Back to Journals » Patient Preference and Adherence » Volume 16
Patient Preferences for Attributes of Biologic Treatments in Moderate to Severe Asthma: A Discrete Choice Experiment Study
Authors Yang M, Chao J, Fillbrunn M, Mallya UG, Wang MJ, Franke L, Cohn L, Kamat S
Received 24 March 2022
Accepted for publication 9 August 2022
Published 23 September 2022 Volume 2022:16 Pages 2649—2661
DOI https://doi.org/10.2147/PPA.S365117
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Johnny Chen
Min Yang,1 Jingdong Chao,2 Mirko Fillbrunn,1 Usha G Mallya,3 Min-Jung Wang,1 Leigh Franke,1 Lauren Cohn,4,5 Siddhesh Kamat2
1Analysis Group, Inc, Boston, MA, USA; 2Regeneron Pharmaceuticals, Inc, Tarrytown, NY, USA; 3Sanofi, Boston, MA, USA; 4Section of Pulmonary, Critical Care and Sleep Medicine, Yale School of Medicine, New Haven, CT, USA; 5VA Connecticut Healthcare System, West Haven, CT, USA
Correspondence: Min Yang, Analysis Group, Inc, 111 Huntington Avenue, Fourteenth Floor, Boston, MA, 02199, USA, Tel +1-617-425-8487, Fax +1-617-425-8001, Email [email protected]
Purpose: Multiple biologics are available for moderate to severe asthma. Given the important relationship between patient engagement in healthcare decision-making and health outcomes, patient preference is an increasingly important consideration. This study elicited patients’ preferences for attributes of biologic therapies for moderate to severe asthma.
Patient and Methods: A discrete choice experiment (DCE) questionnaire was designed to collect data from an existing survey panel of adults with moderate to severe asthma in the United States. Patients were asked to select their preferred hypothetical treatment from profiles with varying attributes related to efficacy, safety, and administration convenience. Conditional logit regression models were used to quantify patient preferences.
Results: Of 301 eligible patients who completed the survey, the mean age was 46.7± 15.1 years and 71.8% were female. Patients had asthma for 22.5± 16.3 years on average, and most (97.3%) had experienced ≥ 1 asthma attack in the past 12 months. Among treatment attributes examined, patients most valued the absence of a black box warning for the risk of a life-threatening allergic reaction, effectiveness of reducing severe asthma exacerbations, and improvement in lung function (all p < 0.001). Home administration setting for subcutaneous injections (vs doctor’s office/clinic) (p = 0.009) and ability of a biologic to treat additional chronic condition(s) (p < 0.05) were also considered important. Dosing frequency and type of injection device were not significant factors.
Conclusion: Patients with moderate to severe asthma valued efficacy and safety over convenience attributes when selecting biologic treatments. Awareness of these preferences can facilitate patient-physician shared decision-making when managing moderate to severe asthma in clinical practice.
Keywords: asthma, patient preference, biologic, treatment
Introduction
Asthma is a common, chronic respiratory disease, affecting millions of people in the United States (US). Patients with moderate to severe asthma may experience frequent respiratory symptoms and exacerbations, reversible airflow obstruction, bronchial hyper-responsiveness, and airway inflammation which, if not well-controlled, can lead to substantial morbidity, impaired health-related quality of life, and potentially asthma-related mortality.1–3 The majority (~70%) of patients with moderate to severe asthma have Type 2 inflammation that is often characterized by eosinophilia, increased fractional concentration of exhaled nitric oxide, or allergen-driven asthma.4–7 Inhaled corticosteroids (ICS), long-acting beta agonists (LABA), and leukotriene-modifying agents are the standard of care for the treatment of moderate to severe asthma. However, many patients still fail to have sustained symptom control, and long-term use of systemic corticosteroids is associated with harmful side effects.8,9
With the availability of biologic therapies that specifically target antibodies, inflammatory molecules, or cell receptors, the clinical management of patients with moderate to severe asthma who are inadequately controlled on ICS + additional controllers has substantially improved.4 As of 2022, six biologics have been approved by the US Food and Drug Administration (FDA) for patients with severe or uncontrolled asthma, and the majority of these medications are delivered via subcutaneous injection.10 These biologics include anti-immunoglobulin E therapy (omalizumab) and inhibitors of interleukin 5 (IL-5; mepolizumab and reslizumab), the IL-5 receptor (benralizumab), the IL-4 receptor (dupilumab), and thymic stromal lymphpoietin (tezepelumab). With the increasing number of treatment options available, an appreciation of treatment preferences from the patient perspective can improve patient engagement and inform patient-physician shared decision-making. The Global Initiative For Asthma (GINA)4 emphasizes the importance of patient preferences during a clinical evaluation, because it has implications on the partnership between the patient and the clinician, the adherence behavior to the treatment and ultimately the clinical outcomes of these patients.
Given the evolving treatment landscape for patients with moderate to severe asthma, we conducted a discrete choice experiment (DCE) survey study (INvestigate preferenceS for and wIllinGness to trade for attributes of moderate to severe astHma Treatments [INSIGHT]) to better understand preferences for clinical benefits, risks, and administration-related attributes of subcutaneous biologic therapies, among patients with self-reported moderate to severe asthma in the US.
Methods
Study Population
Study participants consisted of adults with self-reported moderate to severe asthma in the US. All participants were recruited from an existing voluntary panel of patients as members of a well-established market research firm, Schlesinger Group, with a global presence for health-care surveys. Patients were eligible for the survey if they met the following criteria: (1) were at least 18 years of age; (2) had moderate or severe asthma (meeting at least one of the following criteria in the past 12 months, as informed by the GINA4 and clinical input: use of ICS plus at least one additional controller [eg, LABA, leukotriene modifiers, tiotropium] and asthma exacerbation [ie, requiring an oral steroid burst, urgent care/emergency department visits, or hospitalization], or oral corticosteroid [OCS] use for at least 60 days, or use of any FDA-approved biologic therapies); and (3) were literate in English. In line with the empirical recommendation by the International Society for Pharmacoeconomics and Outcomes Research Good Research Practices for Conjoint Analysis Task Force,11 the target sample size for the DCE was 300 participants. All participants provided informed consent and confirmed eligibility to participate in the study prior to proceeding with the survey. This study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments and was granted an exemption from a full review by the New England Institutional Research Board. The determination of exemptions is based on the nature of the study; studies based on surveys (such as presented herein), interviews, or focus groups, are eligible for exemption.
Survey Design
The survey was designed to collect information on patient characteristics and preferences for biologic treatments from patients with self-reported moderate to severe asthma. The survey collected information on participants’ demographics (age, sex, race/ethnicity, level of education, and employment status), asthma-related treatment history and disease status (past-year asthma attacks, Asthma Control Test [ACT]), general health status, and comorbidities. Treatment history included use of biologics, ICS, LABA, ICS/LABA combination, long-acting muscarinic antagonists, leukotriene modifiers, OCS, short-acting beta agonists (SABA), treatment with an add-on short course of OCS, and asthma-related healthcare resource use (emergency department visits, urgent care visits, and hospitalizations). An add-on short-course of OCS was defined as add-on corticosteroid pills for three days or longer in patients not on maintenance steroids, or a short course of add-on steroid pills for three days or longer at two times or higher than usual dose, for patients on maintenance steroids. The ACT is a self-administered questionnaire that assesses the frequency of shortness of breath, general asthma symptoms, use of rescue medications, impact of asthma on daily functioning, and overall self-assessment of asthma control. Scores range from 5 (poor control) to 25 (complete control), and a score of <20 indicates uncontrolled asthma.12,13
Patient Preferences
The second part of the survey consisted of questions pertaining to patient preferences for subcutaneous biologic injections for asthma, designed using the DCE approach. Treatment attributes and associated levels used in this study were informed by a targeted literature review of biologic treatments that had been approved for moderate to severe asthma by the US FDA at the time of this study, along with input from clinical experts and patients. The FDA drug labels, Phase 3 clinical trial publications, and literature on the experiences of patients with moderate to severe asthma, along with consultation with clinical experts (one round of interview with a clinical expert and one round of survey discussion with another) informed the initial selection of the key attributes and the associated levels, focusing on efficacy, safety, and convenience. The relevance and importance of the attributes and levels were further informed through 1-on-1 phone discussions with ten patients who met the same eligibility criteria. During the interviews, patients provided feedback on relevance, relatability, and interpretability of the attributes. The finalized seven attributes, each with two to four levels (Table 1), were broadly related to treatment efficacy (ie, effects on reducing severe asthma attacks and improving lung function), other clinical benefits or risks (ie, treating additional chronic condition[s]), presence or absence of a black box warning for the risk of a life-threatening allergic reaction, or convenience of a subcutaneous biologic treatment for asthma (ie, subcutaneous injection frequency, type of injection device, and setting for having subcutaneous injection).
 |
Table 1 List of Treatment Attributes and Levels Associated with Biologics Used to Treat Asthma |
A brief tutorial was designed using layman language and visual aids to provide a basic description for each of the treatment attributes used and familiarize participants with the concept of choosing a preferred treatment profile between two options on a choice card. The tutorial was debriefed with the patients and revised as needed based on feedback. In the final survey, the participants would review the tutorial before proceeding to complete the DCE questions.
Sixteen choice cards, each showing two hypothetical treatment profiles, were generated based on a balanced and efficient design, using the SAS (SAS Institute, Cary, NC, US) DCE macro package. An example choice card is displayed in Figure 1. The 16 choice cards were randomly divided into two blocks to reduce response burden. Each block also included two additional choice cards to evaluate internal validity and consistency of participants’ responses. Study participants were randomly assigned to one of the two blocks, such that the number of participants within each block was balanced. All choice cards were randomized in the order of appearance during the survey.
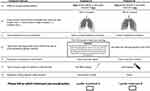 |
Figure 1 Example of a DCE choice card. Abbreviations: DCE, discrete choice experiment; FDA, Food and Drug Administration. |
Statistical Analysis
Participants’ demographics, medical and treatment history, asthma-related disease status, health status, and comorbidities were summarized using relative frequencies for categorical variables, and means, medians, and standard deviations (SDs) for continuous variables.
Conditional logit regression models were used to analyze patient preferences, and regression coefficients and 95% confidence intervals for preference weights were reported. Categorical attributes were modeled using dummy coding. A multiplicative interaction term between injection device type and frequency of injection was included in the model as these two attributes were hypothesized to be conceptually related (eg, preference for the frequency of injections may vary depending upon the type of injection device); in such cases, interaction terms are recommended.14,15
Regression coefficients were also used to assess the relative importance of treatment attributes in participants’ preference decisions among all attributes and among attributes that were significantly associated with treatment preference. To facilitate the interpretation, the relative importance of treatment efficacy outcomes was quantified as follows: 60% reduction in the frequency of severe asthma attacks (an efficacy estimate found in clinical trials)16,17 and 250 mL improvement in lung function (an improvement that is considered moderately better by patients).18 The relative importance of an attribute in participants’ treatment choices was estimated by dividing the difference in part-worth utilities between selected levels of an attribute by the sum of the part-worth utility differences across all attributes. A p-value of 0.05 was used to determine statistical significance. All analyses were performed using SAS (SAS Institute, Cary, NC, US).
Results
Study Population Characteristics
A total of 301 adults with self-reported moderate to severe asthma met the study inclusion criteria and completed the survey (12.5% of those adults with a self-reported asthma who accessed the survey invitation). Participants’ characteristics and general health status are summarized in Table 2, and their clinical and treatment histories are summarized in Table 3. The mean ± SD age of the participants was 46.7 ± 15.1 years, 71.8% were female, and the majority were white/Caucasian (80.1%) and non-Hispanic (85.7%) (Table 2). At the time of the survey, approximately one-third (33.2%) of participants had quit smoking and 41.2% had never smoked (Table 2). On average, participants had multiple chronic comorbidities (mean ± SD: 3.4 ± 2.6), and the most frequently reported comorbidity was anxiety/depression (54.8%) followed by overweight/obesity (34.9%), gastroesophageal reflux disease (33.6%), and hypertension (31.9%) (Table 3). Allergic inflammatory conditions were also common, including atopic dermatitis (17.6%), allergic urticarial (16.3%), chronic rhinosinusitis with nasal polyps (5.3%), and eosinophilic esophagitis (3.3%). In the past 12 months, the majority (97.3%) of participants had experienced at least one asthma attack, with a mean ± SD of 6.4 ± 9.3 attacks. The mean ± SD ACT score was low (14.0 ± 4.5), indicative of poor asthma symptom control, with the majority of participants (85.4%) having uncontrolled asthma (ACT < 20).
 |
Table 2 Demographic Characteristics of Survey Participants |
 |
Table 3 Clinical Characteristics and Treatment History of Participants in the Past 12 Months |
Among these participants, the most common asthma treatments received in the past 12 months were ICS/LABA (82.4%), SABA (74.4%), and OCS used regularly or as needed (61.8%) (Table 3). Only 12% of the participants had received a biologic for asthma in the past 12 months, while the majority (89.4%) received at least one add-on short course of OCS. For health-care resource use, 61.8%, 53.5%, and 38.5% had at least one asthma-related urgent care visit, emergency department visit, and hospitalization, respectively, in the past 12 months.
Preferences for Asthma Treatment
Among the participants, 80.4% replied with consistent responses to the pair of consistency questions. Overall, patients strongly preferred attributes related to greater levels of reduction in the frequency of severe asthma attacks, greater levels of improvement in lung function, and no black box warning issued by the FDA for the risk of a life-threatening allergic reaction (all p < 0.001) (Table 4). Participants preferred treatments administered at home rather than at a doctor’s office or clinic (p = 0.009) and those that could also treat one or more additional chronic conditions over those that did not have such a benefit (all p < 0.05). Dosing frequency and the type of device used for the subcutaneous injections did not significantly affect participants’ preferences for asthma treatments.
 |
Table 4 Preferences for Attributes of Biologic Treatments for Asthmaa |
Attributes’ Relative Importance
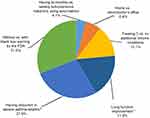
The relative importance of the treatment attributes as a result of the choices made in the DCE questions is illustrated in Figure 2. Across all the attributes, treatment efficacy (severe asthma attack reduction and lung function improvement) accounted for close to half of the choice decision weight while absence/presence of a black box warning accounted for 31% of the weight. The next most important attributes to patients were the ability to treat three chronic conditions (vs no additional chronic conditions; 13.1%) and the ability to administer a subcutaneous injection at home (vs at a doctor’s office/clinic; 6.4%). The combination of having bi-monthly over weekly subcutaneous injections using an auto-injector (vs prefilled syringe) had a minimal impact on the treatment decision (4.1%).
Discussion
Research has shown that patient engagement in healthcare decisions reflecting their treatment preferences can improve adherence to treatment regimens and lead to better clinical outcomes.19–21 GINA guidelines also recommend that the choice of treatment should account for patient preferences, in addition to risk factors and practical considerations.4 Using DCE, a well-established preference research methodology, our study surveyed a large sample of US adults with self-reported moderate to severe asthma to assess key attributes that may influence their preferences for subcutaneous biologic therapies. Most of the existing DCE studies in patients with asthma date back a decade or more and include attributes that are no longer relevant to newer biologics treatments, such as hygiene of an inhaler mouthpiece, while more recent DCE studies have evaluated a limited set of attributes.22–24 With an increasing number of biologic treatment options available for moderate to severe asthma, a better understanding of patient preferences to facilitate shared decision-making is needed.
The population in our study was primarily composed of participants with uncontrolled asthma (85.4%), with only 12% who had received a biologic therapy for asthma in the past 12 months. Compared to the recent real-world studies in patients with severe asthma (ie, the SARP III cohort25 and the ARIETTA study6), our sample was relatively younger (mean age 47 vs 50 years and median age 46 vs 54 years, respectively), and had higher proportions of women (72% vs 67% in both studies), former smokers (33% vs not reported and 18%, respectively), and white/Caucasian patients (80% vs 67% and 89%, respectively).26,27 The proportion of patients who self-reported at least one asthma-related urgent care visit (61.8%), emergency department visit (53.5%), or hospitalization (38.5%) in the past 12 months reflect the large proportion of patients in the present cohort with uncontrolled moderate to severe asthma. The findings are generally similar to those of a claims database analysis by Suruki et al which reported that 53.7% of US patients with uncontrolled severe asthma experienced at least one exacerbation (asthma-related emergency department visits, hospitalization, or oral corticosteroid use) in their 12-month study period.28 In addition, a study by Griswold et al found that 73% of patients with asthma presenting across 83 US emergency departments had at least one emergency department visit in the past year.29 However, a more recent US claims database analysis by Burnette et al reported lower rates among US patients with severe uncontrolled asthma—26.2% of had an asthma-related emergency department visit and 6.3% had a hospitalization in their 12-month study period (may potentially be related to the availability of more biologics for these patients in recent years).30
It is worth noting that among the participants with moderate to severe asthma in our survey, slightly over half had self-reported anxiety or depression. Depression has been previously noted as a common comorbid condition among patients with asthma and is closely correlated with symptom control.31,32 Furthermore, symptoms of anxiety and depression are associated with non-adherence to medications, including both intentional and unintentional non-adherent behaviors.33,34 Therefore, an in-depth understanding of patient characteristics and their preferences for various features of biologic treatments could assist healthcare providers in an informed shared decision-making. This, in turn, may improve treatment adherence and ultimately result in better adherence and downstream treatment outcomes for these patients.
We found that both treatment efficacy attributes (reduction of severe asthma attacks and lung function improvement) were very important in preference decisions. This finding is corroborated by other DCE studies on asthma treatment preferences.23,24 For example, the study by Johansson et al found that the most important attribute for asthma treatments was the number of symptom-free-days per month.24 Similarly, the study by Lloyd et al reported that the reduction of severe asthma attacks and the number of days per week without symptoms were the most important attributes.23 The current study findings are expected given the large number of patients with uncontrolled asthma (85.4%), who may have a strong desire for effective treatments and consider efficacy as a key factor in determining their treatment preference.
With the increasing complexity in clinical management of moderate to severe asthma, particularly when patients have multiple comorbidities, clearly informing benefits and risks of treatment options can provide additional aid for treatment decision. This study’s finding of the strong influence of a black box warning for the risk of a life-threatening allergic reaction on patient preference is unsurprising as asthma itself is an allergic chronic inflammatory disorder. Interviews with patients revealed that patients desired full information about the medication choices and wanted to be made aware of important pros and cons. Some patients had the awareness already and pointed out the value of having the knowledge for the risk of triggering a life-threatening allergic reaction for a balanced consideration.
Additionally, some of the convenience factors (eg, the ability of a therapy to treat one or more additional chronic conditions and an at-home setting for administering subcutaneous injections) were also considered important from a patient’s perspective. The desire to co-treat additional conditions is reasonable considering many patients with asthma have other respiratory or allergic/immune-mediated comorbid conditions.35,36 For patients with such comorbidities, a treatment that can target multiple chronic conditions would be preferable. To a slightly lesser extent, participants preferred being able to administer a subcutaneous injection at home rather than at the clinic or doctor’s office. This finding is likely explained by the inconvenience of traveling to and waiting at the physician’s office, which could lead to more disruption in daily routines. In addition, an at-home administration setting avoids the cost of an outpatient visit or co-pay, reducing the associated financial burden on the patient as well as barriers to treatment. However, the preference for the location of administration varies across studies and is likely to be tied to the route and ease of administration of the biologic. For example, one study found that a roughly equal number of survey participants with asthma preferred biologic administration at home (46%) vs in a healthcare practitioner’s office or clinic (54%).22 While our study only recruited patients in the US, other considerations affecting patients’ preference could be the characteristics of the national or regional health insurance system and the type of reimbursement for biologics.
Based on the literature and input from clinical experts and patients, our study included attributes of biologics for asthma in three categories: treatment efficacy, clinical benefits and risks, and convenience. Among these categories, treatment efficacy and clinical risks were the most important factors in survey participants’ treatment decisions, followed by additional clinical benefit. While the administration setting for the subcutaneous injection mattered to participants, the type of subcutaneous injection device and administration frequency were the only non-significant factors found in our study, indicating that participants did not consider these attributes as important when making a treatment choice decision. Having a holistic appreciation of an individual’s preference could facilitate an informative dialogue between patients and physicians in finalizing the management plan for asthma control.
Incorporating patient perspectives has become increasingly important for the development of new therapies and guiding treatment decisions in clinical practice. For a chronic disease without a cure, such as moderate to severe asthma, maintenance therapies that can suade patients to adherence to treatment is of paramount importance for maintaining disease control, avoiding poor outcomes, and reducing costly healthcare encounters. Our study serves as the initial attempt in quantifying preferences of patients with severe or uncontrolled asthma when navigating biologic treatment options. As the understanding of the mechanism of asthma and treatment mode of action further deepens, continued research is warranted to further appreciate patients’ preferences along with the evolving landscape of complex treatment options.
The findings from our study should be interpreted in consideration of several limitations. First, as with all DCE studies, the preferred choices for hypothetical treatments for asthma do not necessarily reflect the asthma treatments the participants may have received in the real world. Second, attributes in our study were identified based on clinical importance and convenience of treatment management through literature review while taking into account the inputs from clinical experts and patients. However, some of the attributes may not be intuitively comprehensible for patients, for example, the concepts of lung function and FDA black box warning. On the other hand, with the proven effectiveness of the advanced therapies, improvement in lung function (as measured by forced expiratory volume in one second [FEV1]) has become one of the key endpoints assessed in clinical trials. Practically, FEV1 can have a direct impact on a person’s wellbeing, which is the emphasis in patient-centric care. A prior study demonstrated that the average minimal “patient perceivable improvement” for FEV1 is 230 mL.18 To facilitate the interpretation of the lung function concept to be more relatable to patients, the tutorial provided the context to the participants to help them make an informed choice decision:
Many asthma patients have trouble breathing. A breathing test can help measure how easy or difficult it is to get air in and out of your lungs. New treatments could increase how much you can breathe out in 1 second after a deep inhalation, as measured in milliliters (mL). For reference, one cup is about 250 mL. For many asthma patients, a 250 mL increase would be a noticeable improvement.
The wording was reviewed and debriefed with the patients to ensure the interpretability and relatability before the survey fielded for data collection.
Similar efforts were made for the interpretability of the FDA black box warning from the patient perspective. The final description on black box warning was shown as follows:
Some people can be allergic to certain medications. In rare occasions, such allergic reactions to a medication can be serious or even lead to death, thus requiring close monitoring and immediate treatment. When there is reasonable evidence that a drug has been associated with a serious life-threatening adverse event, the FDA issues the black box warning. It is the strictest warning by the FDA to particularly alert doctors to consider benefits and risks when they prescribe the medication. While all medications provide full disclosure, some new asthma treatments may have been associated with reports of severe allergic reactions even after 1 year on treatment. The FDA issues the black box warning for such a medication because of these reports.
The wording was also reviewed and debriefed with the patients to ensure the interpretability and relatability before the survey fielded for data collection.
Third, we conducted 1-on-1 interviews with 10 patients with moderate to severe asthma in the US. However, given the heterogeneity of the disease manifestations and variability in treatment strategies that the patients had experienced, the finalized attributes and levels may not be as comprehensive as we would wish to. In order to manage the response burden, not all attributes that could potentially influence preference decisions were included in our choice card design (eg, participant’s knowledge of asthma and treatments, insurance, out of pocket expenses). Future research could consider these and other attributes of biologic treatments for asthma to help form a deeper understanding of patient preferences. Fourth, participant eligibility was determined using self-reported information (eg, occurrence of asthma attacks in the past 12 months) and may be subject to recall bias. In particular, as the cohort largely comprised patients with uncontrolled asthma and survey answers were self-reported, health-care resource use such as urgent care, emergency department visit, or hospitalization based on a recall over the past 12 months may not be entirely accurate. To help minimize potential recall bias, we provided clear explanations and definitions for terms or events (eg, asthma attacks) that may have subjective interpretation and allowed participants unlimited time to answer the screening questions. Lastly, we included any patient with self-reported moderate to severe asthma to reflect such a patient profile in the real world; patients may or may not have prior experiences with biologic therapies and some patients had chronic pulmonary disease. In addition, in our participant sample, approximately 20% patients indicated having chronic pulmonary disease, which could include chronic obstructive pulmonary disease (not captured specifically). The generalizability of the study findings would warrant caution.
Conclusions
Adults with moderate to severe asthma highly value the safety and efficacy of the treatments while concern less in terms of convenience/administrative characteristics. With multiple biologic therapy options available for patients with moderate to severe asthma in the US, a deeper understanding of patients’ preferences for treatments can play an important role in shared decision-making and impact adherence to these treatments as well as health outcomes. In the current era of patient-centric care, these findings provide supportive evidence to healthcare providers and policy makers to appreciate patients’ preferences in asthma biologic treatment management.
Abbreviations
ACT, Asthma Control Test; CI, confidence interval; DCE, discrete choice experiment; FDA, Food and Drug Administration; FEV1, forced exhalation volume in one second; GINA, Global Initiative for Asthma; ICS, inhaled corticosteroids; INSIGHT, INvestigate preferenceS for and wIllinGness to trade for attributes of moderate to severe astHma Treatments; LABA, long-acting beta agonists; OCS, oral corticosteroids; SABA, short-acting beta agonists; SD, standard deviation; US, United States.
Data Sharing Statement
The associated data to this study are presented in the tables and figures of the manuscript.
Ethics Approval
This study was conducted in accordance with the Helsinki Declaration of 1964 and its later amendments, and was granted an exemption from a full review by the New England Institutional Research Board in November 2019.
Acknowledgments
We wish to thank all of the study participants for their contribution to the study. We are thankful to Vera Mastey, an employee of Regeneron, for her valuable input in the development of the manuscript. Medical writing assistance was provided by Shelley Batts, PhD and Loraine Georgy, PhD, employees of Analysis Group, Inc. Funding for this assistance was provided by Regeneron and Sanofi.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Financial support for the study was provided by Regeneron Pharmaceuticals, Inc. and Sanofi. Regeneron and Sanofi participated in the study concept, interpretation of data, review, and approval of the publication.
Disclosure
Min Yang and Mirko Fillbrunn, are employees of Analysis Group, Inc., and Min-Jung Wang and Leigh Franke were employees of Analysis Group, Inc. at the time of the study conduct, which has received consultancy fees from Regeneron Pharmaceuticals, Inc. and Sanofi for this study. Jingdong Chao and Siddhesh Kamat are employees of Regeneron Pharmaceuticals, Inc. and own Regeneron Pharmaceuticals, Inc. stocks. Usha G. Mallya was an employee of Sanofi at the time of the study conduct and owns Sanofi stocks. Lauren Cohn is an employee of Yale School of Medicine, Section of Pulmonary, Critical Care and Sleep Medicine, Yale School of Medicine and the VA Connecticut Healthcare System, and received consulting fees from AstraZeneca, BioHaven, Genentech, GlaxoSmithKline, Novartis, Pieris, Regeneron and Sanofi. Dr. Cohn has been a speaker for the following companies: Genentech, GlaxoSmithKline. The authors report no other conflicts of interest in this work.
References
1. McCracken JL, Veeranki SP, Ameredes BT, Calhoun WJ. Diagnosis and management of asthma in adults: a review. JAMA. 2017;318(3):279–290. doi:10.1001/jama.2017.8372
2. Bateman E, Hurd S, Barnes P, et al. Global strategy for asthma management and prevention: GINA executive summary. Eur Respir J. 2008;31(1):143–178. doi:10.1183/09031936.00138707
3. Murphy KR, Bender BG. Treatment of moderate to severe asthma: patient perspectives on combination inhaler therapy and implications for adherence. J Asthma Allergy. 2009;2:63. doi:10.2147/JAA.S4214
4. Global Initiative for Asthma. Global strategy for asthma management and prevention; 2020. Available from: https://ginasthma.org/gina-reports/gina-2020-full-report_-final-_wms/.
5. Fahy JV. Type 2 inflammation in asthma – present in most, absent in many. Nat Rev Immunol. 2015;15(1):57–65. doi:10.1038/nri3786
6. Buhl R, Korn S, Menzies-Gow A, et al. Prospective, single-arm, longitudinal study of biomarkers in real-world patients with severe asthma. J Allergy Clin Immunol Pract. 2020;8(8):2630–2639. e2636. doi:10.1016/j.jaip.2020.03.038
7. Wang E, Wechsler ME, Tran TN, et al. Characterization of severe asthma worldwide: data from the international severe asthma registry. Chest. 2020;157(4):790–804. doi:10.1016/j.chest.2019.10.053
8. Bateman ED, Boushey HA, Bousquet J, et al. Can guideline-defined asthma control be achieved? The gaining optimal asthma control study. Am J Respir Crit Care Med. 2004;170(8):836–844. doi:10.1164/rccm.200401-033OC
9. Volmer T, Effenberger T, Trautner C, Buhl R. Consequences of long-term oral corticosteroid therapy and its side-effects in severe asthma in adults: a focused review of the impact data in the literature. Eur Respir J. 2018;52:4. doi:10.1183/13993003.00703-2018
10. Doroudchi A, Pathria M, Modena BD. Asthma biologics: comparing trial designs, patient cohorts and study results. Ann Allergy Asthma Immunol. 2020;124(1):44–56. doi:10.1016/j.anai.2019.10.016
11. Bridges JF, Hauber AB, Marshall D, et al. Conjoint analysis applications in health – a checklist: a report of the ISPOR good research practices for conjoint analysis task force. Value Health. 2011;14(4):403–413. doi:10.1016/j.jval.2010.11.013
12. American Thoracic Society. Asthma Control Test (ACT); 2020. Available from: https://www.thoracic.org/members/assemblies/assemblies/srn/questionaires/act.php.
13. Schatz M, Zeiger RS, Drane A, et al. Reliability and predictive validity of the asthma control test administered by telephone calls using speech recognition technology. J Allergy Clin Immunol. 2007;119(2):336–343. doi:10.1016/j.jaci.2006.08.042
14. Hauber AB, Gonzalez JM, Groothuis-Oudshoorn CG, et al. Statistical methods for the analysis of discrete choice experiments: a report of the ISPOR conjoint analysis good research practices task force. Value Health. 2016;19(4):300–315. doi:10.1016/j.jval.2016.04.004
15. Nicolet A, Groothuis-Oudshoorn CGM, Krabbe PFM. Does inclusion of interactions result in higher precision of estimated health state values? Value Health. 2018;21(12):1437–1444. doi:10.1016/j.jval.2018.06.001
16. Tepper RS, Wise RS, Covar R, et al. Asthma outcomes: pulmonary physiology. J Allergy Clin Immunol. 2012;129(3):S65–S87. doi:10.1016/j.jaci.2011.12.986
17. Schmidt HH, Waddington-Cruz M, Botteman MF, et al. Estimating the global prevalence of transthyretin familial amyloid polyneuropathy. Muscle Nerve. 2017;57(5):829–837. doi:10.1002/mus.26034
18. Santanello N, Zhang J, Seidenberg B, Reiss T, Barber B. What are minimal important changes for asthma measures in a clinical trial? Eur Respir J. 1999;14(1):23–27. doi:10.1034/j.1399-3003.1999.14a06.x
19. Agency for Healthcare Research and Quality. The CAHPS ambulatory care improvement guide: strategy 6I: shared decision making; 2017. Available from: https://www.ahrq.gov/sites/default/files/wysiwyg/cahps/quality-improvement/improvement-guide/cahps-ambulatory-care-guide-full.pdf.
20. Shay LA, Lafata JE. Where is the evidence? A systematic review of shared decision making and patient outcomes. Med Decis Making. 2015;35(1):114–131. doi:10.1177/0272989X14551638
21. Bachert C, Vignola AM, Gevaert P, Leynaert B, Van Cauwenberge P, Bousquet J. Allergic rhinitis, rhinosinusitis, and asthma: one airway disease. Immunol Allergy Clin North Am. 2004;24(1):19–43. doi:10.1016/S0889-8561(03)00104-8
22. Gelhorn HL, Balantac Z, Ambrose CS, Chung YN, Stone B. Patient and physician preferences for attributes of biologic medications for severe asthma. Patient Prefer Adherence. 2019;13:1253. doi:10.2147/PPA.S198953
23. Lloyd A, McIntosh E, Rabe KF, Williams A. Patient preferences for asthma therapy: a discrete choice experiment. Prim Care Respir J. 2007;16(4):241–248. doi:10.3132/pcrj.2007.00052
24. Johansson G, Stallberg B, Tornling G, et al. Asthma treatment preference study: a conjoint analysis of preferred drug treatments. Chest. 2004;125(3):916–923. doi:10.1378/chest.125.3.916
25. Teague WG, Phillips BR, Fahy JV, et al. Baseline features of the severe asthma research program (SARP III) cohort: differences with age. J Allergy Clin Immunol Pract. 2018;6(2):545–554. e544. doi:10.1016/j.jaip.2017.05.032
26. Castro M, Corren J, Pavord ID, et al. Dupilumab efficacy and safety in moderate-to-severe uncontrolled asthma. N Engl J Med. 2018;378(26):2486–2496. doi:10.1056/NEJMoa1804092
27. Russell RJ, Chachi L, FitzGerald JM, et al. Effect of tralokinumab, an interleukin-13 neutralising monoclonal antibody, on eosinophilic airway inflammation in uncontrolled moderate-to-severe asthma (MESOS): a multicentre, double-blind, randomised, placebo-controlled Phase 2 trial. Lancet Respir Med. 2018;6(7):499–510. doi:10.1016/S2213-2600(18)30201-7
28. Suruki RY, Daugherty JB, Boudiaf N, Albers FC. The frequency of asthma exacerbations and healthcare utilization in patients with asthma from the UK and USA. BMC Pulm Med. 2017;17(1):74. doi:10.1186/s12890-017-0409-3
29. Griswold SK, Nordstrom CR, Clark S, Gaeta TJ, Price ML, Camargo CA
30. Burnette A, Wang Y, Rane P, et al. Real-world asthma-related health care resource utilization and costs among patients with severe uncontrolled asthma 2022 AMCP Meeting. Chicago, IL; 2022.
31. Di Marco F, Verga M, Santus P, et al. Close correlation between anxiety, depression, and asthma control. Respir Med. 2010;104(1):22–28. doi:10.1016/j.rmed.2009.08.005
32. Di Marco F, Santus P, Centanni S. Anxiety and depression in asthma. Curr Opin Pulm Med. 2011;17:1. doi:10.1097/MCP.0b013e3283411440
33. DiMatteo MRL, Croghan TW. Depression is a risk factor for noncompliance with medical treatment meta-analysis of the effects of anxiety and depression on patient adherence. Arch Intern Med. 2000;160(14):2101–2107. doi:10.1001/archinte.160.14.2101
34. Sundbom LT, Bingefors K. The influence of symptoms of anxiety and depression on medication nonadherence and its causes: a population based survey of prescription drug users in Sweden. Patient Prefer Adherence. 2013;7:805–811. doi:10.2147/PPA.S50055
35. Boulet LP. Influence of comorbid conditions on asthma. Eur Respir J. 2009;33(4):897–906. doi:10.1183/09031936.00121308
36. Boulet LP, Boulay M. Asthma-related comorbidities. Expert Rev Respir Med. 2011;5(3):377–393. doi:10.1586/ers.11.34
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.