Back to Journals » Patient Preference and Adherence » Volume 8
Patient preference and ease of use for different coagulation factor VIII reconstitution device scenarios: a cross-sectional survey in five European countries
Authors Cimino E, Linari S, Malerba M, Halimeh S, Biondo F, Westfeld M
Received 24 March 2014
Accepted for publication 24 June 2014
Published 12 December 2014 Volume 2014:8 Pages 1713—1720
DOI https://doi.org/10.2147/PPA.S64709
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Ernesto Cimino,1 Silvia Linari,2 Mara Malerba,3 Susan Halimeh,4 Francesca Biondo,5 Martina Westfeld5
1Dipartimento Medicina Clinica e Sperimentale, Universita’ degli Studi di Napoli Federico II, Naples, Italy; 2Agenzia per l’ Emofilia, AOU Careggi di Firenze, Florence, Italy; 3Fondazione Cà Granda Ospedale Maggiore Policlinico, Centro Emofilia e Trombosi “A Bianchi Bonomi”, Milan, Italy; 4CRC Coagulation Research Centre GmbH, Duisburg, Germany; 5Pfizer Italia, Rome, Italy
Introduction: Hemophilia A treatment involves replacing the deficient coagulation factor VIII. This process may involve multiple steps that might create a barrier to adherence. A new dual-chamber syringe (DCS; FuseNGo®) was recently introduced with the aim of simplifying reconstitution.
Aim: This study aimed to identify factors associated with adult patients’ preferences for different coagulation factor VIII reconstitution systems and to test ease of use and patient preference for the DCS.
Methods: A cross-sectional survey of adults with hemophilia A in five European countries was conducted; a subset of subjects also participated in a practical testing session of the DCS.
Results: Among the 299 survey participants, the device scenario requiring the least equipment and reconstitution steps (the DCS) received a median preference rating of 71 out of 100 (0 being “the least desirable” and 100 “the most desirable” rating). This was significantly higher than the other scenarios (the next highest achieved a median of 50 points; P<0.001). Participants would be more likely to use this device prophylactically (P<0.001). Among the 98 participants who tested the DCS, 57% preferred this device over their current device, 26% preferred their current device, and 17% had no preference. The DCS was rated as easier to use than current treatment devices (median score 9/10 versus 7/10 for current treatment, P=0.001).
Conclusion: The survey indicates that the prefilled DCS, FuseNGo®, requiring the least equipment and fewest reconstitution steps, was preferred by patients and was the device most likely to be used prophylactically; the practical device testing supports these results.
Keywords: hemophilia, factor VIII, patient preference, reconstitution, dual-chamber syringe
Corrigendum for this paper has been published
Introduction
One of the most severe consequences of hemophilia is recurrent bleeding into joints which can reduce movement and cause chronic pain and stiffness.1 Hemophilia A treatment involves replacing coagulation factor VIII (FVIII) by intravenous infusion.2 Prophylactic therapy in patients with severe hemophilia is primarily administered to prevent bleeding and the development of chronic arthropathy.1 The two currently used prophylaxis protocols with long-term follow-up data are the Malmö protocol (25–40 IU/kg per dose) and the Utrecht protocol (15–30 IU/kg per dose), each used three times weekly (on alternate days) for hemophilia A.2
Inadequate treatment, one of the causes of which may be poor adherence, leads to poorer outcomes in hemophilia A, including spontaneous bleeding and joint damage or destruction.2 Poor adherence may be due to a number of factors, for example, unsatisfactory access to health care, difficulties with treatment at home, and a lack of skills for self-treatment.3 Other barriers include perceived negative consequences of treatment (for example, pain or discomfort caused by infusions) and lack of perceived benefit to symptoms.3 In addition, the perceived costs of treatment including the time consumed for complex treatments and lack of patient satisfaction with treatment devices also contribute to non-adherence.1,3–5
Lack of time and inconvenience have been cited in surveys as primary barriers for both prophylaxis and early episodic treatment of hemophilia.6 Many parents and school-age children find it challenging to incorporate a prophylactic regimen into a busy morning schedule.6 The storage and administration of coagulation factor concentrate also present significant barriers to early treatment during a bleeding episode.6 Need for refrigeration is a further inconvenience, since most coagulation factor concentrates require storage in a refrigerator and reconstitution at room temperature before use.6 Coagulation factor concentrates are supplied as lyophilized powder and diluent. Devices to reconstitute the lyophilized FVIII with diluent may involve multiple steps. For example, some currently available products involve a preparation method where FVIII must be reconstituted using two vials (one containing diluent, the other containing the lyophilized FVIII powder), a double-sided needle to transfer the diluent into the FVIII vial, and a syringe into which the reconstituted FVIII product is transferred.5 Preparation and infusion of a single dose of coagulation factor concentrates may be as brief as 2–5 minutes for some formulations or greater than 50 minutes for others.6
On the basis of this evidence, a reconstitution device that is quick and easy to use and generates high levels of patient satisfaction could potentially increase adherence and therefore reduce morbidity associated with hemophilia. A previous post-marketing surveillance study has shown that patients have improved satisfaction after switching to a needleless system due to ease of use, perceived safety from needle stick injuries, and speed of reconstitution.7 The adherence of patients, compared with their previous reconstitution method before the switch, was rated as “better” in 72.7% of patients, “equal” in 20.5%, and “worse” in 2.3%. The authors suggested that such a reconstitution method could improve patients’ adherence to therapy, especially for those receiving long-term prophylaxis.7
A new prefilled dual-chamber syringe (DCS; FuseNGo®; Pfizer Ltd., Sandwich, Kent, United Kingdom) has been recently introduced for the reconstitution of ReFacto AF® (recombinant FVIII, Pfizer Ltd.). The DCS incorporates both lyophilized powder and diluent in one syringe. By pushing the syringe plunger, the diluent is transferred into the chamber containing the lyophilized powder, the powder is dissolved and the solution is ready to be used.8 The aims of its development were to make treatment preparation less burdensome which potentially helps patients to be more adherent with their prescribed treatment regimen.
The aim of this survey was to identify factors associated with adult patients’ preferences for different FVIII reconstitution systems and to test ease of use and patient preference for a new DCS.8
Patients and methods
A cross-sectional survey was conducted in Austria, Germany, Italy, Spain, and the United Kingdom (UK) among adults (aged 18–65 years) with hemophilia A who were using their current FVIII product either prophylactically or on-demand at least once a month. They were required to have used their current treatment at least 20 times to be eligible for this survey. They were recruited via haemophilia patient organisations (Austria, Spain, and the UK), or hemophilia treatment centers (Germany and Italy). A nurse or clinician checked whether each patient fulfilled the inclusion and exclusion criteria for the study.
Two methods of data collection were used. First, a questionnaire was developed to elicit patients’ preferences for different FVIII reconstitution scenarios reflecting reconstitution devices with various levels of complexity. Second, practical testing of the DCS was carried out among a sub-sample of those who completed the questionnaire to assess the ease of use of and preference for the DCS.
The majority of participants (90%) completed the preference questionnaire online. In two centers in Italy, the questionnaire was administered as a paper-based version because of problems with Internet access.
Development of the questionnaire involved a comprehensive literature review to identify the impact of hemophilia A on patients’ lives and any factors associated with patient preference,5,7,9–15 incorporation of items from a validated questionnaire designed to measure treatment adherence,16 and questions designed to establish the factors driving patient behavior in relation to their FVIII treatment (based on the theory of planned behavior).17
The questionnaire was primarily designed to capture patients’ preferences for different reconstitution systems used to prepare and administer FVIII treatment. The questionnaire covered demographic data, duration of hemophilia and time on current treatment, number of joint bleeds and amount of pain due to bleeds, prophylactic or on-demand use of FVIII, ease of use of current treatment and time taken, effect of treatment on activities, beliefs about hemophilia and its treatment, and preferences for five different treatment scenarios that represent existing treatment devices. All of the scenarios were presented in an unbranded, anonymized (ie, non-product-specific) way. The scenarios were presented using pictures (Figure 1) with a written description of the reconstitution steps, based on the patient information leaflets of the different products.
A subset of participants (in all countries except Austria) additionally tested the reconstitution of the DCS (using demonstration kits not containing any active drug substance). The correct procedure for reconstitution using the DCS was demonstrated by a trained hemophilia nurse, physician or researcher. Each participant then received demonstration kits of the device (from which the infusion needles had been removed) and was asked to follow the reconstitution as demonstrated four times. Participants practiced the reconstitution only and stopped before attaching the infusion set. No product was administered. The time it took to prepare the FVIII to the point of injection was recorded on each occasion and then compared with the time to prepare their current treatment (based on recall). Following the testing, participants completed a questionnaire giving ratings for different aspects of ease of use of the DCS and their current treatment device and stated their preference for the DCS or their current treatment device.
Data were analyzed using SPSS for Windows version 19.0 (IBM Corporation, Armonk, NY, USA). Descriptive statistics were used to quantify ease of use and preference ratings for treatment device scenarios. As the data were not normally distributed, non-parametric tests were used to compare ratings for the DCS against participants’ current reconstitution system. Univariate analyses (Wilcoxon signed-rank tests) were used to identify factors associated with preference for the DCS. All significant factors from the online questionnaire were entered in a multivariate regression model to identify factors associated with high levels of preference for the DCS.
A maximum sample size of 120 patients was required to demonstrate a significant difference in preference between ReFacto AF® FuseNGo® (Pfizer Ltd.) and current devices with 95% power, and 2-sided significance at 0.01.
The protocol and study materials were reviewed and approved by research ethics committees in accordance with individual country requirements and regulations. Online or written informed consent was obtained from each subject.
Results
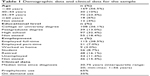
A total of 299 patients were recruited from Austria (n=34), Germany (n=35), Italy (n=49), Spain (n=74), and the UK (n=107). Participants were exclusively male and most (88.6%; n=265) were Caucasian. Demographic data are shown in Table 1.
|
Table 1 Demographic data and clinical data for the sample |
The median time since diagnosis was 30.75 years (interquartile range [IQR] 20, min–max 1 to 56 years). Current treatment included a range of both recombinant and plasma-derived FVIII products and all participants had used their current treatment at least 20 times. One hundred and ninety-four (65%) used FVIII prophylactically and 105 (35%) used treatment on-demand (see Table 1).
Preferences for five anonymized treatment device scenarios were rated on a scale between 0 (the least desirable) and 100 (the most desirable). Preference scores ranged from a median of 29 (IQR 40) for scenario 4 (the double-sided needle) to 71 (IQR 53) for scenario 5 (the DCS). Preference scores for scenario 5 were significantly higher than for any of the other scenarios (all scenarios 1 to 4 versus scenario 5, P<0.001) (Figure 2).
The likelihood of using treatment prophylactically was rated on a scale between 0 (very unlikely) and 10 (very likely) for each treatment device. Likelihood scores ranged from a median of 5 for scenarios 1, 3 and 4, and 6 for scenario 2 (all IQR 4 or 5) to a median likelihood score of 8 (IQR 4) for scenario 5. Likelihood scores for scenario 5 (the DCS) were significantly higher than for the other scenarios (all scenarios 1 to 4 versus scenario 5, P<0.001) (Figure 3).
Participants were asked how likely they would be to use each of the treatment device scenarios more frequently than their current treatment, rated on a scale of 0 (very unlikely) to 10 (very likely). Scenario 5 received the highest likelihood scores among all the scenarios, regardless of whether respondents were currently prophylactic or on-demand users (all scenarios 1 to 4 versus scenario 5, P<0.001) (Figure 4).
High preference ratings for the DCS scenario were associated in univariate analyses with longer duration of hemophilia A (P=0.003) and less time on current treatment (P=0.004).
A sub-sample of 98 participants tested the DCS using non-product-containing demonstration kits. The median time taken to prepare the DCS was less than 1 minute (IQR 27 seconds), compared with around 4 minutes (IQR 285 seconds) for current treatment (reported from recall). Time to prepare the DCS decreased slightly over the four timed attempts.
Patients participating in the device testing were asked to rate the ease of preparation, holding the device, depressing the plunger, storage, and disposal for their current treatment and the DCS. Each attribute was rated on a scale between 0 (not at all easy) and 10 (extremely easy). Ratings were significantly higher for the DCS than current treatment for preparation of the coagulation factor concentrate, ease of holding, storing, and ease of disposal (Table 2).
Patients participating in the device testing had to provide an overall preference rating between 0 (least desirable) and 10 (most desirable) for their current treatment device and the DCS. The median rating score for the DCS was 8 (IQR 3, min–max 1–10), compared with 7 (IQR 3, min–max 0–10, P=0.001) for current treatment, indicating high treatment satisfaction with both devices but a preference for the DCS device (P<0.001).
Participants in the device testing stage were also asked to rate the likelihood of using treatment prophylactically or of increasing the frequency of prophylactic use using the new DCS. Likelihood was rated between 0 (very unlikely) and 10 (very likely). For all those involved in the practical device testing, the median score for the likelihood of using the DCS prophylactically was 7.5 (IQR 4). Participants who used their current treatment prophylactically would wish to continue prophylactic use with the DCS (median likelihood score 8, IQR 4). Sixteen out of 24 (67%) current on-demand users had a score of 5 or more, suggesting that they might change to prophylactic use with the new device. The median score for the likelihood of increasing the frequency of use was 8 (IQR 4). Participants who used their current treatment prophylactically were more likely than participants using treatment on-demand, to consider using the DCS more frequently (median score of current prophylactic users 8, IQR 4, for current on-demand users 6, IQR 5), although these differences were not statistically significant.
Following device testing, participants indicated which device (the DCS or current treatment) they preferred and the reasons for their preference. Of the 98 respondents, 57.1% (n=56) preferred the DCS; 25.5% (n=25) preferred their current treatment; and 17.3% (n=17) had no preference. The main reason for preferring the DCS was that it was perceived as easier and quicker to prepare, while reasons for preferring their current treatment over the DCS included familiarity and perceptions that the new treatment was difficult.
Discussion
Adherence to any long-term medical regimen is challenging.4 In hemophilia in particular, FVIII replacement therapy is required long-term to prevent or resolve bleeding, especially into joints, which over time can lead to joint destruction.1 There are a number of known barriers to adherence to hemophilia treatments, including the time required for therapy, and a lack of satisfaction with treatment devices.2–5
This cross-sectional survey examined patient preferences for five different reconstitution systems, including one representing a new DCS (FuseNGo®; Pfizer Ltd.)8 and then tested ease of use of the DCS with practical device testing. The DCS was preferred to alternative device scenarios, and to participants’ current treatment, and was most likely to encourage prophylactic use and increased frequency of prophylactic use of FVIII replacement.
The latter aspect is of particular interest as recent publications have shown a benefit of individualized prophylaxis guided by pharmacokinetic dosing principles.18 The prophylaxis regimen that was most efficient in maintaining higher trough levels of FVIII and reducing the weekly time below 1 IU dL−1 FVIII activity was a daily dosing regimen.18 However, maintaining adherence with such a frequent dosing regimen could be a challenge. The results of this survey indicate that more frequent dosing could be supported by the DCS.
Also, in a more general setting, the increased convenience of the device may have a positive effect on adherence in patients with hemophilia A and so potentially decrease the morbidity associated with the condition.1,7 These findings are similar to those from a post-marketing surveillance study of another needleless system, which reported high patient satisfaction, and that patients preferred the new system to their old reconstitution system.7 It will be interesting to further explore the relationship between convenience and adherence.
There are some limitations to the study that should be considered when interpreting the results. First, there might have been a difference in participant selection across the different countries. Whilst in countries, where patients were recruited through the haemophilia patient organizations, the selection was random (through mailings to all members); in other countries, where recruitment was performed by the center, there might have been a selection of specific patients to participate.
In Austria, Spain, and the UK, participants completed the survey questionnaire online. In Germany, participants completed the questionnaire online either at home or within the clinical centers. In Italy, participants completed the questionnaires in the hemophilia treatment centers either online or, where Internet access was a problem, completed a paper version of the questionnaire that was collected by their physician or nurse.
The differences in methods of recruitment and data collection may have resulted in slightly different results/responses on the questionnaires either because the populations of participants were slightly different, or because the presence of the physician/nurse may have influenced how participants answered some of the questions. However, examination of the data by country suggests that any biasing effects of the difference in methodology were minimal as there were few differences between countries overall.
Second, patient preference ratings may have been influenced by testing the new device in the presence of the treating physician or the hemophilia nurse. However, whilst preference ratings for the new device were highest in Germany (73%) where the device testing was conducted in the presence of the physician or nurse in the center, they were comparable between Italy (65%), where the device was tested in the presence of the physician, and Spain (62%), where device testing was conducted in a neutral environment. Thus the influence on the overall results can be considered minimal.
Comparative assessment of the DCS with participants’ current treatment was limited in some aspects of the assessment (most notably the estimate of the time taken to prepare FVIII for injection) by its reliance on participant’s recall of their experience with current treatment (compared with timed use of the DCS). Ideally, patients’ use of their current treatment would have been measured in the same way as the test device to enable an immediate comparison. However, other aspects of the assessment relied on general impressions/assessments that were less affected by recall bias. Moreover, the inclusion of participants who regularly used their current factor concentrate and had been using it for at least 20 times prior to enrollment ensured that patients were familiar with their current treatment and limited recall bias. In addition, the fact that patients were asked to reconstitute a dummy device might have resulted in working more quickly with less attention to careful aseptic technique.
The survey questionnaire used was designed for the purposes of the study but was not subjected to rigorous psychometric and clinimetric testing. Items for the questionnaire were based on a literature review and expert opinion but did not include any cognitive debriefing with people with hemophilia, or any standardized assessment of the validity, reliability, and responsiveness of the questionnaire. This may have resulted in redundant or missing items and there was certainly evidence of floor and ceiling effects with some questions and scaling. However, the questionnaire has subsequently been psychometrically evaluated, refined, and validated to produce a 14-item questionnaire (HaemoPREF)19 for use in future research to assess hemophilia patients’ experiences of their current clotting factor treatment.
Strengths of the study included the involvement of a meaningful sample of participants from five different European countries. In addition, the study included patients who were receiving on-demand treatment as well as patients using prophylaxis. The fact that the preference rating was done using unbranded, anonymized device scenarios limited the bias that might have been introduced by listing product names or types with the scenarios.
Conclusion
Preference ratings for the DCS (FuseNGo®; Pfizer Ltd.) were higher than for any other treatment device both in ratings of anonymized treatment device scenarios and in practical device testing. Higher preference ratings were associated with longer duration of hemophilia A and shorter duration of current treatment. The likelihood of prophylactic use was highest for the DCS scenario, as were scores for the likelihood of increasing frequency of use. Higher frequency of use, and prophylactic rather than on-demand use for FVIII replacement, with easier reconstitution and higher patient satisfaction with the treatment device, may potentially contribute to improved adherence to therapy and therefore reduced morbidity in hemophilia A.2 In further research the questionnaire developed for this study has been psychometrically evaluated, refined, and validated to produce a 14-item questionnaire (HaemoPREF)19 and will be used in a real world study in hemophilia A.
Acknowledgments
The authors would like to thank all patients who took part in this survey as well as the patient organizations in the participating countries: Asociación Malagueña de Hemofilia (AMH), Asociación Gallega de Hemofilia (AGADHEMO), Asociación de Hemofilia de la Comunidad de Madrid (ASHEMADRID), Asociación de Hemofilia de la Comunidad Valenciana (ASHECOVA), Österreichische Hämophilie Gesellschaft (ÖHG), The Haemophilia Society UK, Deutsche Hämophiliegesellschaft (DHG). The authors would like to further thank all hemophilia center staff who helped conduct this survey and the device testing sessions.
The authors acknowledge Alison Carr from Hamell for providing medical writing support funded by Pfizer and Hamell for performing the statistical analysis.
Author contributions
EC, SL, MM, and SH performed the research and reviewed the paper. FB and MW were involved in the study design and reviewed the paper. All authors contributed toward data analysis, drafting, and revising the manuscript.
Disclosure
All participating hemophilia treatment centers and patient organizations received a fee from Pfizer for the conduct of this survey (based on local regulations). FB and MW are employees of Pfizer. The other authors report no conflicts of interest in this work.
References
Thornburg CD. Prophylactic factor infusions for patients with hemophilia: challenges with treatment adherence. J Coagul Disord. 2010;2:9–14. | ||
World Federation of Hemophilia. Guidelines for the management of haemophilia. World Federation of Hemophilia; 2012. Available from: www1.wfh.org/publications/files/pdf-1472.pdf. Accessed January 6, 2014. | ||
Remor E. Predictors of treatment difficulties and satisfaction with haemophilia therapy in adult patients. Haemophilia. 2011;17(5):e901–e905. | ||
Hacker MR, Geraght S, Manco-Johnson M. Barriers to compliance with prophylaxis therapy in haemophilia. Haemophilia. 2001;7(4):392–396. | ||
Vidovic N, Musso R, Klamroth R, Enriquez MM, Achilles K. Postmarketing surveillance study of KOGENATE® Bayer with Bio-Set® in patients with haemophilia A: evaluation of patients’ satisfaction after switch to the new reconstitution system. Haemophilia. 2010;16(1):66–71. | ||
Saxena K. Barriers and perceived limitations to early treatment of haemophilia. J Blood Med. 2013;4:49–56. | ||
Musso R, Santoro R, Coppola A, et al. Patient preference for needleless factor VIII reconstitution device: the Italian experience. Int J Gen Med. 2010;3:203–208. | ||
European Medicines Agency. Summary of Product Characteristics ReFacto AF powder and solvent for solution for injection. European Medicines Agency; 2012. Available from: http://www.ema.europa.eu/docs/en_GB/document_library/EPAR_-_Product_Information/human/000232/WC500049008.pdf. Accessed January 6, 2014. | ||
Fletcher M. The impact of haemophilia on the patient and family. In: Rizza C, Lowe G, editors. Haemophilia and other inherited bleeding disorders. London: WB Saunders Co Ltd, 1997. | ||
Rosendaal F, Smit C, Varekamp I, et al. Modern haemophilia treatment: medical improvements and quality of life. J Intern Med. 1990;228(6):633–640. | ||
Miners A, Sabin C, Tolley K, et al. Assessing health-related quality-of-life in individuals with haemophilia. Haemophilia. 1999;5(6):378–385. | ||
Solovieva S. Clinical severity of disease, functional disability and health-related quality of life. Three year follow-up study of 150 Finnish patients with coagulation disorders. Haemophilia. 2001;7(1):53–63. | ||
Trippoli S, Viaiani M, Linari S, et al. Multivariate analysis of factors influencing quality of life and utility in patients with haemophilia. Haematologica. 2001;86(7):722–728. | ||
Szende A, Schramm W, Flood E, et al. Health-related quality of life in adult haemophilia patients: a systematic review and evaluation of instruments. Haemophilia. 2003;9(6):678–687. | ||
Beeton K, Neal D, Lee C. An exploration of health-related quality of life in adults with haemophilia – a qualitative perspective. Haemophilia. 2005;11(2):123–132. | ||
Carr AJ, Hughes RA, Vincent K, Carr M, Thwaites C. The impact and implications of new treatments in arthritis: validation of a questionnaire to measure the ‘real life’ effectiveness of medication. Rheumatology. 2004;43(suppl):ii83. | ||
Ajzen I. The theory of planned behavior. Organizational Behavior and Human Decision Processes. 1991;50:179–221. | ||
Collins PW, Björkman S, Fischer K, Blanchette V, Oh M, Scroth P, et al. Factor VIII requirement to maintain a target plasma level in the prophylactic treatment of severe hemophilia A: influences of variance in pharmacokinetics and treatment regimens. J Thromb Haemost. 2010;8(2):269–275. | ||
Teal S, Brohan E, Hettema Y, Humphrey L, Willgoss T, Hudgens S, et al. Development and psychometric evaluation of a novel tool for assessing patient perception and preference for haemophilia treatment (HaemoPREF). Haemophilia. 2014;20:666–673. |
 © 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2014 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.





