were to start a medication, which would you choose? Why? Followed by probing questions to gather context around comments raised. More focused questions were asked if they did not naturally come out in conversation. Two research team members (RH, JD), both female, were present at each session. One team member led the presentation (RH), while the second team member led the discussion (JD). Data was audio recorded and transcribed verbatim in a de-identified manner for analysis. Data was collected until saturation was met and no new ideas emerged. Member checking was conducted by paraphrasing the comments shared back to the participants to confirm the intent and meaning was understood. To maintain the privacy of fellow focus group attendees, transcripts were not shared with participants.
Data Analysis
Two research team members (RH, JD) independently reviewed the transcript for accuracy. Both deductive and inductive thematic coding strategies were used to draft a coding template from the collected data. Deductive coding for themes identified in the literature (eg, cost, side effects, weight loss) and inductive coding was used for themes that emerged from the data. A final coding template was agreed upon through discussion. The results are reported thematically. Some quotes are edited for clarity.
Results
A total of 25 individuals consented to participate. Of these, 21 individuals attended (18 people participated in one of four focus groups and 3 people participated in interviews), with 85.7% identifying as female, and 76.2% being ≥40 years of age. The average BMI was 44.3 kg/m2. (Table 2). Participants were recruited across Canada, but 76% of respondents resided in Atlantic Canada.
 |  |  |
Table 2 Participant Characteristics |
Analysis of the transcripts resulted in five broad themes: 1) Cost, 2) Regimen, 3) Side Effects, 4) Benefits, and 5) Non-medication attributes. (Figure 1) Where possible themes were designed to reflect an attribute of choice, as per the multi-attribute utility theory. However, it became apparent that there were other issues that impacted patients’ experiences and ability to consider medications as an alternative, these were captured together under the theme “non-medication attributes”.
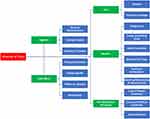 |
Figure 1 Coding tree of identified themes. |
Cost
Cost was one of the main concerns. Many participants mentioned that they were open to trying a new weight loss medication but cost would be a big deterrent. One participant shared how they were taking naltrexone/bupropion and saw results but had to stop due to cost.
I was on it for about three months. And it was working. But the cost of it was really too high. It’s a good medication. It helped a bit with the depression [and] controlling my appetite. But at $300/month. It’s just a little too much. (Participant OMP15)
However, some participants expressed a willingness to pay. One participant stated, “If it actually helped, I would pay for it”. (OMP24)
Lack of coverage was also mentioned. One participant said, “Every medication I have talked to [my doctor] about and he’s given me a recommendation, I go to the pharmacy, and nothing is covered, absolutely nothing”. (OMP22) One participant shared their experience with dulaglutide, a medication in the same class as liraglutide that also causes weight loss, though not officially indicated for this purpose. They said:
I was on Trulicity (dulaglutide) and the Trulicity was helping and my A1C was the best it’s been since I was 16 years old. At that time, my specialist was giving me samples from the drug company and that dried up. So, therefore I had to go off it and now my A1C’s gone back up… [With the Trulicity] I was maintaining [my weight] to where I was… I was taking [Novorapid] and then I started to take… Trulicity and then I went off all daytime insulin. So, right now I’m only taking nighttime insulin, but I can see a difference in my A1C so I can see me eventually going back on the daytime insulin because I could feel the difference. (OMP22)
Regimen
Opinions on the route of administration – subcutaneous injection versus oral tablets – varied considerably among participants. Some participants were against injections. “As soon as they mentioned… injections… this raised a whole other word of anxiety for me”. (OMP16) Many participants had been taking injectable medications previously and found they were not something to be intimidated by. “I was a little nervous about how big the needle was going to be, but when I opened up the box and saw it was just this little, tiny thing I was fine”. (OMP20) One participant seemed to even prefer injections over oral.
I don’t necessarily like taking pills. Sometimes when I take any [oral] medication… I’ll get nauseated. [For injection] I think that I wouldn’t have that same worked up feeling that I’m going to get nauseated if I take it. (OMP18)
Although opinions on dosage form varied considerably, thoughts on the dosing schedule were mostly unanimous. Participants preferred a schedule that was as easy as possible, preferably once a day. “I would want the least amount of times, the easiest to remember, and I wouldn’t want to have to feel like I have to take it exactly at certain times”. (OMP16) Many participants mentioned that it would be difficult to have a complicated dosing schedule due to lifestyle. “Me being on shift work, it would be better if I could just take it once a day.” (OMP23)
In relation to the length of therapy, some participants were hesitant about taking the medications long term. One participant said, “I mean, preferably wouldn’t be on something lifelong”. (OMP6) Other participants viewed obesity like any other chronic disease, suggesting
If I had a medical condition, that required me to take medication, I would take that medication for the rest of my life. So, why wouldn’t I consider taking a medication for the rest of my life for this disease? (OMP18)
Side Effects
For some participants, their main concern was not the immediate side effects once starting, but the effects on their bodies long term. “That would be a concern for me, is how much my [health care provider] knew about what long term effects of these medications are”. (OMP16) Other participants were more concerned about the short-term side effects that would have impacts on their day-to-day life. For example, side effects could exacerbate pre-existing conditions, as one person said “The potential side effect of diarrhea would definitely be a very, very, very big turnoff for me because I also have irritable bowel syndrome.” (OMP10)
Many participants expressed that some side effects would be intolerable due to lifestyle. “With my work lifestyle, I wouldn’t be able to handle [diarrhea], because I’m too much on the go and in the community”. (OMP22) Another participant talked about needing to be mentally alert during their day. “The only thing I’d be worried about would be dizziness in terms of driving”. (OMP1) Several participants quickly ruled out orlistat due to the high risk of gastrointestinal issues. “[Orlistat] would be a hard pass for me. I have no interest in the potential oily spotting and urgency all the time”. (OMP6)
Surprisingly, some participants mentioned side effects as being a positive aspect of the medication. One participant talked about aversion therapy with orlistat:
The orlistat [is a good idea]… I took the medication… and the side effects when I did ingest fat, you would end up with oily stools and accidents. So, it’s almost like aversion therapy where you… just don’t want to have anything happen like that. (OMP1)
It was clear throughout the sessions that preferences regarding side effects were very individualized, based on previous experiences, thoughts about each side effect, and personal situation.
Benefits
When it came to individual weight loss goals, participants had different expectations as to what they would consider success. “I would like to be at [a] normal BMI” (OMP25) said one. Throughout the sessions, information was shared about how a 5–10% weight loss was seen in trials and led to clinically meaningful results. For some participants, this was in line with their goals. “I’d go for the 5–10% range”. (OMP6) Others stated they were not focused on numbers but rather on feelings. “The way I feel about it, anything would help… I try not to focus on numbers”. (OMP22) Opposingly, some participants felt this was not enough. “I wouldn’t be thrilled with [5%]”. (OMP24) One participant said they would be disappointed initially but would not give up on the medication. “I would be disappointed that it wasn’t more, but I don’t think it’s a realistic expectation to just give up”. (OMP25) This revealed that while the trial data matched some participant’s weight goals, it was a mismatch for others.
Increase in energy was a common goal of using the medications and losing weight. One participant shared
I don’t have much energy so I can’t go out and do the things I want to do… so if I can have something… to give me that boost… I probably would be a lot better. (OMP4)
Participants also mentioned living with obesity hindering some of the activities they enjoyed doing.
It’s common for people to say – Oh, when I lose weight, I’m going to do this and that. You tend to put your life on hold because you don’t have self-esteem. So, I feel that if I were to lose weight, that I would be able to do the activities I used to do and stop putting my life on hold. (OMP1)
Finally, several participants desired improvement in other medical conditions. One participant stated, “I think for me it would be to try to keep healthy so that I’m not taking any meds and no diabetes”. (OMP3)
Non-Medication Attributes
Although there were many attributes of the medications that participants spoke about, there were attributes that were independent of the medications and related to personal circumstances and lived experiences.
Several barriers to access were highlighted. Most participants spoke to a lack of awareness about these medications from their family physician and other health care professionals. “My doctor never, ever offered an option for weight loss medication”. (OMP3) For many, stigma from these care providers about obesity was a huge barrier in getting access to medications and hindered participant’s relationships with their providers. One participant shared,
My doctor’s old school… [obesity] is lifestyle. So, you’ve got to change your lifestyle, you’ve got to change your eating… She won’t prescribe anything because, it’s lifestyle. (OMP23)
Another participant experienced a situation where their doctor was dismissive towards their concerns.
Whenever I try to bring up the conversation with my doctor… he’s like you’re young, you don’t have any issues… just keep trying [to lose weight]. It’s just really frustrating every time I go. (OMP25)
The sentiment was summarized by one participant. “We’re all in the same boat, except for the doctors, that this is something you live with for the rest of your life… like any disease”. (OMP12)
Concomitant diseases that participants were living with influenced their choices as it limited their options or made one more favorable. Participants mentioned not being able to consider an option due to contraindications. “I wouldn’t be able to do the liver one because I have fatty liver”. (OMP12) In other situations, concomitant diseases such as diabetes meant that participants could receive coverage for a medication that treated both obesity and diabetes. One participant said, “I’ve been very fortunate that just recently, my insurance company allowed me to have Ozempic (semaglutide) [for diabetes and weight loss]”. (OMP15)
Finally, social influence affected participant’s preferences. Participants had heard both positive and negative comments about medications from those around them. One participant mentioned seeing results from a relative that made them interested.
My sister-in-law is actually [taking medication] and she’s had great success with it. She’s looking the best… feeling the best she has in many, many years… So when I saw that, I [thought], maybe I’d like to try that. (OMP5)
Conversely, negative side effects that friends reported shield participants away from a medication.
I know a friend… has a nurse friend who’s taking a medication… I’m thinking it’s orlistat. And… they’re constantly trying to find her… and she’s in the washroom. (OMP5)
Preference Measurement Instrument
In line with the multi-attribute utility theory, the data collected in these sessions helped to identify the characteristics that patient’s factor into their decision to start a new therapy for obesity management. From these characteristics a preliminary set of attributes and levels (Table 3) has been developed that can be used for a DCE in later stages of this research. This DCE will enable the quantification of relative preference weights for each of the included attributes and the trade-offs people are willing to make between attributes.
 |
Table 3 Preliminary Attributes and Levels |
Discussion
This study, to our knowledge, was the first to engage with patients to understand from their perspective what factors were important to them when considering a Health Canada approved weight loss medication. Throughout the sessions, participants had differing opinions regarding many themes discussed, however, there was consensus in several important ones. Main concerns for participants were stigma from their health care providers, costs of the medications, and lack of coverage from their insurance providers. It was also identified that participants desired a simple regimen to facilitate adherence.
Stigma from health care providers was identified by most participants as a barrier to accessing appropriate care. This is consistent with what is seen in the literature. Primary care providers have been shown to view obesity as largely a behavioral problem, caused by inactivity and overeating.14 They have been identified to hold beliefs that patients living with obesity are less likely to be adherent with treatment, and have a strong anti-fat bias.15–17 Additionally, evidence suggests that patients who report experiencing such weight bias in the healthcare setting have poorer treatment outcomes and are more likely to avoid future care.18 Findings from our study support the existence of widespread weight stigma among health care providers which is directly impacting patient care.
The idea that the sole factor contributing to obesity is lifestyle leads to beliefs that normal weight is achievable through lifestyle changes or the addition of medication. We identified that many participants wanted to achieve weight loss that would allow them to have a normal BMI. These expectations are highlighted in the literature. In a study assessing patient expectations regarding weight loss, patients reported that their “dream” was 38% weight loss, and they would be disappointed with 17% weight loss. The same study showed that after 48 weeks of treatment, 47% of patients did not even achieve a “disappointed” weight.19 Study participants identified improvement in health conditions as a main benefit of losing weight. Research shows that this can be seen with 5–10% weight loss.20–22
This highlights an important gap in public knowledge about clinically meaningful weight loss. With an obvious mismatch in expectations and actual weight loss achieved in clinical trials, it is important for patients who decide to start a weight loss medication to have realistic expectations. Research has shown that when patients feel they failed in achieving their goal, they give up on that weight loss strategy and often gain back much of the weight (if not more) that they had lost.23–25 This yo-yo weight fluctuation can have worse health outcomes than if they never lost the weight at all.26,27
Our study suggested that cost and coverage by insurance companies is a major barrier to accessing medication. Affordability was the main theme identified and overshadowed other attributes of preference. Currently, medications for weight management are seen as elective. Unlike medications for other chronic diseases, patients must overcome large barriers to get them covered or pay completely out-of-pocket.8 Some participants did mention they were willing to pay if they were able to achieve weight loss, however it was not clear if these individuals would continue to be willing to pay long term after weight loss plateaued. There are no published studies that examine patient’s willingness-to-pay (WTP) for these three medications, however other WTP studies done on weight loss strategies do not align with their high costs. For example, Doyle et al showed that patients in the United States and United Kingdom were willing to pay $10.49 USD per one percentage point of weight loss that pharmacotherapy could provide.28 Another study showed that individuals were willing to pay $49.60 USD/month for weight loss coaching.29 These values fall short of the costs associated with approved weight loss medications.
Participants talked about how a simpler regimen would be preferred. Easier regimens have been shown to improve adherence and health outcomes. Ingersoll and Cohen conducted a literature review to assess adherence in several chronic diseases. In diabetes management, the adherence rate of patients taking a once daily regimen was 79% compared to 38% for three times daily. Patients in the once daily regimen had better diabetes control, measured by their A1C.30 Similar results are seen in other studies.31,32 Although there was consensus for a simpler regimen, there was heterogeneity in the preference for injection versus oral. Most participants seemed to prefer an oral medication, though many were open to injection, previous experience with injections contributed to preferences. Preference for oral over injectable therapies is also well cited in the literature in many other diseases.33,34
Finally, there were varying preferences in which side effects participants could tolerate, highlighting differences in lifestyle and previous experiences with medications. Although intolerable to many, orlistat was seen positively as a form of aversion therapy, which uses negative physical or emotional associations to encourage behavior change.35 McCarthy speaks to this “inadvertent aversion therapy” and mentions several studies where patients lowered their dietary fat intake to avoid unpleasant gastrointestinal side effects.36 These opinions may be a reason that orlistat is still a recommended weight loss medication for a small subset of the population.
There were several limitations in this study. First, recruitment was done via social media. This meant potential participants who did not have social media accounts would not necessarily hear about the study. Second, our study had participants reach out to the research team if they were interested. This may bias the sample towards those that were willing to take the medications. We may not understand the thoughts of individuals that were unwilling to try medications or were indifferent. Third, patient education was provided prior to asking questions. This could bias responses to those that the participants thought the research team wanted to hear. Finally, although this study aimed to capture preferences from across Canada and across various demographic characteristics, the sample was mainly female participants, had a higher level of education, and primarily from Atlantic Canada. While there is no reason to believe preferences would differ across provinces, it is possible that findings in this may not be transferable to reflect the opinions of Canadians in other non-Atlantic provinces, of other genders or those with lower levels of education.
This study highlights the attributes of preference that patients in our study consider when selecting a new weight loss medication. It also identifies barriers to the access of these medications in Canada which should be considered when caring for patients who experience obesity. Although important attributes have been identified, it is unclear what tradeoffs patients would make between them. Further research such as a DCE using the identified attributes and levels could be conducted to understand and quantify these tradeoffs.
Conclusion
Patients would consider many factors when choosing a weight loss medication. Cost and insurance coverage were the biggest concerns. Lack of coverage and stigma from care providers were identified as barriers that prevented patients from asking about and accessing medications. This highlights a gap in public and provider education about managing obesity as a chronic disease and recommending evidence-based treatments. This disconnect must be addressed to make progress in obesity management. Although consensus on these issues occurred, there was diversity among preferences in terms of regimen, desired benefits, and tolerable side effects. Due to the diverse array of opinions, it is essential that shared decision making occurs during the initiation of a new medication for weight management.
Ethical Approval
Full ethics approval was granted by the Memorial University of Newfoundland Health Research Ethics Board (File #20210659) and is compliant with the Declaration of Helsinki.
Consent to Participate
Informed consent was obtained from all individual participants in the study, including consent to be audio-recorded and use of direct quotations. Consent was provided verbally due to restrictions with geography and Covid-19 that prevented in-person meetings with participants.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was funded internally through Memorial University.
Disclosure
All authors declare that they have no conflicts of interest in this work.
References
1. Twells LK, Janssen I, Kuk JL Canadian adult obesity clinical practice guidelines: epidemiology of adult obesity. [Internet]. Obesity Canada; 2020 [cited July 15, 2021]. Available from: http://obesitycanada.ca/wp-content/uploads/2021/05/2-Epidemiology-of-Adult-Obesity-5-with-links.pdf.
2. Statistics Canada. Overweight and obese adults, 2018 [Internet]. Government of Canada; 2019 [cited July 15, 2021]. Available from: https://www150.statcan.gc.ca/n1/pub/82-625-x/2019001/article/00005-eng.htm.
3. Wharton S, Lau DCW, Vallis M, et al. Obesity in adults: a clinical practice guideline. CMAJ. 2020;192(31):E875–91. doi:10.1503/cmaj.191707
4. Brauer P, Connor Gorber S, Shaw E, et al. Recommendations for prevention of weight gain and use of behavioral and pharmacologic interventions to manage overweight and obesity in adults in primary care. CMAJ. 2015;187(3):184–195. doi:10.1503/cmaj.140887
5. Xenical Drug Monograph [Internet]. Cheplapharm Arzneimittel GmbH; 2017 [cited July 15, 2021]. Available from: https://pdf.hres.ca/dpd_pm/00041463.PDF.
6. Saxenda Drug Monograph [Internet]. Novo Nordisk Canada; 2021 [cited July 15, 2021]. Available from: https://pdf.hres.ca/dpd_pm/00060180.PDF.
7. Contrave Drug Monograph [Internet]. Bausch Health; 2020 [cited July 15, 2021]. Available from: https://pdf.hres.ca/dpd_pm/00056659.PDF.
8. Sharma AM, Tarride JE, Twells L, Langlois MF, Kirk S, Ramos Salas X Obesity Canada report card on access to obesity treatment for adults in Canada 2019. [Internet]. Obesity Canada; 2019 [cited July 15, 2021]. Available from: https://obesitycanada.ca/resources/reportcard/.
9. Von Winterfeldt D, Fischer GW. Multi-attribute utility theory: models and assessment procedures. In: Wendt D, Vlek C, editors. Utility, Probability, and Human Decision Making. Theory and Decision Library (An International Series in the Philosophy and Methodology of the Social and Behavioral Sciences). Vol. 11. Dordrecht: Springer; 1975. doi:10.1007/978-94-010-1834-0_3
10. Pederson SD, Manjoo P, Wharton S Canadian adult obesity clinical practice guidelines: pharmacotherapy in obesity management. [Internet]. Obesity Canada; 2020 [cited July 15, 2021]. Available from: https://obesitycanada.ca/wp-content/uploads/2021/05/Pharmacotherapy-v6-with-links.pdf.
11. McAlister FA, O’Connor AM, Wells G, Grover SA, Laupacis A. When should hypertension be treated? The different perspectives of Canadian family physicians and patients. CMAJ. 2000;163(4):403–408.
12. Rozier MD, Ghaferi AA, Rose A, Simon NJ, Birkmeyer N, Prosser LA. Patient preferences for bariatric surgery: findings from a survey using discrete choice experiment methodology. JAMA Surg. 2019;154(1):e184375. doi:10.1001/jamasurg.2018.4375
13. Ho MP, Gonzalez JM, Lerner HP, et al. Incorporating patient-preference evidence into regulatory decision making. Surg Endosc. 2015;29(10):2984–2993. doi:10.1007/s00464-014-4044-2
14. Foster GD, Wadden TA, Makris AP, et al. Primary care physicians’ attitudes about obesity and its treatment. Obes Res. 2003;11(10):1168–1177. doi:10.1038/oby.2003.161
15. Phelan SM, Burgess DJ, Yeazel MW, Hellerstedt WL, Griffin JM, Van Ryn M. Impact of weight bias and stigma on quality of care and outcomes for patients with obesity. Obes Rev. 2015;16(4):319–326. doi:10.1111/obr.12266
16. Hebl MR, Xu J. Weighing the care: physicians’ reactions to the size of a patient. Int J Obes. 2001;25(8):1246–1252. doi:10.1038/sj.ijo.0801681
17. Thille P. Managing anti-fat stigma in primary care: an observational study. Health Commun. 2019;34(8):892–903. doi:10.1080/10410236.2018.1439276
18. Rubino F, Puhl RM, Cummings DE, et al. Joint International Consensus statement for ending stigma of obesity. Nat Med. 2020;26(4):485–497. doi:10.1038/s41591-020-0803-x
19. Foster GD, Wadden TA, Vogt RA, Brewer G. What is a reasonable weight loss? Patients’ expectations and evaluations of obesity treatment outcomes. J Consult Clin Psychol. 1997;65(1):79–85. doi:10.1037/0022-006X.65.1.79
20. Neter JE, Stam BE, Kok FJ, Grobbee DE, Geleijnse JM. Influence of weight reduction on blood pressure. Hypertension. 2003;42:878–884. doi:10.1161/01.HYP.0000094221.86888.AE
21. Ryan DH, Yockey SR. Weight Loss and Improvement in comorbidity: differences at 5%, 10%, 15%, and over. Curr Obes Rep. 2017;Jun(2):187–194. doi:10.1007/s13679-017-0262-y
22. Lean ME, Leslie WS, Barnes AC, et al. Primary care-led weight management for remission of type 2 diabetes (DiRECT): an open-label, cluster-randomised trial. Lancet Lond Engl. 2018;391(10120):541–551. doi:10.1016/S0140-6736(17)33102-1
23. Pietiläinen KH, Saarni SE, Kaprio J, Rissanen A. Does dieting make you fat? A twin study. Int J Obes. 2012;36(3):456–464. doi:10.1038/ijo.2011.160
24. MacLean PS, Higgins JA, Giles ED, Sherk VD, Jackman MR. The role for adipose tissue in weight regain after weight loss. Obes Rev. 2015;16(Suppl 1):45–54. doi:10.1111/obr.12255
25. Dalle Grave R, Calugi S, Molinari E, et al. Weight loss expectations in obese patients and treatment attrition: an observational multicenter study. Obes Res. 2005;13(11):1961–1969. doi:10.1038/oby.2005.241
26. Montani JP, Schutz Y, Dulloo AG. Dieting and weight cycling as risk factors for cardiometabolic diseases: who is really at risk? Obes Rev. 2015;16:7–18. doi:10.1111/obr.12251
27. Graci S, Izzo G, Savino S, et al. Weight cycling and cardiovascular risk factors in obesity. Int J Obes Relat Metab Disord. 2004;28(1):65–71. doi:10.1038/sj.ijo.0802537
28. Doyle S, Lloyd A, Birt J, et al. Willingness to pay for obesity pharmacotherapy. Obes (Silver Spring). 2012;20(10):2019–2026. doi:10.1038/oby.2011.387
29. Alavi R, Appel L, Brancati F, Clark J, Mohr P, Daumit G. Willingness to Pay (WTP) for weight loss coaching: results from the POWER trial. Value Health. 2011;14(3):A67–8. doi:10.1016/j.jval.2011.02.380
30. Ingersoll KS, Cohen J. The impact of medication regimen factors on adherence to chronic treatment: a review of literature. J Behav Med. 2008;31(3):213–224. doi:10.1007/s10865-007-9147-y
31. Srivastava K, Arora A, Kataria A, Cappelleri JC, Sadosky A, Peterson AM. Impact of reducing dosing frequency on adherence to oral therapies: a literature review and meta-analysis. Patient Prefer Adherence. 2013;7:419–434. doi:10.2147/PPA.S44646
32. Saini SD, Schoenfeld P, Kaulback K, Dubinsky MC. Effect of medication dosing frequency on adherence in chronic diseases. Am J Manag Care. 2009;6:e22–33.
33. Igarashi A, Bekker Hansen B, Langer J, et al. Preference for oral and injectable GLP-1 RA therapy profiles in Japanese patients with type 2 diabetes: a discrete choice experiment. Adv Ther. 2021;38(1):721–738. doi:10.1007/s12325-020-01561-1
34. Taylor PC, Betteridge N, Brown TM, et al. Treatment mode preferences in rheumatoid arthritis: moving toward shared decision-making. Patient Prefer Adherence. 2020;14:119–131. doi:10.2147/PPA.S220714
35. Shafir H Aversion therapy: how it works & what to expect [Internet]. Choosing Therapy; 2021 [cited July 15, 2021]. Available from: https://www.choosingtherapy.com/aversion-therapy/.
36. McCarthy WJ. Orlistat and weight loss. Am J Clin Nutr. 2000;71(3):846–847. doi:10.1093/ajcn/71.3.846a
37. Castle W, Kreutz G, Lumpkin M, et al. Guidelines for preparing core clinical-safety information: second edition [Internet]. Council for International Organizations of Medical Sciences Working Groups III and V; 1999 [cited August 20, 2021]. Available from: https://cioms.ch/wp-content/uploads/2018/03/Guidelines-for-Preparing-Core-Clinical-Safety-Info-Drugs-Report-of-CIOMS-Working-Group-III-and-V.pdf.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
