Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 15
Patient Characteristics and Healthcare Resource Utilization Among Patients with COPD New to LAMA/LABA Fixed-Dose Combination Treatment in US-Based Real-World Practice
Authors Ding B , Kallenbach L, Slipski L , Wilk A , O'Brien D, Guranlioglu D
Received 16 November 2019
Accepted for publication 21 March 2020
Published 16 April 2020 Volume 2020:15 Pages 775—786
DOI https://doi.org/10.2147/COPD.S238408
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Bo Ding,1 Lee Kallenbach,2 Lukas Slipski,2 Alan Wilk,2 Dan O’Brien,2 Deniz Guranlioglu3
1AstraZeneca, Gothenburg, Sweden; 2Practice Fusion, San Francisco, CA, USA; 3AstraZeneca, Cambridge, UK
Correspondence: Bo Ding
AstraZeneca, Pepparedsleden 1, Gothenburg SE 431 83, Mölndal, Sweden
Tel +46 31 776 2406
Email [email protected]
Introduction: This retrospective, observational cohort study utilized an integrated dataset from an electronic health records system and a claims database to describe demographic and clinical characteristics, healthcare resource utilization (HCRU), and treatment patterns in COPD patients initiating long-acting muscarinic antagonist (LAMA)/long-acting β2-agonist (LABA) fixed-dose combination (FDC) treatment in the USA.
Methods: Patients were aged ≥ 40 years and had a COPD diagnosis (Practice Fusion system) and ≥ 1 prescription of LAMA/LABA FDC therapy, with an index date (first prescription) 1 May 2014– 31 December 2017. For the HCRU analysis, patients had ≥ 2 claims from the Symphony Health database within 12 months before index. All analyses of outcomes relating to demographic and clinical characteristics, HCRU, and treatment patterns were descriptive.
Results: Patients initiating LAMA/LABA FDCs (n=8224) had a mean age of 67.9 years, 52.8% were female, and mean BMI was 29.2 kg/m2. The most common comorbidities were cardiovascular disease (74.3%), hypertension (64.0%), and hyperlipidemia (45.6%). In the 12 months prior to index, 53.1% of patients had used inhaled therapy: 23.4% short-acting therapy only, 16.7% short-acting and maintenance therapy, and 13.1% maintenance therapy only. Amongst users of inhaled therapies, the pMDI was the most frequently used device (64.3%, n=2812/4370). Of 7050 patients included in the HCRU analysis, 79.8% had COPD-related costs; mean cost/patient was $4174. Mean COPD-related costs per patient for moderate and severe exacerbations were $910 and $23,208, respectively. Per-patient costs included $23,032 for inpatient visits, $2358 for emergency visits, $4432 for outpatient visits, and $1989 for pharmacy claims.
Conclusion: This observational study is the first to describe the real-world demographic and clinical characteristics and HCRU of patients initiating LAMA/LABA FDC treatment in the USA. Patients were generally elderly and overweight, with comorbidities of CVD, hypertension, and hyperlipidemia. Inpatient visits were the largest contributor to COPD-related costs per patient in the year prior to initiation of LAMA/LABA FDCs.
Keywords: muscarinic antagonist, β2-agonist, demographic characteristics, disease characteristics, cost
Plain Language Summary
Patients with COPD may be prescribed inhaled medications known as bronchodilators to relieve symptoms and prevent flare-ups (“exacerbations”) of the disease. For most patients, guidelines recommend starting therapy with a single short- or long-acting inhaled bronchodilator. If this does not control symptoms, a combination of two long-acting bronchodilators (a long-acting muscarinic antagonist [LAMA] and a long-acting β2-agonist [LABA] in a fixed-dose combination [FDC]) may be prescribed.
In this study, we used data from electronic health records and an insurance claims database to describe the characteristics of patients in the USA with COPD who were starting LAMA/LABA FDC therapy. We investigated the types of medication patients were taking in the 12 months prior to starting LAMA/LABA FDC therapy, as well as their extent of healthcare resource use (eg, hospital visits, pharmacy claims).
We found that patients starting LAMA/LABA FDCs were generally elderly and overweight, and had cardiovascular disease, high blood pressure, and high blood lipids. Around half of patients had used other inhaled medications for COPD in the 12 months prior to their first prescription of LAMA/LABA FDC; the most commonly prescribed inhaled treatment was a short-acting bronchodilator and the most commonly prescribed inhaler type was a pressurized metered-dose inhaler. Most patients (~80%) had costs related to their COPD in the 12 months before starting LAMA/LABA FDC therapy; the average cost per patient was $4174. The greatest costs were due to inpatient hospital visits and for exacerbations.
Future research could look at the impact of starting LAMA/LABA FDC therapy on healthcare resource use.
Introduction
COPD is a leading cause of global mortality, with an estimated 3.2 million disease-related deaths worldwide in 2017.1 COPD is also a leading cause of disability,2 and was ranked seventh globally in terms of disease burden measured by years of life lost in 2017.1 In the USA in 2016, COPD was ranked as the third leading cause of disability after ischemic heart disease and lung cancer.3 Moreover, COPD is associated with a substantial economic burden and, by 2020, the national medical costs attributable to COPD in the USA are projected to increase to $49.0 billion (from $32.1 billion in 2010).4
The treatment of COPD aims to reduce symptoms and the risk of exacerbations, and improve overall health status. The Global Initiative for Chronic Obstructive Lung Disease (GOLD) recommends initial pharmacological treatment with a short- or long-acting bronchodilator, depending on an individual’s symptom burden and risk of exacerbations.5 For patients who experience persistent breathlessness or exacerbations despite maintenance treatment with a single long-acting bronchodilator, treatment escalation to a long-acting muscarinic antagonist (LAMA)/long-acting β2-agonist (LABA) combination is recommended.5
Bronchodilators can be administered via a variety of inhaler devices, such as pressurized metered-dose inhalers (pMDIs), dry powder inhalers, soft mist inhalers, and nebulizers.5 Treatment regimens for patients should be individualized, taking into consideration the individual’s symptom severity, exacerbation risk, side-effects, comorbidities, drug availability and cost, response, preferences, and ability to use different inhaler devices.5 Use of LAMA/LABA fixed-dose combination (FDC) therapies is a pragmatic, simplified choice compared with using the individual LAMA and LABA components in separate inhalers. Previous real-world data have demonstrated that using multiple inhalers is associated with lower adherence and persistence with treatment; simplifying triple therapy regimens may result in increased adherence and persistence, improving health outcomes in patients with COPD.6 LAMA/LABA FDC therapies currently approved for the maintenance treatment of COPD in the USA are umeclidinium/vilanterol 62.5/25.0 µg (Anoro® Ellipta®; approved December 2013),7 tiotropium/olodaterol 5.0/5.0 µg (Stiolto® Respimat®; approved May 2015),8 glycopyrrolate/formoterol fumarate 18/9.6 μg (Bevespi Aerosphere™; approved April 2016),9 and indacaterol/glycopyrrolate 27.5/15.6 µg (Utibron™ Neohaler®; approved October 2015).10 These treatments have demonstrated benefits in patients with moderate-to-very severe COPD for lung function and health status, including patient-reported outcomes, versus placebo11–14 and their specific monocomponents.11–15
Real-world evidence on prescribing trends and clinical characteristics of patients initiating treatment with LAMA/LABA FDC therapies is generally limited to data from studies that focus on the effectiveness of treatment in the period following initiation,16,17 or is confined to evaluations of a very limited number of FDC therapies, as determined by limited clinical availability in the study population.16,18 To our knowledge, this is the first study to describe the demographic and clinical characteristics, healthcare resource utilization (HCRU), and treatment patterns in patients with COPD in the USA who have initiated any LAMA/LABA FDC treatment.
Materials and Methods
Study Design and Data Sources
This was a retrospective, observational cohort study in adults (≥40 years) with COPD that utilized an integrated dataset linking both an electronic health records (EHR) system and an insurance claims database. Practice Fusion’s EHR system (Practice Fusion, San Francisco, CA, USA), when linked with the Symphony Health claims database (Symphony Health, Blue Bell, Pennsylvania, PA, USA), provides a nationwide database of pharmacy, outpatient/medical, and hospital/inpatient claims data. The EHR system provides clinical data for approximately 6% of all ambulatory care, primary care, and specialist practices in the USA.19 We consider this to be comparable to the overall US population in terms of age, gender, and geographic location, as described in the national Ambulatory Medical Care Survey 2014.20 The Symphony Health claims database includes billing-related healthcare data submitted by approximately 30,000 pharmacies, 1000 hospitals, 800 outpatient facilities, and 80,000 physician practices, according to the claims supplier (S Redmond, personal communication, October 2017). Claims include insured populations participating in commercial health plans, as well as claims from patients participating in public insurance programs (eg, Medicaid and Medicare). The Symphony Health claims database and Practice Fusion EHR system are a complete record for approximately half of the patients; the other half may access healthcare outside this system. Exacerbations, HCRU, and costs are derived from Symphony Health claims database.
Patients
Patients (≥40 years of age at the index date) were included in the study cohort if they had a diagnosis of COPD on the Practice Fusion system at any time at, or prior to, the index date and at least one prescription of LAMA/LABA FDC therapy, with the date of first prescription (ie, index date) occurring between 1 May 2014 and 31 December 2017 (Figure 1). Patients also were required to have at least one visit to an ambulatory care, primary care, or specialist practice in the EHR system 12–24 months prior to the index date, and at least one visit ≥3 months following the index date. To be included in the HCRU analysis, patients were also required to have at least two claims from the Symphony Health claims database in the 12 months prior to the index date.
 |
Figure 1 Study design. Abbreviations: FDC, fixed-dose combination; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist. |
Study Endpoints
The primary objective of this study was to describe baseline demographic and clinical characteristics, HCRU, and treatment patterns in patients with COPD who initiated LAMA/LABA FDC treatment. Demographic characteristics analyzed in this study included age, body mass index (BMI), sex, race, ethnicity, insurance type, smoking status, and geographic region. Clinical characteristics included the Charlson Comorbidity Index (CCI), which predicts the 1-year mortality risk for a patient who may have a range of comorbid conditions; scores can be described continuously and categorically (0, 1, 2–3, 4+).21,22 In addition, comorbid conditions were evaluated based on diagnostic flags; comorbid conditions included anemia, anxiety, asthma, bronchiectasis, cardiovascular disease, depression, hypercholesterolemia, hyperlipidemia, hypertension, lung cancer, nasal polyps, osteoporosis, pulmonary fibrosis, sleep apnea, and type 2 diabetes; patients could have multiple comorbid diagnoses. Spirometry data (ie, forced expiratory volume in 1 second [FEV1] and forced vital capacity [FVC]) in the 12 months prior to the index date were also extracted, along with the proportion of patients for whom such data were available. Other clinical characteristics evaluated were the number of outpatient physician visits in the 12 months prior to the index date, including the number of visits to a primary care physician or pulmonologist (from the EHR system), and the number of patients with a prescription for COPD therapy in the 12 months prior to the index date. COPD therapy encompassed short-acting bronchodilators alone; LABA, LAMA, inhaled corticosteroid (ICS), LABA + LAMA in combination or separate inhalers, ICS + LABA in combination or separate inhalers, and ICS + LABA + LAMA in combination or separate inhalers (all either given alone or together with a short-acting β2-agonist [SABA] and/or short-acting muscarinic antagonist [SAMA]); any ICS use; and any regimen that did not include an ICS.
HCRU was calculated using both all-cause and COPD-related claims data (Symphony Health). All-cause claims were not restricted by diagnosis requirements, whereas COPD-related claims were identified by the inclusion of a COPD International Classification of Diseases (ICD)-9 or ICD-10 code on the claim. For the inpatient summaries, COPD-related inpatient hospitalizations were identified by a COPD diagnosis code in either the principal or first diagnosis position on the claim. HCRU also included an assessment of the number of patients with moderate or severe exacerbations. Moderate exacerbations were defined as an outpatient/emergency department (ED)/urgent care visit with a COPD diagnosis and pharmacy claim for oral corticosteroids and/or antibiotics on the same day as or within ≤10 days after the visit. Severe exacerbations were defined as a COPD-related inpatient hospitalization with a primary diagnosis of COPD. In addition, the number of patients who visited an ED (defined as patient-days) and the number with inpatient hospitalization, plus the number of visits/inpatient days and total associated costs for each, were evaluated. The number of patients with outpatient visits/procedures and pharmacy claims (including the number of claims and total associated costs) were also analyzed. Overall costs were calculated as an aggregation of all the above costs. All costs are reported as US dollars per patient per year.
Statistical Analysis
Statistical analysis was performed using the SAS statistical analysis tool (9.4, SAS Institute Inc., Cary, NC, USA). The demographic and clinical characteristics of study patients (including the type of treatment prescribed) are described as counts and percentages for categorical variables and as measures of central tendency (mean, median, standard deviation [SD], and interquartile range) for continuous variables.
Results
Study Population
In total, 1,258,889 patients with a diagnosis of COPD had claims data and, of these, 19,926 initiated LAMA/LABA FDC therapy (Figure 2). Overall, 8224 patients fulfilled all inclusion criteria for the overall study population and 7051 of these patients had at least two claims in the Symphony Health database in the 12 months prior to the index date. Data for one patient were excluded from the HCRU analysis as that person was considered an extreme outlier (HCRU approximately 10 SDs greater than the mean); therefore, the HCRU analysis population comprised 7050 patients.
Demographic Characteristics
The mean (SD) age of patients in the EHR cohort (n=8224) was 67.9 (10.7) years, and 52.8% of patients were female (Table 1). The mean (SD) BMI was 29.2 (7.6) kg/m2, suggesting that most patients were overweight or obese. The majority of patients were white (60.9%), non-Hispanic (91.6%), and former or current smokers (78.3%).
 |
Table 1 Demographic Characteristics of Patients Initiating LAMA/LABA FDC Treatment (EHR Practice Fusion Patient Cohort) |
Disease Characteristics
Patients had a mean (SD) CCI continuous score of 2.5 (1.9) (COPD contributes a value of 1 to the CCI; a mean of 2.5 indicates that on average patients had at least one additional comorbid condition). The most frequently reported comorbid diagnoses in patients initiating LAMA/LABA FDCs were cardiovascular disease (74.3%), hypertension (64.0%), and hyperlipidemia (45.6%) (Table 2). The mean (SD) number of outpatient visits in the year preceding the index date was 7.9 (6.7) visits per patient and, of these, the mean (SD) number of visits to primary care practitioners and pulmonologists was 6.6 (6.5) and 0.8 (2.2), respectively. Only 103 patients (1.3%) had spirometry (ie, FEV1/FVC) test results available, and the mean (SD) number of tests for these patients in the year prior to the index date was 1.3 (0.7) per patient. In terms of COPD treatment history, short-acting bronchodilators were used by 40.0% of patients in the 12 months prior to the index date; 18.1% had received ICS alone or in combination with other therapies; 11.2% had used a LAMA ± SABA and/or SAMA; and 1.3% had used LABA ± SABA and/or SAMA (Table 2).
 |
Table 2 Clinical Characteristics of Patients Initiating LAMA/LABA FDC Treatment |
Just over half of patients (n=4370; 53.1%) had used inhaled therapies within 12 months of the index date (Figure 3; Table 3), including 23.4% of patients on short-acting only therapy, 16.7% on short-acting and a maintenance therapy, and 13.1% on maintenance therapy only (Table 3). Of the users of inhaled therapy, 75.3% (n=3292/4370) used a short-acting bronchodilator with or without maintenance treatment, of whom the majority used a pMDI (76.3% [n=2513/3292]; Figure 3). A dry powder inhaler was the most commonly used inhaler device by patients receiving maintenance-only inhaled therapy (68.1% [n=734/1078]; Figure 3). Overall, the most frequently used devices were pMDIs (64.3% [n=2812/4370];Table 3; Figure 4).
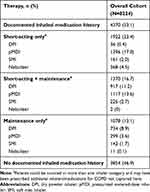 |
Table 3 Inhaled Therapy Use in the 12 Months Prior to the Index Date |
HCRU in the 12 Months Prior to the Index Date
Of the patients who met all inclusion criteria for the HCRU analysis (n=7050; excluding one outlier), 79.8% had COPD-related resource costs in the 12 months prior to the index date, and the mean cost per patient was $4174. All-cause claims were filed by 99.9% of patients, with a mean cost per patient of $19,690 (Table 4).
 |
Table 4 Healthcare Resource Utilization in the 12 Months Prior to the Index Date |
Overall, 14.8% and 4.5% of patients had at least one moderate or severe exacerbation, respectively. Patients with moderate exacerbations had a mean occurrence of 1.8 events 12 months prior to the index date at a mean cost per patient of $910, whereas patients with severe exacerbations had a mean occurrence of 1.2 events with a mean cost per patient of $23,208. Overall, 17.2% of patients had moderate and severe exacerbations, with a mean occurrence of 1.9 events per patient and a mean cost per patient of $6818.
In total, 421 patients (6.0%) had a COPD-related ED visit (with ICD codes for COPD indicated on the claim) in the 12 months prior to the index date. These patients had a mean occurrence of 1.6 visits at a mean cost per patient of $2358. There were 1058 patients (15.0%) who had a visit to the ED for any reason (mean 2.0 visits per patient). For these patients, the mean cost per patient for the ED visit was $3149.
Few patients (4.5%) had ≥1 day of COPD-related inpatient hospitalization (with COPD indicated on the claim as the principal or first reason). These patients were hospitalized for a mean of 5.3 days at a cost per patient of $23,032. More patients (17.0%) were treated in an inpatient facility for any reason, and the mean cost per patient (for a mean of 6.9 inpatient days) was $34,208.
In total, 2834 patients (40.2%) had a COPD-related visit to, or procedure in, an outpatient facility (mean 12.3 visits). For these patients, the mean cost per patient was $4432. The majority of patients (79.1%) had a visit to, or procedure in, an outpatient facility for any reason during the 12 months prior to the index date, at a cost per patient of $9527.
Approximately two-thirds of patients (67.8%) had a COPD-related pharmacy claim (mean number of prescriptions: 8.2) at a mean cost per patient of $1989. All-cause pharmacy claims were reported for 90.6% of patients, and the mean cost per patient was $7004 (mean number of prescriptions: 50.4).
Discussion
This retrospective, observational cohort study evaluated the demographic and clinical characteristics, HCRU, and prior treatment patterns of patients with COPD who initiated LAMA/LABA FDC treatment in the USA. Patients were included if they had at least one prescription for LAMA/LABA FDC therapy, with an index date (first prescription) between 1 May 2014 and 31 December 2017. During this period, four LAMA/LABA FDC therapies were approved in the USA (umeclidinium/vilanterol 62.5/25.0 µg [Anoro® Ellipta®],7 tiotropium/olodaterol 5.0/5.0 µg [Stiolto® Respimat®],8 glycopyrrolate/formoterol fumarate 18/9.6 μg [Bevespi Aerosphere™],9 and indacaterol/glycopyrrolate 27.5/15.6 µg [Utibron™ Neohaler®]).10
The results of this study suggest that, in a real-world setting, patients who are newly prescribed LAMA/LABA FDC therapies are generally elderly and overweight, with most patients having a smoking history and comorbid conditions, particularly cardiovascular disease, hypertension, and hyperlipidemia. These findings are generally aligned with the established characteristics of US and European patients with COPD in clinical studies of LAMA/LABA FDCs.11–15
The majority of patients (75.3%) who had used an inhaled medication in the 12 months prior to the index date used a short-acting bronchodilator with or without maintenance treatment; 76.3% of these patients used a pMDI. Overall, the use of pMDIs was common in the 12 months prior to initiation of LAMA/LABA FDCs, with approximately two-thirds of patients using these devices. This indicates that most patients had experience and familiarity with pMDIs before they initiated LAMA/LABA FDC therapy. Patients with COPD may use two or three inhaled medications simultaneously.23,24 Use of a device that the patient is familiar with can result in consistency of use and improved clinical results.14,25-29 Nevertheless, education of patients in the correct use of inhalation devices is a crucial consideration, regardless of their prior experience.5 It was observed that patients were primarily managed in primary care (mean visits of 6.6 [6.5] vs 0.8 [2.2] pulmonologist visits). Primary care providers are likely to rely on external guidance (eg, GOLD annual updates5) to inform treatment discussion, highlighting the importance of consensus documents.
The second aspect of this study was to characterize the prior HCRU in patients with COPD initiating LAMA/LABA FDCs. In the 12 months prior to initiation of these therapies, mean total COPD-related and all-cause HCRU costs were $4174 and $19,690, respectively. Few patients had COPD-related inpatient days or ED visits; however, the cost per patient for inpatient visits was the largest contributor to COPD-related costs, greatly exceeding costs for outpatient visits, ED visits, and pharmacy resource use.
The findings of this study in US patients are generally consistent with the results of other non-interventional studies. A longitudinal prospective study (the DACCORD study) described the baseline demographics and disease characteristics of patients with COPD initiating LAMA/LABA FDCs in clinical practice in Germany.16 Consistent with the present findings, patients in the DACCORD study were generally overweight at baseline; however, the proportion of men was higher than in the present results. Similar to the current study, the more common comorbidities in the DACCORD study included cardiovascular disease and type 2 diabetes.16 A retrospective, observational study in US patients evaluated exacerbation rates following initiation of LAMA/LABA or LABA/ICS combinations using insurance claims data from 2004 to 2014. Patients initiating LAMA/LABA were elderly, approximately 54% were male, and the most common comorbidities included hypertension and cardiovascular disease.17 These findings are generally in line with the demographics, clinical characteristics, and HCRU reported in this study. A Canadian population-based study in patients ≥65 years of age, evaluated trends in COPD treatments before and after initiation of LAMA/LABA combination therapies using administrative claims data from 2010 to 2016. Similar trends in prior treatment to those seen in the present study were observed. For example, the Canadian study reported that prior to initiation of LAMA/LABA combination therapy, 40.0% of patients had no COPD therapy prescribed in the 180-day period prior to the index date (compared with 46.9% of patients in this study who received no inhaled therapy in the previous 12 months prior to the index date); 5.0% had received a SAMA and 41.8% had received a SABA (compared with 40.0% of patients receiving short-acting bronchodilators in this study); and 6.8% had used ICS monotherapy (compared with 4.2% in this study).18
The costs reported in this study were very similar to those found in a retrospective, US claims-based study that evaluated healthcare costs and utilization among patients with COPD who were newly initiating ICS/LABA combination therapy with budesonide/formoterol or fluticasone/salmeterol between 1 March 2009 and 31 March 2012.30 COPD-related costs in the first year following the index date were $4326–4846 and all-cause costs were $21,580–24,483.
The strengths of this study include the large sample of patients with COPD in a real-world setting across the USA. The results of this study are limited by their descriptive nature without multivariate modeling. Moreover, as this was a retrospective, observational study, potential confounding effects due to unknown variables, including lifestyle and medication adherence, cannot be ruled out. It is also possible that additional ongoing prescriptions obtained outside the EHR may impact on baseline medication use. In addition, few clinical measures of COPD were captured that would confirm the diagnosis (eg, airflow limitation severity). Data captured in an EHR system reflect routine clinical practice rather than mandatory assessments at pre-specified time points, which may have an impact on the quality and timing of available data. In addition, differentiation between patients without medication use and patients with missing history was not possible. The drug exposure definition used was based on written prescription orders with no confirmation of whether the prescription was filled at the pharmacy or taken by the patient. As a result, measures of drug use related to timing are based on order dates and may be imprecise with respect to actual medication start and stop dates. Future research could look at the impact of starting LAMA/LABA FDC therapy on healthcare resource use.
Conclusions
This retrospective, observational cohort study is the first to describe the real-world demographic and clinical characteristics, as well as HCRU, of patients with COPD who initiated any LAMA/LABA FDC treatment in the USA. The patients were generally elderly and overweight, and had a high prevalence of cardiovascular disease, hypertension, and hyperlipidemia. In the year prior to initiating LAMA/LABA FDC therapy, the most frequently prescribed inhaled therapy was a short-acting bronchodilator with or without a maintenance therapy, and a pMDI was the most commonly used device. From the HCRU findings, it appeared that the largest contributor to COPD-related costs per patient in the year prior to LAMA/LABA FDC initiation was inpatient visits.
Abbreviations
BMI, body mass index; CCI, Charlson Comorbidity Index; ED, emergency department; EHR, electronic health records; FDC, fixed-dose combination; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; HCRU, healthcare resource utilization; ICD, International Classification of Diseases; ICS, inhaled corticosteroid; LABA, long-acting β2-agonist; LAMA, long-acting muscarinic antagonist; pMDI, pressurized metered-dose inhaler; SABA, short-acting β2-agonist; SAMA, short-acting muscarinic antagonist; SD, standard deviation.
Data Sharing Statement
Data underlying the findings described in this manuscript may be obtained in accordance with AstraZeneca’s data-sharing policy, described at: https://astrazenecagrouptrials.pharmacm.com/ST/Submission/Disclosure.
Ethics and Consent Statement
This study was performed in compliance with Good Clinical Practice and Good Pharmacoepidemiology Practice. Institutional review board approval was granted by the New England Independent Review Board (#120180176), 197 First Avenue, Suite 250, Needham, MA 02494, USA. Patient consent was not sought for this study as the institutional review board found that this research meets the requirements for a waiver of consent under 45 CFR 46.116(d).
Acknowledgments
The authors wish to thank Alina Bogdanov for providing oversight and quality assurance support, and Nova Hammerquist for providing project management support throughout the project. Medical writing support, under the direction of the authors, was provided by Karleen Nicholson, PhD, on behalf of CMC Connect, McCann Health Medical Communications, funded by AstraZeneca, Gaithersburg, USA in accordance with Good Publication Practice (GPP3) guidelines.31
Author Contributions
All authors contributed to the conception and design of this analysis, data acquisition, data analysis, and interpretation. All authors contributed to the drafting of this article, were involved in critically revising the manuscript for important intellectual content, and approved the final version for publication. All authors agree to be accountable for all aspects of the work and ensure that questions related to the accuracy or integrity of the work are appropriately investigated and resolved.
Funding
This study was supported by AstraZeneca. Employees of the sponsor were involved in various aspects of the conception and design of the studies, acquisition of data, analysis and interpretation of data, and input into manuscript development. The sponsor did not place any restrictions on the authors about the statements made in the final article.
Disclosure
BD and DG are employees of AstraZeneca. DG holds AstraZeneca stock and/or stock options. LK, LS, AW, and DO are employees of Practice Fusion. The authors report no other conflicts of interest in this work.
References
1. GBD 2017 Causes of Death Collaborators. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788.
2. GBD 2015 Chronic Respiratory Disease Collaborators. Global, regional, and national deaths, prevalence, disability-adjusted life years, and years lived with disability for chronic obstructive pulmonary disease and asthma, 1990–2015: a systematic analysis for the Global Burden of Disease Study 2015. Lancet Respir Med. 2017;5(9):691–706. doi:10.1016/S2213-2600(17)30293-X
3. US Burden of Disease Collaborators. The state of US health, 1990–2016: burden of diseases, injuries, and risk factors among US States. JAMA. 2018;319(14):1444–1472. doi:10.1001/jama.2018.0158
4. Ford ES, Murphy LB, Khavjou O, Giles WH, Holt JB, Croft JB. Total and state-specific medical and absenteeism costs of COPD among adults aged ≥ 18 years in the United States for 2010 and projections through 2020. Chest. 2015;147(1):31–45. doi:10.1378/chest.14-0972
5. Global Initiative for Chronic Obstructive Lung Disease. Global strategy for the diagnosis, management, and prevention of chronic obstructive pulmonary disease (2020 report); 2020. Available from: https://goldcopd.org/wp-content/uploads/2019/11/GOLD-2020-REPORT-ver1.0wms.pdf.
6. Bogart M, Stanford RH, Laliberté F, Germain G, Wu JW, Duh MS. Medication adherence and persistence in chronic obstructive pulmonary disease patients receiving triple therapy in a USA commercially insured population. Int J Chron Obstruct Pulmon Dis. 2019;14:343–352. doi:10.2147/COPD.S184653
7. GlaxoSmithKline. Anoro® Ellipta® Prescribing Information; 2019. Available from: https://www.gsksource.com/pharma/content/dam/GlaxoSmithKline/US/en/Prescribing_Information/Anoro_Ellipta/pdf/ANORO-ELLIPTA-PI-MG-IFU.PDF.
8. Boehringer Ingelheim Pharmaceuticals Inc. Stiolto™ Respimat® Summary of Product Characteristics; 2018. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2018/206756s009lbl.pdf.
9. AstraZeneca Pharmaceuticals LP. Bevespi Aerosphere™ Prescribing Information; 2019. Available from: http://www.azpicentral.com/bevespi/bevespi_pi.pdf.
10. Novartis Pharmaceuticals Corporation. Utibron™ Neohaler® Prescribing Information; 2017. Available from: https://www.accessdata.fda.gov/drugsatfda_docs/label/2017/207930s002lbl.pdf.
11. Mahler DA, Kerwin E, Ayers T, et al. FLIGHT1 and FLIGHT2: efficacy and safety of QVA149 (indacaterol/glycopyrrolate) versus its monocomponents and placebo in patients with chronic obstructive pulmonary disease. Am J Respir Crit Care Med. 2015;192(9):1068–1079. doi:10.1164/rccm.201505-1048OC
12. Donohue JF, Maleki-Yazdi MR, Kilbride S, Mehta R, Kalberg C, Church A. Efficacy and safety of once-daily umeclidinium/vilanterol 62.5/25mcg in COPD. Respir Med. 2013;107(10):1538–1546. doi:10.1016/j.rmed.2013.06.001
13. Martinez FJ, Rabe KF, Ferguson GT, et al. Efficacy and safety of glycopyrrolate/formoterol metered dose inhaler formulated using co-suspension delivery technology in patients with COPD. Chest. 2017;151(2):340–357. doi:10.1016/j.chest.2016.11.028
14. Lipworth BJ, Collier DJ, Gon Y, et al. Improved lung function and patient-reported outcomes with co-suspension delivery technology glycopyrrolate/formoterol fumarate metered dose inhaler in COPD: a randomized Phase III study conducted in Asia, Europe, and the USA. Int J Chron Obstruct Pulmon Dis. 2018;13:2969–2984. doi:10.2147/COPD
15. Buhl R, Maltais F, Abrahams R, et al. Tiotropium and olodaterol fixed-dose combination versus mono-components in COPD (GOLD 2-4). Eur Respir J. 2015;45(4):969–979. doi:10.1183/09031936.00136014
16. Worth H, Buhl R, Criée CP, Kardos P, Lossi NS, Vogelmeier CF. GOLD 2017 treatment pathways in ‘real life’: an analysis of the DACCORD observational study. Respir Med. 2017;131:77–84. doi:10.1016/j.rmed.2017.08.008
17. Samp JC, Joo MJ, Schumock GT, Calip GS, Pickard AS, Lee TA. Comparative effectiveness of long-acting beta2-agonist combined with a long-acting muscarinic antagonist or inhaled corticosteroid in chronic obstructive pulmonary disease. Pharmacotherapy. 2017;37(4):447–455. doi:10.1002/phar.2017.37.issue-4
18. Parkin L, Khuu W, Stanbrook MB, Tadrous M, Martins D, Gomes T. Trends in the utilisation of COPD therapeutic regimens before and after the introduction of LAMA/LABA combination products: a population-based study. Respir Med. 2018;143:1–7. doi:10.1016/j.rmed.2018.08.001
19. IQVIA. Physician office usage of electronic health records software. A market insights report; 2018. Available from: https://www.iqvia.com/locations/united-states/library/fact-sheets/iqvia-market-insight-report-ehr-software-adoption.
20. Centers for Disease Control and Prevention’s National Center for Health Statistics (NCHS). National Ambulatory Medical Care Survey: 2014 State and National Summary Tables; 2014. Available from: https://www.cdc.gov/nchs/data/ahcd/namcs_summary/2014_namcs_web_tables.pdf.
21. Charlson ME, Pompei P, Ales KL, MacKenzie CR. A new method of classifying prognostic comorbidity in longitudinal studies: development and validation. J Chronic Dis. 1987;40(5):373–383. doi:10.1016/0021-9681(87)90171-8
22. Deyo RA, Cherkin DC, Ciol MA. Adapting a clinical comorbidity index for use with ICD-9-CM administrative databases. J Clin Epidemiol. 1992;45(6):613–619. doi:10.1016/0895-4356(92)90133-8
23. Batterink J, Dahri K, Aulakh A, Rempel C. Evaluation of the use of inhaled medications by hospital inpatients with chronic obstructive pulmonary disease. Can J Hosp Pharm. 2012;65(2):111–118. doi:10.4212/cjhp.v65i2.1118
24. Sriram KB, Percival M. Suboptimal inhaler medication adherence and incorrect technique are common among chronic obstructive pulmonary disease patients. Chron Respir Dis. 2016;13(1):13–22. doi:10.1177/1479972315606313
25. Khassawneh BY, Al-Ali MK, Alzoubi KH, et al. Handling of inhaler devices in actual pulmonary practice: metered-dose inhaler versus dry powder inhalers. Respir Care. 2008;53(3):324–328.
26. van der Palen J, Klein JJ, van Herwaarden CL, Zielhuis GA, Seydel ER. Multiple inhalers confuse asthma patients. Eur Respir J. 1999;14(5):1034–1037. doi:10.1183/09031936.99.14510349
27. Dolovich MB, Ahrens RC, Hess DR, et al. Device selection and outcomes of aerosol therapy: evidence-based guidelines. American College of Chest Physicians/American College of Asthma, Allergy, and Immunology. Chest. 2005;127(1):335–371. doi:10.1378/chest.127.1.335
28. Bosnic-Anticevich S, Chrystyn H, Costello RW, et al. The use of multiple respiratory inhalers requiring different inhalation techniques has an adverse effect on COPD outcomes. Int J Chron Obstruct Pulmon Dis. 2016;12:59–71.
29. Price D, Chrystyn H, Kaplan A, et al. Effectiveness of same versus mixed asthma inhaler devices: a retrospective observational study in primary care. Allergy Asthma Immunol Res. 2012;4(4):184–191. doi:10.4168/aair.2012.4.4.184
30. Davis JR, Kern DM, Williams SA, et al. Health care utilization and costs after initiating budesonide/formoterol combination or fluticasone/salmeterol combination among COPD patients new to ICS/LABA treatment. J Manag Care Spec Pharm. 2016;22(3):293–304. doi:10.18553/jmcp.2016.22.3.293
31. Battisti WP, Wager E, Baltzer L, et al. Good publication practice for communicating company-sponsored medical research: GPP3. Ann Intern Med. 2015;163(6):461–464. doi:10.7326/M15-0288
32. US Census Bureau. OUR DIVERSE POPULATION: race and Hispanic Origin, 2000; 2000. Available from: https://www.census.gov/prod/2001pubs/cenbr01-1.pdf.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.



