Back to Journals » Journal of Asthma and Allergy » Volume 16
Effect of Biologic Therapies on Airway Hyperresponsiveness and Allergic Response: A Systematic Literature Review
Authors Spahn JD, Brightling CE, O’Byrne PM, Simpson LJ, Molfino NA, Ambrose CS , Martin N, Hallstrand TS
Received 28 March 2023
Accepted for publication 28 June 2023
Published 21 July 2023 Volume 2023:16 Pages 755—774
DOI https://doi.org/10.2147/JAA.S410592
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Luis Garcia-Marcos
Joseph D Spahn,1 Christopher E Brightling,2 Paul M O’Byrne,3 Lisa J Simpson,4 Nestor A Molfino,5 Christopher S Ambrose,6 Neil Martin,2,7 Teal S Hallstrand8
1Respiratory and Immunology, BioPharmaceuticals Medical, AstraZeneca, Wilmington, DE, USA; 2Institute for Lung Health, NIHR Leicester Biomedical Research Centre, Department of Respiratory Sciences, University of Leicester, Leicester, UK; 3Firestone Institute for Respiratory Health, St Joseph’s Hospital and Department of Medicine, McMaster University, Hamilton, Ontario, Canada; 4PharmaGenesis London, London, UK; 5Global Development, Amgen, Thousand Oaks, CA, USA; 6Respiratory and Immunology, BioPharmaceuticals Medical, AstraZeneca, Gaithersburg, MD, USA; 7Respiratory and Immunology, BioPharmaceuticals Medical, AstraZeneca, Cambridge, UK; 8Division of Pulmonary, Critical Care and Sleep Medicine, and the Center for Lung Biology, Department of Medicine, University of Washington, Seattle, WA, USA
Correspondence: Joseph D Spahn, AstraZeneca, 1800 Concord Pike, Wilmington, DE, 19803, USA, Tel +1 303-886-5257, Email [email protected]
Background: Airway hyperresponsiveness (AHR) is a key feature of asthma. Biologic therapies used to treat asthma target specific components of the inflammatory pathway, and their effects on AHR can provide valuable information about the underlying disease pathophysiology. This review summarizes the available evidence regarding the effects of biologics on allergen-specific and non-allergen-specific airway responses in patients with asthma.
Methods: We conducted a systematic review in accordance with Preferred Reporting Items for Systematic Reviews and Meta-Analysis (PRISMA) guidelines, including risk-of-bias assessment. PubMed and Ovid were searched for studies published between January 1997 and December 2021. Eligible studies were randomized, placebo-controlled trials that assessed the effects of biologics on AHR, early allergic response (EAR) and/or late allergic response (LAR) in patients with asthma.
Results: Thirty studies were identified for inclusion. Bronchoprovocation testing was allergen-specific in 18 studies and non-allergen-specific in 12 studies. Omalizumab reduced AHR to methacholine, acetylcholine or adenosine monophosphate (3/9 studies), and reduced EAR (4/5 studies) and LAR (2/3 studies). Mepolizumab had no effect on AHR (3/3 studies), EAR or LAR (1/1 study). Tezepelumab reduced AHR to methacholine or mannitol (3/3 studies), and reduced EAR and LAR (1/1 study). Pitrakinra reduced LAR, with no effect on AHR (1/1 study). Etanercept reduced AHR to methacholine (1/2 studies). No effects were observed for lebrikizumab, tocilizumab, efalizumab, IMA-638 and anti-OX40 ligand on AHR, EAR or LAR; benralizumab on LAR; tralokinumab on AHR; and Ro-24-7472 on AHR or LAR (all 1/1 study each). No dupilumab or reslizumab studies were identified.
Conclusion: Omalizumab and tezepelumab reduced EAR and LAR to allergens. Tezepelumab consistently reduced AHR to methacholine or mannitol. These findings provide insights into AHR mechanisms and the precise effects of asthma biologics. Furthermore, findings suggest that tezepelumab broadly targets allergen-specific and non-allergic forms of AHR, and the underlying cells and mediators involved in asthma.
Keywords: airway hyperresponsiveness, allergic response, biologic, systematic review, asthma, randomized placebo-controlled trial
Introduction
Airway hyperresponsiveness (AHR) is a hallmark pathophysiologic feature of asthma and constitutes a heightened responsiveness to inhaled bronchoconstrictors and/or the increased production of mediators of bronchoconstriction.1–3 Historically, treatment strategies to normalize AHR have been associated with clinically important outcomes in asthma, including reduction in exacerbation rates and histopathologic features of remodeling and inflammation.4
Direct AHR in humans is related to the degree of baseline airflow obstruction and has been further related to changes in the amount of airway smooth muscle,5 in addition to the infiltration of airway smooth muscle by inflammatory cells, such as mast cells.6,7 The interaction between airway smooth muscle and mast cells, driven by inflammation, can ultimately lead to airway remodeling. In mice, the type-2 (T2) cytokine interleukin (IL)-13 plays a central role in the development of direct AHR to methacholine.8–10 Indirect AHR has been associated with the severity of cellular inflammation of the airways, particularly a shift in the number and type of mast cells in the airway epithelium11,12 and infiltration of the epithelium with eosinophils.13 Epithelial cytokines, including IL-33 and thymic stromal lymphopoietin (TSLP), represent upstream regulators of inflammation, including both T2 and non-T2 inflammatory pathways.14
AHR is assessed by bronchoprovocation testing.1,2 The tests can be subdivided according to whether the bronchoconstrictor is administered during the test or is endogenously generated in the airways, and whether the response requires allergen sensitization. Direct challenge tests use exogenous agonists (such as methacholine, acetylcholine or histamine) that directly interact with receptors on airway smooth muscle and other cells, resulting in bronchoconstriction.1 Indirect challenge tests involve triggering the endogenous release of inflammatory mediators by an osmotic challenge (such as mannitol or hypertonic saline), an adenosine monophosphate (AMP) challenge or a hyperpnea challenge (such as eucapnic voluntary hyperventilation or dry-air exercise).2 Direct challenge tests are sensitive for asthma detection but are non-specific because many obstructive lung diseases can yield a positive result.1 Thus, methacholine testing is frequently used to rule out asthma.1 In contrast, assessments such as mannitol and exercise challenges are generally thought to be asthma-specific but less sensitive than direct challenge tests for asthma detection overall.2 Indirect bronchoprovocation can also be conducted using an allergen-specific challenge in patients with allergen sensitization. Allergen challenge testing is used to assess both the early allergic response (EAR), which develops within the first 15 minutes of allergen administration, and the late allergic response (LAR), which starts to develop 3–4 hours after allergen administration and is associated with a period of cellular inflammation and an increase in both direct and indirect AHR.3
In recent years, there has been a growing number of targeted biologic (ie monoclonal antibody [mAb]) therapies approved for the treatment of severe, uncontrolled asthma. Six biologics are currently approved by the US Food and Drug Administration (FDA) for use in the treatment of asthma: omalizumab, an anti-immunoglobulin E (anti-IgE) mAb; mepolizumab, an anti-IL-5 mAb; reslizumab, an anti-IL-5 mAb; benralizumab, an anti-IL-5 receptor mAb; dupilumab, an anti-IL-4 receptor α mAb; and tezepelumab, an anti-TSLP mAb.15 Biologic therapies target specific components of the inflammatory pathway, and their differential effects on AHR, EAR and LAR can provide information on therapeutic effects, as well as the underlying biology of asthma. Studies have been conducted on the effects of individual biologics on AHR, EAR and LAR; however, to our knowledge, there are currently no systematic literature reviews that compare the effects of approved, non-approved (eg investigational and/or off-label) and discontinued biologics on these outcomes in patients with asthma. The objective of this systematic literature review was to summarize the available evidence regarding the effects of biologic therapies on AHR, EAR and LAR in patients with asthma.
Methods
Literature Search
A systematic literature review was conducted and reported in accordance with the Preferred Reporting Items for Systematic Reviews and Meta-Analyses (PRISMA) guidelines,16 though this systematic review has not been registered and a review protocol was not prepared. We systematically searched PubMed and Ovid (MEDLINE and Embase) to identify randomized, placebo-controlled studies published between January 1, 1997 and December 17, 2021 (inclusive) that examined the effects of biologics on AHR, EAR and/or LAR in patients with asthma. The search string contained terms relating to randomized, placebo-controlled clinical trials; crossover studies; asthma; biologic and tumor necrosis factor (TNF)-α therapies; allergic stimuli; AHR challenges; EAR; and LAR (Table S1). No language restrictions were applied. Two reviewers (one author and one non-author) independently screened the results based on the titles and abstracts, and then assessed the eligibility of the publications according to specific inclusion criteria (Table S2).
Inclusion and Exclusion Criteria
Publications were eligible for inclusion if they reported randomized, placebo-controlled studies of biologic therapies in patients with asthma, irrespective of asthma type or severity, and reported measurements of AHR, EAR and/or LAR to allergen-specific and non‑allergen-specific stimuli. The biologics could be approved, non‑approved or discontinued. Both full publications and congress abstracts were eligible for inclusion. Reviews and meta-analyses were excluded.
Data Extraction and Summary
Data were extracted into a standardized form by one reviewer (author), and the second reviewer (non-author) verified the accuracy of data entry. The extracted data included information on the study design (including inclusion and exclusion criteria), the study population (such as baseline demographic and clinical characteristics), the biologics assessed and their effects on AHR, EAR and/or LAR (Tables S3–S5).
Bias Assessment
Risk of bias in the eligible studies was assessed using the revised Cochrane risk-of-bias tool for randomized trials (RoB 2).17 RoB 2 evaluates the risk of bias in the results arising from the randomization process, deviations from the intended interventions, missing outcome data, measurement of outcomes and selective reporting.17
Results
Literature Search, Screening and Publication Selection
The systematic literature search identified 950 publications (Figure 1 and Table S2). Of these, 898 were excluded following screening of the title and abstract. The most common reasons for exclusion were duplication in search retrievals (n = 489) and interventions that did not match the review criteria (n = 237). The remaining 52 publications underwent screening of the full text. Thirty publications met the eligibility criteria and were included in this review (Table 1).18–47 Eighteen studies utilized allergen-specific airway challenge testing for AHR, EAR and/or LAR; the remaining 12 studies used non-allergen-specific testing.
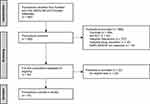 |
Figure 1 PRISMA flow diagram summarizing the selection process for publications included in the review. Abbreviations: AHR, airway hyperresponsiveness; EAR, early allergic response; LAR, late allergic response; PRISMA, Preferred Reporting Items for Systematic Reviews and Meta-Analyses; RCT, randomized controlled trial. Notes: Adapted from Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. Creative Commons.16 |
 |
Table 1 Characteristics of the Included Trials and Publications |
Of the 30 publications, 11 were found to have a low risk of bias (Figure S1). The remaining 19 publications did not report whether analyses were pre-specified or if the trial was pre-registered, and there was thus a potential risk of bias in the selection of results to report.
Characteristics of the Included Studies
Characteristics of the included studies are summarized in Table 1 (see Table S3 for further details). The number of patients was in the range 11–99 per study, and the treatment period duration was in the range 2–52 weeks. In more than half of the included studies (16/30), the population was described as having mild asthma (2 studies), mild allergic asthma (10 studies) or mild atopic asthma (4 studies). In the allergen challenge studies (18/30), patients had predominantly mild and/or allergic asthma (one study did not report the patient population) and patients were not receiving inhaled corticosteroids (ICS) at trial entry in 8 studies. Of the nine studies where ICS use was not directly reported, the study design criteria of three of these studies indirectly suggest that patients were receiving ICS at study entry. One allergen challenge study included patients who were receiving ICS at study entry. In the non-allergen challenge AHR studies (12/30), included patient populations were described as having mild-to-moderate or refractory asthma (7 studies), or moderate-to-severe, uncontrolled asthma (4 studies), with the remaining study assessing a population with persistent asthma. Biologic therapies assessed were: omalizumab (10 studies); mepolizumab (3 studies); tezepelumab (3 studies); etanercept (2 studies); and benralizumab, lebrikizumab, tralokinumab, tocilizumab, efalizumab, pitrakinra, IMA-638, MEMP1972A, CSJ117, recombinant human platelet-activating factor acetylhydrolase (rhPAF-AH), Ro-24-7472 and anti-OX40 ligand (1 study each). No published studies assessing dupilumab or reslizumab were identified.
Patient Baseline Characteristics
Baseline demographic and clinical characteristics of patients who participated in each study arm are summarized in Table 2 (see Table S4 for further details). Mean age of patients was in the range 16–50 years across study arms. Body mass index was reported in six studies, with the mean in the range 25–30 kg/m2. Of the 19 studies that reported smoking history, none included patients that were current smokers. Across the included studies, the pre-bronchodilator forced expiratory volume in 1 second (FEV1) was in the range 2.18–4.09 L, and the pre-bronchodilator FEV1 percentage of predicted normal values was in the range 62–102%.
 |
Table 2 Baseline Demographic and Clinical Characteristics |
Effects of Biologic Therapies on AHR
Twenty-six studies assessed the effect of biologic therapies on AHR in patients with asthma (Figure 2 and Table S5). Omalizumab (anti-IgE mAb) was the most extensively studied biologic and reduced AHR (3/9 studies) to various challenges: methacholine (1/7 studies),18 acetylcholine (1/1 study)21 and AMP (1/2 studies); one of the studies assessed both methacholine and AMP.27 The initial allergen challenge studies that assessed AHR in the late 1990s reported conflicting results.18,19 In a study of 20 patients with mild allergic asthma, omalizumab treatment reduced methacholine responsiveness; the methacholine concentration that provokes a 20% decrease in FEV1 (PC20) increased from 0.73 mg/mL at baseline to 1.34 mg/mL at day 76 of treatment (0.9 doubling dose); p = 0.048.18 In contrast, another study, also in patients with mild allergic asthma, found no change in methacholine PC20.19 Furthermore, another study showed that inhaled omalizumab, administered daily, also failed to affect methacholine reactivity.20 Two studies assessed the effect of omalizumab on both direct (methacholine) and indirect (hypertonic saline or AMP) measures of AHR.23,24 In one of these studies, omalizumab had no effect on either methacholine or hypertonic saline challenge in 18 patients with mild allergic asthma;24 in the second study, in mild-to-moderate asthma, omalizumab significantly reduced AMP reactivity compared with placebo at 4 weeks but not at 12 weeks, and had no effect on methacholine responsiveness compared with placebo.23 Two bronchoscopy studies assessed the effect of omalizumab on airway inflammation and AHR to methacholine.22,25 In 25 patients with mild allergic asthma, 12 weeks of omalizumab treatment reduced airway tissue and sputum eosinophil counts compared with placebo, while having no effect on methacholine responsiveness.25 In another study of 45 patients with mild-to-moderate asthma with at least 2% sputum eosinophils, omalizumab had no effect on methacholine responsiveness despite reductions in the concentration of serum IgE and IgE+ cells in airway tissue and reductions in the abundance of a number of tissue inflammatory cells (eosinophils, cluster of differentiation [CD]3+, CD4+ and CD8+ T lymphocytes and B lymphocytes).22 In a study of 35 patients with moderate-to-severe allergic asthma, acetylcholine PC20 increased from 1.0 mg/mL to 1.43 mg/mL (0.43 doubling concentration) in omalizumab-treated patients, while minimal change was noted with placebo (p < 0.05; omalizumab vs placebo).21 In parallel, serum IL-13 levels decreased significantly in patients receiving omalizumab compared with those receiving placebo. In a crossover study of adults and adolescents (mean age ± standard deviation, 16.4 ± 5.5 years) with poorly controlled asthma and inadequate adherence to ICS therapy, there was a 3.1-fold and a 0.9-fold increase in adenosine PC20 during omalizumab and placebo treatment, respectively (estimated ratio 3.4; p = 0.02).27 None of the reviewed studies assessed the effect of omalizumab on mannitol responsiveness.
The anti-IL-5 mAb mepolizumab was assessed in three studies and had no effect on AHR to histamine (2/2 studies)28,29 or to methacholine (1/1 study).30 In a study of 24 patients with mild allergic asthma, a single dose of mepolizumab did not reduce AHR to histamine, despite lowering the degree of sputum eosinophilia compared with placebo.28 In a bronchoscopy study of 24 patients with mild asthma who underwent histamine challenges at screening and after three intravenous doses of mepolizumab, AHR was not reduced compared with placebo despite reductions in blood and airway tissue eosinophil counts.29 Lastly, methacholine challenges were assessed in 34 of 61 patients with refractory eosinophilic asthma who received intravenous mepolizumab or placebo over 12 months. Despite reductions in blood and sputum eosinophil counts with treatment, mepolizumab had no effect on FEV1 or AHR.30
The anti-TSLP mAb tezepelumab, which was assessed in three studies, reduced AHR to methacholine (1/1 study)32 and to mannitol (2/2 studies).33,34 In a phase 1 allergen challenge study of 31 patients with mild allergic asthma and normal lung function (FEV1, 95.4% and 97.6% of predicted normal values for tezepelumab and placebo, respectively), tezepelumab treatment was associated with a 0.7 doubling concentration increase in methacholine PC20 compared with placebo (p < 0.05).32 Reductions in sputum and blood eosinophil counts and in fractional exhaled nitric oxide (FeNO) level were also noted in the tezepelumab-treated group.32 In a phase 2 mechanistic study of 99 adults with moderate-to-severe, poorly controlled asthma receiving medium-to-high dose ICS (FEV1, 69% of predicted normal values), reduction in airway submucosal eosinophil count was 89% with tezepelumab and 25% with placebo.33 There was also a significant reduction in AHR to mannitol in patients who received tezepelumab compared with placebo, both in terms of the mannitol dose that provokes a reduction of at least 15% in FEV1 (PD15) (197.4 mg vs 58.6 mg, difference of 138.8 mg; p = 0.03) and doubling doses (1.41 vs 0.57, difference of 0.84; p = 0.04, n = 48).33 Tezepelumab treatment also numerically reduced the concentrations of blood eosinophils, serum IL-5 and IL-13, and FeNO levels.33 In a second phase 2 study in 40 patients with poorly controlled asthma despite treatment with ICS (FEV1, 88.7% of predicted normal values), tezepelumab treatment decreased eosinophil counts in tissue, bronchiolar lavage fluid and sputum. There was a 0.9 doubling dose improvement in PD15 (1.9 and 1.0 for tezepelumab and placebo, respectively; p = 0.06), with a higher proportion of tezepelumab recipients able to normalize mannitol responsiveness than those receiving placebo (45% vs 15%; p = 0.04).34
The TNF receptor etanercept reduced AHR to methacholine in one of two studies.39 In a randomized, double-blind, placebo-controlled, crossover pilot study in 10 patients with refractory asthma (FEV1, 62% of predicted normal values), etanercept treatment improved PC20 compared with placebo, being associated with a 2.3 versus a −1.2 doubling concentration of methacholine (mean difference, 3.5; p = 0.05). This effect occurred despite no change in sputum eosinophil percentage, sputum eosinophil cationic protein concentration and FeNO level; however, a reduction in sputum histamine concentration was observed.39 In a second study in 21 patients with mild-to-moderate allergic asthma (FEV1, 92% and 96% predicted normal for etanercept and placebo, respectively), etanercept had no effect on responsiveness to methacholine (PD20 95% confidence interval: −0.202, 0.053 mg; p = 0.4).40
Anti-IL-13 mAbs were assessed in three studies and did not reduce AHR in any of these. In allergen challenge studies, neither IMA-638 nor lebrikizumab had an effect on post-allergen increases in AHR, as measured by methacholine.35,42 In a phase 2 mechanistic study, 79 patients with inadequately controlled, moderate-to-severe asthma received tralokinumab 300 mg or placebo every 2 weeks for 12 weeks.36 No reductions were observed in blood and tissue eosinophil counts, although FeNO level and total blood IgE concentration were reduced.36 Methacholine challenges were performed in 39 patients before and after tralokinumab treatment, and there was no significant change in AHR.36 Pitrakinra, an anti-IL-4 receptor antagonist, failed to improve AHR to methacholine in patients with mild allergic asthma when treatment was administered subcutaneously, and to AMP when treatment was administered by inhalation.41 No effect on AHR to methacholine was observed with the anti-IL-6 mAb tocilizumab, the anti-αLβ2 integrin α-chain (anti-CD11a) mAb efalizumab, the anti-M1 prime MEMP1972A or the anti-OX40 ligand (1/1 study each).37,38,43,47 The recombinant IL-12 Ro-24-7472 had no effect on AHR to histamine (1/1 study).46
Effects of Biologic Therapies on EAR and LAR
Eighteen studies assessed biologic therapies and their effects on EAR and/or LAR in asthma (Figure 3 and Table S5). Five were allergen challenge studies of omalizumab.18–20,25,26 Omalizumab reduced the EAR in four studies (4/5 studies)18,19,25,26 and the LAR in two studies (2/3 studies).19,25 Mepolizumab, benralizumab, lebrikizumab, tocilizumab and efalizumab, each assessed in one study, had no effect on the EAR or LAR.28,31,35,37,38 Tezepelumab reduced the EAR and LAR in one study.32 Only the inhaled formulation of pitrakinra (60 mg dose) reduced the LAR; no effects on the EAR were observed with either the subcutaneous or inhaled formulations (1/1 study).41 IMA-638, rhPAF-AH, Ro-24-7472 and anti-OX40 ligand had no effects on the EAR or LAR when reported (1/1 study each).42,45–47 MEMP1972A reduced only the EAR, and CSJ117 reduced only the LAR (1/1 study each).43,44
Discussion
This systematic literature review summarizes, for the first time, the published evidence regarding the effects of targeted biologic therapies on AHR, EAR and LAR in patients with asthma. To the best of our knowledge, there have been no published systematic literature reviews to date comparing the effects of biologic treatments (approved, non-approved and discontinued) on AHR, EAR and LAR in patients with asthma. In most studies, the population was described as having mild, mild allergic or mild atopic asthma, although patients with moderate-to-severe asthma were evaluated in one of 10 omalizumab studies,21 one of three mepolizumab studies,30 the one tralokinumab study36 and two of three tezepelumab studies.33,34 Tezepelumab and omalizumab were the only biologics with positive AHR outcome data for patients with moderate-to-severe asthma. Of the FDA-approved biologics, only omalizumab and tezepelumab reduced AHR as well as both the EAR and LAR. However, the effects of omalizumab on AHR were inconsistent across studies, which differed in dose and delivery, asthma severity at baseline and type of challenge administered. For example, Boulet et al, who reported a positive effect of omalizumab on methacholine reactivity, assessed the effect of intravenous omalizumab (initial dose, 2 mg/kg, then 1 mg/kg on days 7, 14, 28, 56 and 70),18 while other studies used subcutaneously administered omalizumab 0.016 mg/kg per IgE (IU/mL).21–23,25 In six of the nine studies which assessed the effect of omalizumab on AHR, patients with asthma were either naive to steroids or their steroid use was not reported, and these patients predominantly had mild or mild-to-moderate disease. In the remaining three studies which assessed the effect of omalizumab on AHR, patients were receiving ICS treatment from study entry and were described as having mild-moderate, persistent allergic asthma (1 study), moderate-to-severe allergic asthma (1 study) or persistent asthma (1 study). Direct measures of AHR were most often utilized (methacholine, n = 7;18–20,22–25 acetylcholine, n = 121) compared with indirect measures (AMP, n = 223,27). In contrast, the ability of tezepelumab to reduce AHR was observed in all three studies assessed (one methacholine challenge32 and two mannitol challenges33,34); in the two tezepelumab studies where mannitol challenges were used, patients were described as having moderate to severe and/or uncontrolled asthma and were receiving ICS treatment (medium to high dose) from study entry.
A systematic literature review of the effect of approved biologics on airway smooth muscle contractility concluded that omalizumab, tezepelumab and dupilumab can directly modulate the contractility of airway smooth muscle to prevent AHR, whereas mepolizumab and benralizumab have indirect effects.15 However, unlike this review, the publication included data from non-clinical (ex vivo, in vitro and in vivo) and observational studies. Indeed, the conclusion regarding the direct effect of dupilumab and the indirect effects of mepolizumab and benralizumab were based solely on non-clinical data. The authors also commented on in vitro and in vivo data supporting an improvement in AHR with omalizumab; however they highlighted that in several randomized studies19,22,23 and an observational study,48 no beneficial effect on AHR was reported.15 Of note, more recently, Chan et al have demonstrated that benralizumab significantly reduced AHR as measured by hyperresponsiveness to mannitol in patients with severe eosinophilic asthma.49 Although these findings are from a clinical study, it was of open-label design and not placebo-controlled, and, therefore, would not have been eligible for inclusion in this systematic review.
The findings across biologics suggest that inhibition of T2-specific pathways alone (eg those targeting eosinophils, IL-13) is not sufficient to affect AHR meaningfully. Inhibition of pathways that affect mast cell activation appears to be necessary (eg those targeting TSLP, anti-IgE), which is supported by the finding that imatinib, a c-kit tyrosine kinase inhibitor that targets mast cells, reduced AHR in patients with severe refractory asthma (imatinib was not included in the current review because it is a small molecule).50 Reductions in AHR with imatinib were noted without an associated effect on eosinophil counts in blood or bronchiolar lavage fluid or in FeNO level, and there was a negative correlation between peripheral blood eosinophil counts and changes in AHR; these findings suggest a mechanism targeting both T2 and non-T2 pathways in mast cells.50 Mast cells serve as a source of T2 cytokines51 and a shift in mast cells to the airway epithelium has been associated with both T2 gene expression in the airways and indirect AHR in the form of exercise-induced bronchoconstriction (EIB), a form of AHR that correlates with mannitol induced AHR.11 However, non-T2 mast cell-derived products are also implicated in AHR as some patients with EIB do not have high T2 gene expression.11 An example of a non-T2 mast cell derived product may be TNF, which has been implicated in AHR in rodent models.52,53 Consistent with this observation is the finding that etanercept, which acts on TNF, reduced AHR to methacholine in one of two studies.39,40 Prior work has shown that mast cells that infiltrate the airway smooth muscle are associated with direct AHR to methacholine, and it has been suggested that AHR present in paucigranulocytic asthma is the result of mast cell infiltration of airway smooth muscle bundles.54 The effect of tezepelumab on AHR could explain, at least in part, the reduction in exacerbation rates observed in patients with T2-low asthma.55,56
IL-13 has been associated with promoting AHR-related mechanisms in preclinical mouse and in vitro studies,8,9 and roles have been suggested in airway tone and AHR in patients with asthma. It was unexpected that in phase 3 studies, the anti-IL-13 mAbs tralokinumab and lebrikizumab failed to reduce exacerbation rates (primary endpoint) effectively in severe asthma57,58 and, thus, never became approved medications for this indication. Neither of these drugs, nor a third drug of this class, IMA-638, had an impact on AHR.35,36,42 Pitrakinra, which blocks the activity of IL-4 and IL-13, also failed to significantly affect AHR.41
Although allergen challenge tests are not performed in clinical practice owing to their complexity and safety issues concerning the delivery of inhaled allergens to the airways of patients with asthma, they can be used in clinical trials of investigational medications. Allergen challenge studies can probe many of the physiologic and inflammatory manifestations of asthma including reversible airflow limitation, airway inflammation and AHR. The EAR occurs in the 15 minutes following inhalation of an allergen, reaching a maximum at 30 minutes, and recovery within 1–3 hours.3 In the majority of patients, bronchoconstriction recurs 3–4 hours later, reaching a maximum over the next 6–12 hours, a process termed the LAR.3 Associated with the LAR is an increase in AHR that can last for several days to weeks.3 The ability of an asthma medication to inhibit allergen-driven outcomes can provide supportive evidence for its clinical efficacy. The LAR is most often selected as the primary outcome measure because it is most closely associated with airway inflammation and results are very reproducible, allowing for smaller study populations. The allergen challenge model also has an excellent negative predictive value, demonstrated by the lack of clinical efficacy of medications failing to affect allergen challenge outcomes.3,59 ICS are the gold standard comparators for investigational therapies because they significantly reduce the LAR while attenuating AHR and reducing airway inflammation.3 The fact that omalizumab and tezepelumab were the only biologics that influence both the EAR and LAR suggests that they both influence the underlying pathophysiologic mechanisms involved in these processes. Histamine and cysteinyl leukotriene from airway mast cells and basophils are the major mediators of the EAR and LAR.60–62 The LAR is also associated with the influx of allergen-induced inflammatory cells such as eosinophils and basophils.60 Omalizumab may act by blocking the effects of IgE on mast cell degranulation and inhibiting eosinophil influx during the LAR.63 Mast cells and airway smooth muscle cells produce both TSLP and its receptor,64 which could be relevant for the effect of tezepelumab on AHR, where tezepelumab likely inhibits mast cell activation in addition to its broad anti-inflammatory effects.14
Contraindications for allergen challenge tests include uncontrolled, or partially controlled, asthma and FEV1 < 70%.65 Contraindications for methacholine and mannitol challenges include FEV1 < 60% or 1.5 L, cardiovascular disease (myocardial infarction, or stroke in past 3 months, uncontrolled hypertension, aortic aneurysm), recent eye surgery or increased intracranial pressure elevation risk, and hypersensitivity to mannitol or the gelatin used to make the capsules for mannitol challenge testing.1,2 Five non-allergen challenge studies in the current review assessed the effects of biologics on AHR in patients with moderate-to-severe, uncontrolled and/or refractory asthma.21,30,33,36 These studies are especially important for several reasons. First, demonstrating an effect of a new medication, additional to that of medium-to-high-dose ICS therapy, is no small feat, especially because ICS therapy is known to be effective in reducing AHR.66,67 Thus, biologics such as tezepelumab may be acting on top of the effect of corticosteroids, either by reducing inflammation that is resistant to corticosteroid therapy or by targeting non-corticosteroid-dependent pathways. Second, these studies include those patients with asthma who are most likely to benefit from a biologic agent. Third, because they utilize non-allergen challenge agents (direct and indirect), the results are not limited to the effect of the biologic on allergen-driven aspects of AHR.
The cut-off publication date for studies to be included in this review was December 17, 2021 (inclusive). A limitation of any systematic review is the possibility that potentially relevant studies are ongoing or have not been presented or published in full at the cut-off date. Data updates to the review would necessitate restarting the systematic review process according to PRISMA guidelines. A limitation of our review is that no data were available for dupilumab or reslizumab, both of which are approved for asthma. Future studies may further our understanding of AHR in asthma and subsequently help to inform treatment decisions in clinical practice.
Conclusion
These findings provide further insights into the mechanisms of AHR and the clinical benefits of biologic therapies in asthma. Overall, they suggest that upstream regulators of inflammation, such as TSLP, can drive AHR via allergen-specific and non-allergen-specific pathways. Within the limitations of the data summarized in this review, the findings indicate that biologics that target specific, downstream aspects of T2 inflammation alone do not improve AHR; supporting the hypothesis that AHR is driven by mast cell activation and by T2 inflammation acting in conjunction with other factors.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This study was funded by AstraZeneca and Amgen Inc.
Disclosure
Joseph D Spahn, Christopher S Ambrose and Neil Martin are employees of AstraZeneca and own stock or stock options in AstraZeneca. Christopher E Brightling has received grants and consultancy fees from 4DPharma, AstraZeneca, Chiesi, Genentech, GSK, Mologic, Novartis, Regeneron Pharmaceuticals, Roche and Sanofi. Paul M O’Byrne has received grants and consultancy fees from AstraZeneca, GSK, MedImmune and Novartis; consultancy fees from Covis and Teva; speaker fees from AstraZeneca, Chiesi and Menarini; and grants from Bayer and Merck. Lisa J Simpson is an employee of PharmaGenesis London, a HealthScience communications consultancy based in the UK. Nestor A Molfino is an employee of Amgen and owns stock in Amgen. Teal S Hallstrand has received grants from the National Institutes of Health and has received consultancy fees from AstraZeneca. The authors report no other conflicts of interest in this work.
References
1. Coates AL, Wanger J, Cockcroft DW, et al. ERS technical standard on bronchial challenge testing: general considerations and performance of methacholine challenge tests. Eur Respir J. 2017;49(5):1601526. doi:10.1183/13993003.01526-2016
2. Hallstrand TS, Leuppi JD, Joos G, et al. ERS technical standard on bronchial challenge testing: pathophysiology and methodology of indirect airway challenge testing. Eur Respir J. 2018;52(5):1801033. doi:10.1183/13993003.01033-2018
3. Gauvreau GM, El-Gammal AI, O’Byrne PM. Allergen-induced airway responses. Eur Respir J. 2015;46(3):819–831. doi:10.1183/13993003.00536-2015
4. Sont JK, Willems LN, Bel EH, van Krieken JH, Vandenbroucke JP, Sterk PJ. Clinical control and histopathologic outcome of asthma when using airway hyperresponsiveness as an additional guide to long-term treatment. The AMPUL Study Group. Am J Respir Crit Care Med. 1999;159(4):1043–1051. doi:10.1164/ajrccm.159.4.9806052
5. Lauzon AM, Martin JG. Airway hyperresponsiveness; smooth muscle as the principal actor. F1000Res. 2016;5:306. doi:10.12688/f1000research.7422.1
6. Brightling CE, Bradding P, Symon FA, Holgate ST, Wardlaw AJ, Pavord ID. Mast-cell infiltration of airway smooth muscle in asthma. N Engl J Med. 2002;346(22):1699–1705. doi:10.1056/NEJMoa012705
7. Siddiqui S, Mistry V, Doe C, et al. Airway hyperresponsiveness is dissociated from airway wall structural remodeling. J Allergy Clin Immunol. 2008;122(2):335–341. doi:10.1016/j.jaci.2008.05.020
8. Corry DB, Folkesson HG, Warnock ML, et al. Interleukin 4, but not interleukin 5 or eosinophils, is required in a murine model of acute airway hyperreactivity. J Exp Med. 1996;183(1):109–117. doi:10.1084/jem.183.1.109
9. Wills-Karp M, Rani R, Dienger K, et al. Trefoil factor 2 rapidly induces interleukin 33 to promote type 2 immunity during allergic asthma and hookworm infection. J Exp Med. 2012;209(3):607–622. doi:10.1084/jem.20110079
10. Yang G, Volk A, Petley T, et al. Anti-IL-13 monoclonal antibody inhibits airway hyperresponsiveness, inflammation and airway remodeling. Cytokine. 2004;28(6):224–232. doi:10.1016/j.cyto.2004.08.007
11. Altman MC, Lai Y, Nolin JD, et al. Airway epithelium-shifted mast cell infiltration regulates asthmatic inflammation via IL-33 signaling. J Clin Invest. 2019;129(11):4979–4991. doi:10.1172/JCI126402
12. Lai Y, Altemeier WA, Vandree J, et al. Increased density of intraepithelial mast cells in patients with exercise-induced bronchoconstriction regulated through epithelially derived thymic stromal lymphopoietin and IL-33. J Allergy Clin Immunol. 2014;133(5):1448–1455. doi:10.1016/j.jaci.2013.08.052
13. Al-Shaikhly T, Murphy RC, Parker A, et al. Location of eosinophils in the airway wall is critical for specific features of airway hyperresponsiveness and T2 inflammation in asthma. Eur Respir J. 2022;60:2101865. doi:10.1183/13993003.01865-2021
14. Gauvreau GM, Sehmi R, Ambrose CS, Griffiths JM. Thymic stromal lymphopoietin: its role and potential as a therapeutic target in asthma. Expert Opin Ther Targets. 2020;24(8):777–792. doi:10.1080/14728222.2020.1783242
15. Calzetta L, Aiello M, Frizzelli A, et al. The impact of monoclonal antibodies on airway smooth muscle contractility in asthma: a systematic review. Biomedicines. 2021;9(9):1281. doi:10.3390/biomedicines9091281
16. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. doi:10.1136/bmj.n71
17. Sterne JAC, Savovic J, Page MJ, et al. RoB 2: a revised tool for assessing risk of bias in randomised trials. BMJ. 2019;366:l4898. doi:10.1136/bmj.l4898
18. Boulet LP, Chapman KR, Côté J, et al. Inhibitory effects of an anti-IgE antibody E25 on allergen-induced early asthmatic response. Am J Respir Crit Care Med. 1997;155(6):1835–1840. doi:10.1164/ajrccm.155.6.9196083
19. Fahy JV, Fleming HE, Wong HH, et al. The effect of an anti-IgE monoclonal antibody on the early- and late-phase responses to allergen inhalation in asthmatic subjects. Am J Respir Crit Care Med. 1997;155(6):1828–1834. doi:10.1164/ajrccm.155.6.9196082
20. Fahy JV, Cockcroft DW, Boulet LP, et al. Effect of aerosolized anti-IgE (E25) on airway responses to inhaled allergen in asthmatic subjects. Am J Respir Crit Care Med. 1999;160(3):1023–1027. doi:10.1164/ajrccm.160.3.9810012
21. Noga O, Hanf G, Kunkel G. Immunological and clinical changes in allergic asthmatics following treatment with omalizumab. Int Arch Allergy Immunol. 2003;131(1):46–52. doi:10.1159/000070434
22. Djukanović R, Wilson SJ, Kraft M, et al. Effects of treatment with anti-immunoglobulin E antibody omalizumab on airway inflammation in allergic asthma. Am J Respir Crit Care Med. 2004;170(6):583–593. doi:10.1164/rccm.200312-1651OC
23. Prieto L, Gutiérrez V, Colás C, et al. Effect of omalizumab on adenosine 5’-monophosphate responsiveness in subjects with allergic asthma. Int Arch Allergy Immunol. 2006;139(2):122–131. doi:10.1159/000090387
24. Patel BM, Chiang DT, Clark JP, Romero FA, Casale TB. 1022 Effects of omalizumab (Xolair®) on airway hyperresponsiveness. J Allergy Clin Immunol. 2009;123(2):S263. doi:10.1016/j.jaci.2008.12.1020
25. van Rensen EL, Evertse CE, van Schadewijk WA, et al. Eosinophils in bronchial mucosa of asthmatics after allergen challenge: effect of anti-IgE treatment. Allergy. 2009;64(1):72–80. doi:10.1111/j.1398-9995.2008.01881.x
26. Zielen S, Lieb A, De La Motte S, et al. Omalizumab protects against allergen-induced bronchoconstriction in allergic (immunoglobulin E-mediated) asthma. Int Arch Allergy Immunol. 2013;160(1):102–110. doi:10.1159/000339243
27. Hendeles L, Khan YR, Shuster JJ, Chesrown SE, Abu-Hasan M. Omalizumab therapy for asthma patients with poor adherence to inhaled corticosteroid therapy. Ann Allergy Asthma Immunol. 2015;114(1):58–62. doi:10.1016/j.anai.2014.10.012
28. Leckie MJ, ten Brinke A, Khan J, et al. Effects of an interleukin-5 blocking monoclonal antibody on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356(9248):2144–2148. doi:10.1016/S0140-6736(00)03496-6
29. Flood-Page PT, Menzies-Gow AN, Kay AB, Robinson DS. Eosinophil’s role remains uncertain as anti-interleukin-5 only partially depletes numbers in asthmatic airway. Am J Respir Crit Care Med. 2003;167(2):199–204. doi:10.1164/rccm.200208-789OC
30. Haldar P, Brightling CE, Hargadon B, et al. Mepolizumab and exacerbations of refractory eosinophilic asthma. N Engl J Med. 2009;360(10):973–984. doi:10.1056/NEJMoa0808991
31. Gauvreau GM, Sehmi R, FitzGerald J, et al. 500 The effect of benralizumab on allergen-induced responses in subjects with mild allergic asthma. J Allergy Clin Immunol. 2021;147(2):AB157. doi:10.1016/j.jaci.2020.12.563
32. Gauvreau GM, O’Byrne PM, Boulet LP, et al. Effects of an anti-TSLP antibody on allergen-induced asthmatic responses. N Engl J Med. 2014;370(22):2102–2110. doi:10.1056/NEJMoa1402895
33. Diver S, Khalfaoui L, Emson C, et al. Effect of tezepelumab on airway inflammatory cells, remodelling, and hyperresponsiveness in patients with moderate-to-severe uncontrolled asthma (CASCADE): a double-blind, randomised, placebo-controlled, phase 2 trial. Lancet Respir Med. 2021;9(11):1299–1312. doi:10.1016/S2213-2600(21)00226-5
34. Sverrild A, Hansen S, Hvidtfeldt M, et al. The effect of tezepelumab on airway hyperresponsiveness to mannitol in asthma (UPSTREAM). Eur Respir J. 2022;59(1):2101296. doi:10.1183/13993003.01296-2021
35. Scheerens H, Arron JR, Zheng Y, et al. The effects of lebrikizumab in patients with mild asthma following whole lung allergen challenge. Clin Exp Allergy. 2014;44(1):38–46. doi:10.1111/cea.12220
36. Russell RJ, Chachi L, FitzGerald JM, et al. Effect of tralokinumab, an interleukin-13 neutralising monoclonal antibody, on eosinophilic airway inflammation in uncontrolled moderate-to-severe asthma (MESOS): a multicentre, double-blind, randomised, placebo-controlled phase 2 trial. Lancet Respir Med. 2018;6(7):499–510. doi:10.1016/S2213-2600(18)30201-7
37. Revez JA, Bain LM, Watson RM, et al. Effects of interleukin-6 receptor blockade on allergen-induced airway responses in mild asthmatics. Clin Transl Immunology. 2019;8(6):e1044. doi:10.1002/cti2.1044
38. Gauvreau GM, Becker AB, Boulet LP, et al. The effects of an anti-CD11a mAb, efalizumab, on allergen-induced airway responses and airway inflammation in subjects with atopic asthma. J Allergy Clin Immunol. 2003;112(2):331–338. doi:10.1067/mai.2003.1689
39. Berry MA, Hargadon B, Shelley M, et al. Evidence of a role of tumor necrosis factor alpha in refractory asthma. N Engl J Med. 2006;354(7):697–708. doi:10.1056/NEJMoa050580
40. Rouhani FN, Meitin CA, Kaler M, Miskinis-Hilligoss D, Stylianou M, Levine SJ. Effect of tumor necrosis factor antagonism on allergen-mediated asthmatic airway inflammation. Respir Med. 2005;99(9):1175–1182. doi:10.1016/j.rmed.2005.02.031
41. Wenzel S, Wilbraham D, Fuller R, Getz EB, Longphre M. Effect of an interleukin-4 variant on late phase asthmatic response to allergen challenge in asthmatic patients: results of two phase 2a studies. Lancet. 2007;370(9596):1422–1431. doi:10.1016/S0140-6736(07)61600-6
42. Gauvreau GM, Boulet LP, Cockcroft DW, et al. Effects of interleukin-13 blockade on allergen-induced airway responses in mild atopic asthma. Am J Respir Crit Care Med. 2011;183(8):1007–1014. doi:10.1164/rccm.201008-1210OC
43. Gauvreau GM, Boulet LP, Cockcroft DW, et al. Effect of an anti-M1 prime monoclonal antibody, MEMP1972A, in a phase II proof-of-activity allergen challenge study in patients with mild asthma. Am J Respir Crit Care Med. 2012;185:A6793.
44. Gauvreau GM, Hohlfeld JM, Grant S, et al. Efficacy and safety of an inhaled anti-TSLP antibody fragment in adults with mild atopic asthma. Am J Respir Crit Care Med. 2020;201:A4207.
45. Henig NR, Aitken ML, Liu MC, Yu AS, Henderson WR Jr. Effect of recombinant human platelet-activating factor-acetylhydrolase on allergen-induced asthmatic responses. Am J Respir Crit Care Med. 2000;162(2 Pt 1):523–527. doi:10.1164/ajrccm.162.2.9911084
46. Bryan SA, O’Connor BJ, Matti S, et al. Effects of recombinant human interleukin-12 on eosinophils, airway hyper-responsiveness, and the late asthmatic response. Lancet. 2000;356(9248):2149–2153. doi:10.1016/S0140-6736(00)03497-8
47. Gauvreau GM, Boulet LP, Cockcroft DW, et al. OX40L blockade and allergen-induced airway responses in subjects with mild asthma. Clin Exp Allergy. 2014;44(1):29–37. doi:10.1111/cea.12235
48. Tajiri T, Niimi A, Matsumoto H, et al. Comprehensive efficacy of omalizumab for severe refractory asthma: a time-series observational study. Ann Allergy Asthma Immunol. 2014;113(4):470–475 e472. doi:10.1016/j.anai.2014.06.004
49. Chan R, RuiWen Kuo C, Jabbal S, Lipworth BJ. Eosinophil depletion with benralizumab is associated with attenuated mannitol airway hyperresponsiveness in severe uncontrolled eosinophilic asthma. J Allergy Clin Immunol. 2023;151(3):700–705. doi:10.1016/j.jaci.2022.10.028
50. Cahill KN, Katz HR, Cui J, et al. KIT inhibition by imatinib in patients with severe refractory asthma. N Engl J Med. 2017;376(20):1911–1920. doi:10.1056/NEJMoa1613125
51. Murphy RC, Chow YH, Lai Y, et al. Identification of mast cell progenitor cells in the airways of individuals with allergic asthma. Allergy. 2022;78:547–549. doi:10.1111/all.15498
52. Nakae S, Ho LH, Yu M, et al. Mast cell-derived TNF contributes to airway hyperreactivity, inflammation, and TH2 cytokine production in an asthma model in mice. J Allergy Clin Immunol. 2007;120(1):48–55. doi:10.1016/j.jaci.2007.02.046
53. Reuter S, Heinz A, Sieren M, et al. Mast cell-derived tumour necrosis factor is essential for allergic airway disease. Eur Respir J. 2008;31(4):773–782. doi:10.1183/09031936.00058907
54. Sze E, Bhalla A, Nair P. Mechanisms and therapeutic strategies for non-T2 asthma. Allergy. 2020;75(2):311–325. doi:10.1111/all.13985
55. Menzies-Gow A, Corren J, Bourdin A, et al. Efficacy and safety of tezepelumab in adults and adolescents with severe, uncontrolled asthma: results from the phase 3 NAVIGATOR study (Abstract L46). J Allergy Clin Immunol. 2021;147(2):AB249.
56. Menzies-Gow A, Corren J, Bourdin A, et al. Tezepelumab in adults and adolescents with severe, uncontrolled asthma. N Engl J Med. 2021;384(19):1800–1809. doi:10.1056/NEJMoa2034975
57. Panettieri RA Jr, Sjöbring U, Péterffy A, et al. Tralokinumab for severe, uncontrolled asthma (STRATOS 1 and STRATOS 2): two randomised, double-blind, placebo-controlled, phase 3 clinical trials. Lancet Respir Med. 2018;6(7):511–525. doi:10.1016/S2213-2600(18)30184-X
58. Hanania NA, Korenblat P, Chapman KR, et al. Efficacy and safety of lebrikizumab in patients with uncontrolled asthma (LAVOLTA I and LAVOLTA II): replicate, phase 3, randomised, double-blind, placebo-controlled trials. Lancet Respir Med. 2016;4(10):781–796. doi:10.1016/S2213-2600(16)30265-X
59. Boulet LP, Gauvreau G, Boulay ME, O’Byrne P, Cockcroft DW. Clinical Investigative Collaboration CNoCoEA. The allergen bronchoprovocation model: an important tool for the investigation of new asthma anti-inflammatory therapies. Allergy. 2007;62(10):1101–1110. doi:10.1111/j.1398-9995.2007.01499.x
60. Davis BE, Illamperuma C, Gauvreau GM, et al. Single-dose desloratadine and montelukast and allergen-induced late airway responses. Eur Respir J. 2009;33(6):1302–1308. doi:10.1183/09031936.00169008
61. Gauvreau GM, Lee JM, Watson RM, Irani AM, Schwartz LB, O’Byrne PM. Increased numbers of both airway basophils and mast cells in sputum after allergen inhalation challenge of atopic asthmatics. Am J Respir Crit Care Med. 2000;161(5):1473–1478. doi:10.1164/ajrccm.161.5.9908090
62. Parameswaran K, Liang H, Fanat A, Watson R, Snider DP, O’Byrne PM. Role for cysteinyl leukotrienes in allergen-induced change in circulating dendritic cell number in asthma. J Allergy Clin Immunol. 2004;114(1):73–79. doi:10.1016/j.jaci.2004.03.054
63. Holgate S, Smith N, Massanari M, Jimenez P. Effects of omalizumab on markers of inflammation in patients with allergic asthma. Allergy. 2009;64(12):1728–1736. doi:10.1111/j.1398-9995.2009.02201.x
64. Brusselle GG, Koppelman GH. Biologic therapies for severe asthma. N Engl J Med. 2022;386(2):157–171. doi:10.1056/NEJMra2032506
65. Agache I, Antolin-Amerigo D, de Blay F, et al. EAACI position paper on the clinical use of the bronchial allergen challenge: unmet needs and research priorities. Allergy. 2022;77(6):1667–1684. doi:10.1111/all.15203
66. Haahtela T, Järvinen M, Kava T, et al. Comparison of a beta 2-agonist, terbutaline, with an inhaled corticosteroid, budesonide, in newly detected asthma. N Engl J Med. 1991;325(6):388–392. doi:10.1056/NEJM199108083250603
67. Szefler S, Weiss S, Tonascia J, et al. Long-term effects of budesonide or nedocromil in children with asthma. N Engl J Med. 2000;343(15):1054–1063.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


