Back to Journals » Patient Preference and Adherence » Volume 13
Patient and physician preferences for attributes of biologic medications for severe asthma
Authors Gelhorn HL, Balantac Z , Ambrose CS , Chung YN , Stone B
Received 20 December 2018
Accepted for publication 14 June 2019
Published 25 July 2019 Volume 2019:13 Pages 1253—1268
DOI https://doi.org/10.2147/PPA.S198953
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Johnny Chen
Heather L Gelhorn,1 Zaneta Balantac,1 Christopher S Ambrose,2 Yen N Chung,2 Brian Stone3
1Evidera, Bethesda, MD, USA; 2AstraZeneca, Gaithersburg, MD, USA; 3Allergy Partners, San Diego, CA, USA
Objective: Despite the increased availability of biologic treatments indicated for severe asthma, patient and physician preferences for these medications remains largely unknown. The purpose of this study was to understand perceptions of biologic therapies, barriers to care with biologic medications, and preferences for biologic therapy attributes.
Methods: This mixed-methods study involved quantitative surveys and qualitative telephone interviews with patients and physicians from the United States. Participants described preferences for relevant attributes, and barriers to use of biologic medications. Participants rated, ranked, and indicated importance of preferences for different levels of key attributes including: mode of administration, administration setting, dosing frequency, number of injections, and time to onset of effect. Other attributes unique to each group were also included.
Results: A total of 47 patients and 25 physicians participated. Patients ranked out-of-pocket costs, mode of administration, time to onset of efficacy, and administration setting as the most important attributes. Physicians ranked mode of administration, time to onset of efficacy, dosing frequency, and insurance reimbursement/access as most important. Both groups expressed preferences for less frequent administrations (Q8W over Q4W or Q2W) (all P<0.01) and subcutaneous (SC) over intravenous injection (both P<0.0001). Key patient barriers to biologic medications include location of treatment, administration time, scheduling, cost/insurance coverage, number of injections, and mode of administration. Physicians identified patient candidacy, convincing patients, administration setting, mode of administration, cost, and administrative burden as key barriers to initiating therapy; and efficacy, speed of onset, convenience of administration, cost, and patient compliance as barriers to staying on therapy.
Conclusions: Patients and physicians expressed strong preferences for less frequent dosing, SC administration, and faster onset. Cost/insurance coverage and convenience issues were key barriers to use. Increased awareness and understanding of preferences and barriers may be useful in facilitating physician-patient conversations with the goal of individualizing treatment.
Keywords: patient preference, clinician preference, severe asthma, biologic therapy, treatment barriers
Introduction
Severe asthma affects approximately 10% of all asthma patients and is difficult to control with traditional therapies.1,2 The US, real-world Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens (TENOR) study of patients with severe or difficult-to-treat asthma demonstrated high rates of health care use by patients with asthma despite use of multiple long-term controller medications.3 Recent identification of different inflammatory asthma endotypes and the availability of targeted treatments, including biologics, have changed the treatment approach for patients with severe asthma.4–6 However, attributes of available biologic therapies differ, and patient and physician preferences for these attributes remain largely unknown.
While preference research has been conducted among patients with asthma,7,8 none of these prior studies included biologic therapies. However, preference research on biologic medications has been conducted in other disease areas, including arthritis (eg, rheumatoid arthritis, psoriatic arthritis, ankylosing spondylitis),9–24 psoriasis,10,23,25–33 gastrointestinal disorders (eg, Crohn’s disease, ulcerative colitis, irritable bowel syndrome),10,34–36 neurologic disorders (eg, multiple sclerosis),20 immunodeficiency,1,2 cancer (eg, breast cancer, non-Hodgkin’s lymphoma),37–43 osteoporosis,44 and general chronic inflammatory conditions.45 This prior research included surveys, qualitative studies, and discrete choice experiments (DCEs) and often explored topics such as efficacy, safety/adverse effects, administration characteristics, and cost. From research using DCE methodology, the most commonly included attributes were mode of administration,1,2,9,11,16–18,20,27,39 efficacy,9,16,17,20,27,30,33,39,46 and dosing frequency.1,2,9,16,17,20,27,46
Results from prior preference studies in other disease areas reported preference for less frequent dosing. In patients with psoriasis, for example, the majority of both biologic-naïve and biologic-experienced patients preferred the administration frequency of once every 12 weeks over once every 1 or 2 weeks.47 In addition, of patients with osteoporosis, the majority preferred once-monthly treatment over once-weekly treatment, citing ease of following the treatment regimen and tolerance of adverse effects as key decision factors.44 Several preference studies of biologic treatments in other disease areas reported dispreference for intravenous (IV) administration.9,11,13,17
The dosing frequency of currently available biologic therapies indicated for severe asthma ranges from once every 2 weeks to once every 8 weeks, but little is known about the importance of dosing frequency to patients and physicians in severe asthma care. Understanding patient and physician perceptions and preferences, as well as identifying factors influencing treatment behaviors, may help guide current treatment decisions and the development of new therapies for severe asthma. The purpose of this study was to understand patient and physician perceptions, barriers to care, and preferences for various attributes, including dosing frequency, formulation, mode of administration, time to onset of action, and administration burden for biologic therapies used to treat severe asthma.
Methods
This was a quantitative and qualitative mixed-methods study involving interviews and surveys of adult patients with severe asthma and of board-certified physicians with at least 5 years of experience treating patients with severe, uncontrolled asthma. Sample size was determined based on power analyses used for the primary hypothesis of the co-primary and key secondary endpoints.
Sample
Patients were recruited from six clinical sites in the United States. These sites were identified through an asthma clinical database of sites that were successful in recruiting patients in prior studies and selected based on site interest and feasibility of recruitment. Key participant inclusion criteria included age ≥18 years; diagnosis of severe asthma of ≥3 years; a candidate for biologic therapy and one of the following: currently on biologic therapy for ≥3 months, previously on biologic therapy and had discontinued within last 18 months, or recommended for biologic therapy but declined and were biologic-naïve at the time of study participation. Key exclusion criteria included diagnosed with asthma <5 years ago; currently or had ever previously enrolled in a clinical trial to receive biologic therapy; had a cognitive, physical, or psychological impairment that would interfere with ability to provide consent and participate in interview; or had COPD or lung cancer.
Board-certified pulmonologists and allergists from the United States with experience in prescribing biologics for severe, uncontrolled asthma were recruited through a health care market research firm with access to physician panels. Physicians were recruited through email and screened by telephone or online. Key inclusion criteria for physicians included: Medical Doctors (MD) or Doctor of Osteopathic Medicine (DO) with ≥15 years of overall experience in medicine; currently a board-certified allergist or pulmonologist; ≥5 years of experience treating patients with severe asthma; and currently treating ≥5 patients with biologics for severe asthma per year. Physicians with limited English proficiency were excluded. The physicians were not recruited from the 6 sites that assisted with patient recruitment and were not aware of the identity of the study sponsor.
Identification of attributes
The design for this study was developed based on a two-step approach. The first step involved identification of attributes, as informed by a targeted literature review and social media search. The information was then used to develop the content of the study materials used for the interview and survey-administration portion of the study.
The targeted literature review was conducted via PubMed between August and September 2017 for key peer-reviewed literature published between 2012 and 2017 related to patient and physician perceptions and preferences relevant to biologic treatment (for severe asthma or other therapeutic areas). Overall, 44 articles were identified and the studies described utilized various research methodologies, including surveys, qualitative studies, and DCEs (Figure S1). Only 2 articles were specific to asthma.7,8 Different attributes were discussed or utilized in experiments, including efficacy, life expectancy, duration of response, safety/adverse effects (eg, minor or serious adverse events), mode of administration, frequency of administration, time of administration, location of administration, number of injections per administration, and cost.1,2,9,11,16–18,20,27,30,33,39,46 Results of the search were used to inform the development of the study protocol, interview guide, and any relevant and important medication attributes to be included in the preference survey.
Concurrent with the targeted search of the scientific literature, a social media search was conducted to identify real-world concerns of asthma patients with regards to their treatment perceptions, outcomes, and preferences related to biologic medications (Figure S2).48 Posts from November 2003 to September 2017 were extracted and analyzed from healthboards.com (351 posts), drugs.com (44 posts), and WebMD.com (122 posts). All data collection/selection and aggregation activities were carried out through the use of programmed algorithms (R statistical programming). Specific treatment names and other patient identifiable information were removed and replaced with a generic flag prior to data review. Nine main attributes of relevance were discussed in posts (number of posts range: n=212 [efficacy] to n=1 [formulation, or preparation process or procedures for reconstitution of the medication prior to administration]).48 Few posts discussed patients’ direct preferences, but posts often included factual information on the attribute topics indicating that it was relevant from the patient perspective. Overall, the most commonly mentioned positive perceptions about asthma biologics discussed in social media were efficacy in reducing asthma symptoms and the reduced need for using other treatments (Figure S3). The biologic treatment attributes most commonly included in negative comments were related to the cost of the biologics (eg, out-of-pocket), access (eg, insurance coverage, distance, wait times), and adverse effects (Figure S4).
Results from both the literature review and social media data mining helped inform the treatment attributes selected for the design of the study. Eight biologic treatment attributes were evaluated and selected based upon their relevance to the decision to initiate biologic treatment in general or the presence of clear differentiation across available treatments (Table 1). Dosing frequency was a primary area of interest given the existing differences across available biologic treatments for severe asthma.
 |
Table 1 Selected attributes and definitions |
Measures and procedures
Participants completed both a qualitative telephone interview and a quantitative survey to elicit information about their preferences for, and perceived barriers to, treatment with biologic medications. Physicians completed a web-based quantitative survey immediately before the interview (Survey–Physician). A paper-and-pencil quantitative survey (Survey–Patient) was mailed to patients ahead of the telephone interview but completed at the end of the interview to ensure that the patient understood the medication features of interest. The interviews were designed to elicit information from the participants, and interviewers did not comment or make judgments on participants’ responses with the exception of clarifying any misconceptions.
In the qualitative telephone interviews, patients and physicians were asked detailed questions to help understand the rationale for their preferences and to understand their experiences with biologic treatments, including any barriers they may have encountered. A semi-structured interview guide was used to guide the discussions, which included an introduction to the interview session, and followed up with specific questions and probes that were designed to facilitate discussion and optimize consistency across interviews. Patients and physicians also completed a sociodemographic form. Data regarding general clinical characteristics of the patients were collected from clinical sites, including type and date of asthma diagnosis, physical characteristics such as height and weight, pulmonary function test and FeNO level in the past 12 months, inhaled corticosteroids history, and exacerbation history.
In the quantitative survey, patients and physicians were asked to rate, rank, and select preferred attributes or features of biologic medications indicated for the treatment of severe asthma (Survey-Physician and Survey-Patient). The various exercises were (1) rate 8 features of biologic treatment by importance (1=not at all important; 10=extremely important); (2) rank the same 8 features in order of importance (1=most important feature; 8=least important feature); and (3) select a preference for specific levels of medication attributes which were categorical in nature. For example, patients and physicians were asked about their preferences on dosing frequency (every 2, 4, or 8 weeks). Finally, patients and physicians (4) selected their preferred medication from 4 anonymized profiles of existing biologic medications that were described based on their mode of administration (subcutaneous [SC] vs. intravenous [IV]), dosing frequency (every 2, 4 or 8 weeks), number of injections per treatment, and for physicians only, the process required for administration (Tables S1a and S1b).
The protocol was approved by Ethical and Independent Review Services, a central institutional review board (Ethical and Independent Review Services, protocol #17156-01, v1.0 date 17Nov17, approved 21Nov17) prior to initiation of the study.
All participants provided informed consent prior to participating. Patients provided written informed consent (mailed forms) and verbal consent, while physicians provided electronic (online form) and verbal consent.
Analyses
Quantitative analyses
Descriptive statistics were used to characterize the sample using SAS version 9.4 (SAS Institute, Cary, NC, USA), Mean, SDs, and ranges were presented for continuous variables, and frequencies and percentages were presented for categorical variables.
Chi-squared goodness-of-fit tests were used for analyses of the co-primary endpoints, testing whether patients reported statistically significant preferences for (1) 2-, 4-, or 8-week dosing frequency, and (2) the amount of time between dosing relative to a 1-month interval (<1 month between treatments, 1 month between treatments, or >1 month between treatments). Secondary endpoints included the same goodness-of-fit analyses described above for physician preferences on dosing interval, and tests across the full study sample (ie, patients and physicians combined). Additional post-hoc chi-squared goodness-of-fit tests were also conducted to examine preferences by variables of interest, including: travel distance for treatment, state of residence, years since diagnosis, employment status, current corticosteroid status, biologic status, and location of clinical practice.
Qualitative analyses
All interviews were digitally recorded and transcribed. A qualitative analysis software program, ATLAS.ti version 8.0+,49 was used to organize and categorize the text in the interview transcripts. Using a content analysis approach,50 the de-identified data were examined for attributes that patients and physicians consider important when evaluating treatment for their severe asthma. A coding dictionary was developed based on the structure of the interview guide. The first two transcripts were independently coded and the coding was compared for consistency. A constant comparative method—an iterative coding approach moving between consecutive transcripts as new codes emerge51—was used. Participant quotes were then grouped and summarized by thematic code, and coding outputs were generated based on each utilized code.
Results
Sample characteristics
A total of 47 patients and 25 physicians in the US participated in the study. Only 46 patients were included in the final quantitative analysis as one patient had low literacy that prevented accurate collection of quantitative data; however, that patient’s qualitative feedback and sociodemographic and clinical characteristics are reported.
Patients were recruited from a total of 6 clinical sites in different states (Vermont, Kentucky, California, Massachusetts, Virginia, and Arizona) and included approximately 29 patients currently on biologic therapy (4 of whom also had previous experience with a different biologic), 6 patients who were previously but not currently on biologic therapy, and 12 patients who were biologic-naïve. The mean (±SD) age among patients was 49.9±13.1 years, with most being female (74.5%) and non-Hispanic White (74.5%). Eleven patients (23.4%) identified as Black or African‐American. Mean (SD) time since asthma diagnosis was 26.7±14.4 years, and mean patient-reported asthma exacerbations in the last 12 months were 6.5±15.2. Patients also experienced various comorbid health conditions (Table 2). Site-reported clinical information is presented in Table 3. Mean (SD) body mass index was 33.6±8.0 and site-reported patient asthma exacerbations in the last 12 months were 1.2±1.8. Differences in the number of asthma exacerbations between patient and site reports seem to suggest patients may not report all their exacerbation experiences with their doctors, and/or that patients and physicians have different criteria to define an asthma exacerbation. Nine patients had a diagnosis of severe eosinophilic asthma.
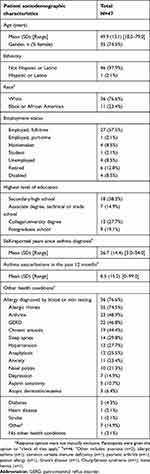 |
Table 2 Patient-reported sociodemographic characteristics (N=47) |
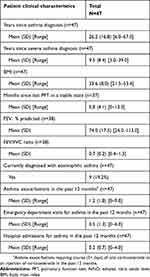 |
Table 3 Site-reported patient clinical characteristics (N=47) |
Physicians included 13 board-certified allergists and 12 board-certified pulmonologists from 15 different US states (Table 4). The mean (SD) age of physician participants was 56.6±6.3 years, and the majority were male (80.0%) and from a group practice (64.0%). Physicians reported seeing between 25 and 350 patients with severe asthma each year (mean (SD) 135.8±93.2) and reported treating severe asthma patients as young as 1-year-old (youngest patient on average: 10.2±7.8 years) and as old as 100 (oldest patient on average: 86.7±10.1 years). The mean (SD) number of severe asthma patients currently on biologic therapy was 28.4±21.4 (range: 5–85). Nearly all physicians (92.0%) indicated that the majority of patients they treat have commercial insurance.
 |
Table 4 Physician sociodemographic characteristics |
Rating and ranking the importance of each attribute
Among patients, there were 4 medication features that were consistently ranked among the top 3 most important attributes: out-of-pocket costs (65.3% ranked within the top 3, average rating 9.2), mode of administration (IV vs. SC, 52.2% ranked within the top 3, average rating 6.8), administration setting (45.7% within the top 3, average rating 7.7), and time to onset of efficacy (39.1% within the top 3, average rating 7.7) (Figure 1, Figure S5).
Among physicians, the highest-ranked attributes were: mode of administration (IV vs. SC, 72% within the top 3, average rating 7.8), time to onset of efficacy (60% within the top 3, average rating 8.0), dosing frequency (every 2, 4 or 8 weeks; 56% within the top 3, average rating 8.1), and insurance reimbursement/access (48% within the top 3, average rating 8.1) (Figure 2, Figure S6).
 |
Figure 2 Physicians’ rankings of importance of biologic medication attributes (N=25). |
Preference between attribute levels
For specific medication features that can be described through categorical levels and that are relevant to both patients and physicians, the survey also included specific questions asking the participants to indicate which level of each feature they would prefer (Figure 3). Both patients and physicians preferred less frequent administration choosing every 8 weeks versus every 4 or 2 weeks (patients P<0.0001, physicians P<0.0001), as well as more than once a month versus once a month or less than once a month (patients P<0.0001, physicians P<0.01). Frequency of treatment preferences was similar when tested across different patient variables, including travel distance for treatment, state of residence, years since diagnosis, employment status, corticosteroid status, or biologic treatment status. Similarly, preferences were similar among physicians practicing in urban vs. suburban locations.
 |
Figure 3 Preferences for biologic characteristics. Only n=46 were included in final analysis. One patient was excluded due to low literacy that prevented accurate collection of quantitative data. |
For the mode of administration, there was an overwhelming preference by both patients (100%) and physicians (96%) for SC over IV injection (P<0.0001). There were mixed preferences for who administers the medication (ie, self- or clinician-administered), and where the medication is administered (ie, at home or in a health care setting such as a clinic). Both patients and physicians were split in their preferences regarding who administers the medication; slightly >50% in each group indicated preferences for health care practitioner (HCP) administration in an office or clinic (preferred by 54% of patients, 52% of physicians) versus at home by patient self-administration or caregiver administration (preferred by 46% of patients, 48% of physicians). Of those who endorsed home administration, most physicians preferred patient administration to caregiver administration. Patients, in contrast, were split on who they preferred to have administered the medication at home, with 24% of patients preferring self-administration at home and 22% preferring a family member or other caregiver to administer the treatment at home.
Preference among 4 biologic treatment profiles
In the last exercise of the survey, both patients and physicians were provided with anonymized profiles (A, B, C, and D) reflecting 4 different biologic treatments that aligned with those currently available for the treatment of severe, uncontrolled asthma at the time of the study (Tables S1a and S1b). The order of presentation of the profiles was randomized. The participants were asked to select the profile that they felt was most preferable. The vast majority of both patients and physicians indicated a preference for (Profile A) the product that was injected subcutaneously, has a dosing frequency of every 8 weeks (after the first 3 doses are given every 4 weeks), requires 1 injection each treatment, and the preparation process requires 30 mins wherein the medication is removed from the refrigerator and allowed to reach room temperature prior to administration (Figure 4).
Key qualitative insights on dosing frequency
Qualitative results provided important additional insights into the rationale behind patient and physician preferences for the biologic medication features. Both patients and physicians were consistent in their preferences for less frequent administration. Patients who preferred longer periods between administration reported convenience as a factor behind their preference, citing that less frequent visits meant less trips to the doctor and more money saved. Physicians who preferred 8 weeks (or more than once a month between treatments) reasoned the longer period between administration would be more convenient for patients, resulting in reduced patient burden and improved compliance. Moreover, physicians were aware that patients generally prefer less frequent injections. One physician also indicated that another advantage of fewer visits meant less administrative burden from the clinical site’s perspective.
Although they represented the minority (24–40%, depending on the question), some physicians noted that they had concerns about the longer intervals between treatments (8 weeks or more than once a month) because patients might forget appointments and because they liked the ability to more closely monitor patients on treatment. However, many other physicians indicated shorter periods between treatments meant more time spent by patients on scheduling and physician visits, causing greater interruption and burden to patient quality of life (eg, missed work or daily events), and potentially greater out-of-pocket costs due to more frequent physician visits. Of patients who preferred 4 weeks (or 1 month between treatments), many indicated that 2 advantages of the monthly treatment are that it fits better into their schedule and is easier for them to remember. Some patients also noted this timeframe is what they are used to and most comfortable with.
Key barriers to biologic medications
Patients and physicians mentioned attributes related to convenience, including setting of administration, time required for the medication to be administered, and scheduling treatment as potential barriers to starting or staying on biologic therapy.
Key barriers to biologic medications for biologic-experienced patients included where they receive treatment, administration time, scheduling treatments, and number of injections. Patients preferred administration at a specialist’s office (54%) as opposed to a primary care physician’s office reporting a perception that specialists are more knowledgeable and better equipped to handle situations when compared to primary care physicians. Patients also reported challenges with the inconvenience of traveling to receive treatment and long waiting times at the physician office. Among those who were experienced with biologic therapy, doctor visits varied from 30 mins to several hours. Although most biologic-experienced patients in the study confirmed insurance coverage of most, if not all, of the cost for their biologic therapies, a number did say that co-payments for each doctor’s visit can also be an issue.
001-006: I mean, it’s, it takes an hour to do it. I mean, that’s definitely a challenge, you know? I mean, it’s definitely, you have to schedule it. It’s not like taking a pill at night before you go to bed, you know?
006-013: It is a challenge because you’re giving up, for one thing, going to the physician and actually traveling to them, and then having to sit there for an hour.
006-013: Unfortunately when you are retired and on Medicare and you have only your social security … so cost is a very big part of it that I can’t start that.
What do you not like about biologic therapy? 001-004: That I have to get it every three weeks, that I have to kind of re-up with the insurance and paperwork, and dealing with like the specialty pharmacy.
For biologic-naïve patients, side-effects and mode of administration (i.e., receiving an injection) were key barriers. These patients also mentioned cost, time commitment, scheduling convenience, and speed of onset.
Ultimately, what led your decision not to take biologic therapies? 001-002: My insurance company wouldn’t pay for it and I couldn’t afford the out-of-pocket with the whole price.
002-019: Cost, how it would be, you know, administered, and I guess the frequency in where I would have to go do it. Cost I would say would probably be the biggest as to whether, you know, my insurance would cover any portion of it, and if I could afford to do it.
005-004: If they could find another form of taking it and putting it in a liquid form or a pill form, I would if it’s proven. A needle, if I have to take it every day—I have the great—my body cringes—I just have the greatest fear of the needle. I had a bad episode with a shot and a needle got stuck in my foot, and it just terrified me after that happened.
006-004: I just want to feel it—I want to feel it—relief in like 5 minutes, I just want to know it’s working fast. I like the medicine to work fast most of the time.
006-012: Because I read all the side effects, the possible side effects of it.
Barriers to biologic medications from the physician perspective that were related to initiating treatment included patient testing to assess candidacy for the treatment, convincing patients of the need for the biologic medication, administration setting (eg, clinician office vs. at-home administration), mode of administration, and cost—particularly the administrative burden of obtaining insurance approval.
100-008: Again, the commitment to it, the timeframe in the office. You know, that may be something where you argue, yeah, home would it be better … Typically the biggest barrier is, A, to recognize your illness to be severe enough to need biologics and, B, you know, logistically, again barring the one that’s at home, just the ones that need office visits and time spent.
100-014: If I prescribe an inhaler or a pill, the patient goes to the pharmacy and they pick it up and that’s the end of it. With these biologics, it’s drama after drama after drama and phone call after phone call, and denial and this, and they don’t send the drug. They send the wrong drug. They send it to the wrong location. It’s endless aggravation.
100-017: Yeah, it takes time and then the nurses, the three nurses are already competent in doing that. It’s just the time issue. Sometimes, especially when we are trying to get a prior authorization for a new start …
100-026: From the start, it’s a very complicated process to get it approved. There are obviously different insurances; each of them has their requirements. Some of them have their own forms. It is all very complicated … So it’s still burden on the staff.
Barriers to biologic medications from the physician perspective that related to staying on treatment included speed of onset and effectiveness, convenience of administration, out-of-pocket costs and insurance, and compliance. Similar to patients, physicians (84%) most commonly preferred that patients see specialists. Physicians reported a wide range of variability in the percentage of patients who are affected by these barriers. Some physicians only mentioned a small subset of patients, up to 5%, while others reported that 20% of their patients had some barriers to staying on treatment. In regard to product formulation, several physicians mentioned disadvantages of reconstitution, a step required by 2 of the product profiles, noting the requirement for patients to be onsite before the clinic can begin reconstitution, increased visit time, increased cost and resources, and the increased risk for errors and contamination due to the preparation step.
100-025: I would say the main barrier is a lack of efficacy and a timeframe that the patient would—I would say there’s two barriers. Efficacy is a barrier in two different ways. One is how long it takes to work and two it doesn’t work at all anyway …
100-001: They feel better, so they don’t want it. They don’t understand it’s the drug that’s making them feel better, so the misconception that they are all cured and don’t need it, that’s one—and that also goes to the doctor not having a good endpoint like a serologic marker.
100-003: To be willing to undergo the injections and to be patient enough to wait for them to work, and I think in there is the convenience factor. You know, if you’ve got to get injected three times a week, that’s going to be a much less compliant patient potentially than somebody that can take a shot every two months.
100-022: … the needle phobia, the fear of a new product, though that tends to wane. But just the commitment, the cost, the schedule, and then above it all if they find the doctor six, twelve months that it’s not helping significantly, well they’re going to be less inclined to continue with it.
Discussion
This study found that both patients and physicians have strong and consistent preferences for SC administration, less frequent dosing, and faster onset of efficacy with biologic therapy for severe asthma. Both patients and physicians indicated that less frequent administration meant less interference and impact on daily activities, fewer appointments, lower out-of-pocket costs, and reduced administrative burden to HCPs. These findings are consistent with the literature, as current research on biologic therapies in other disease areas also support patient preferences favoring the least frequent dosing interval.44,47
Regardless of whether or not patients had experience with biologic therapy, SC administration was preferred over IV administration. IV administration was considered more intrusive and less convenient in terms of both time and cost. Physicians also reasoned that SC was less resource-intensive, was less invasive, was not dependent on the condition and availability of veins, was more preferred by patients, had fewer adverse events or better tolerability, was easier to monitor, avoided the use of IV cannulas or infusion pumps, and was more convenient overall.
Cost and insurance reimbursement are significant barriers for both patients and physicians. These barriers were overcome by those who were on therapy but tended to consistently reemerge over time. Examples include issues concerning insurance changes, insurance re-authorizations, affordability, acceptable locations for administration, and availability of assistance programs. Nevertheless, for many physicians, the largest concern related to insurance reimbursement and access was not obtaining the drug for a patient, but rather obtaining the drug in a timely manner that would avoid a gap in treatment. While many physicians indicated that patient out-of-pocket cost does not factor into their decision to place a patient on biologic therapy, physicians did acknowledge that patient out-of-pocket cost can be a challenge to a patient’s decision to start or stay on a biologic therapy. Although most biologic-experienced patients in the study confirmed insurance coverage of most, if not all, of the cost for their biologic therapies, a number did say that co-payments for each doctor’s visit can be an issue. For several patients, rising costs were one of the biggest reasons for stopping a particular treatment. Changes in insurance coverage, lapses in insurance coverage, and approval requirements were all reported as contributing to the rising out-of-pocket costs for patients. Costs were also a significant barrier for the naïve patients, several of whom indicated that this was the primary reason that they did not initiate biological therapy.
Attributes related to convenience, including setting of administration, time required for the medication to be administered, and scheduling treatment were also discussed as potential barriers to starting or staying on biologic therapy. Both groups preferred administration at a specialist’s office (54% patients, 84% physicians) as opposed to a primary care physician, reporting a perception that specialists are more trained and knowledgeable with respect to these medicines, have more support personnel, and are better equipped to handle adverse reactions and emergencies. Some patients noted less trust in their primary care physician’s ability to treat them, lack of organization at their regular doctor’s office, as well as better service and comfort with their specialist as other reasons for their preference. Despite this preference, the most commonly reported challenge for staying on therapy was the inconvenience of traveling to receive treatment and long waiting times at the physician office. Among patients naïve to biologic therapy, scheduling convenience and time commitment were also among the most common factors that prevented them from initiating therapy.
A small number of patients in the study made incorrect assumptions about the attributes of some attributes. In particular, 3 patients believed treatment administered by IV worked faster compared to other forms of administration. Additionally, when patients were asked to select a preference between every 2, 4, or 8 weeks, 6 (including both biologic-naïve and biologic experienced) patients had a misconception that more time between treatments meant they were likely to be less effective. Finally, 2 patients from the naïve group also believed longer periods between treatments automatically meant larger doses/injection volumes. These misconceptions were clarified by the interviewers, but the fact that they were identified in this interview-based study raises an important consideration for all researchers who design and engage in preference elicitation studies. These incorrect patient assumptions also highlight the importance and need for patient education and discussions with physicians regarding the specific characteristics of available treatment options.
Limitations
The results of this study should be interpreted in consideration of the following limitations. First, this study used ranking and rating approaches to assess preferences, rather than a conjoint analysis/DCE. This was partly due to the mixed-methods nature of the study and the small target sample size. Therefore, the results provide less information on the relative importance of each attribute when considered as a set. The strength of the design included the ability to better understand the specific rationale for the preferences that were elicited based on the qualitative interviews. In addition, the study did not include some important attributes that could potentially influence patient and physician choice, including product-specific efficacy and safety based on specific patient characteristics; these attributes were excluded as they are already established as important and because they cannot be generalized across all patients since they will vary based on each patient’s individual clinical profile. Due to the study’s focus on preference ratings for individual attributes rather than a DCE, the exclusion of these attributes did not bias the study. Because this was a smaller qualitative study with specific recruitment targets, there was not sufficient power to look at differences in the ratings and rankings by patient characteristics. For example, for biologic treatment status, there were insufficient data among those who had previously been on biologic therapy and those who were naïve to biologic therapies to make any meaningful comparisons between these groups. In addition, there was some overlap between the current and previous groups, where some members of the current group had also been on a previous biologic. Recruitment methods may have resulted in some selection biases. For example, patients who agreed to participate may have had a greater likelihood of seeking treatment. Similarly, physicians recruited through the research panel may not represent the average physician. Efforts were made to recruit a diverse, representative, and unbiased sample.
Conclusions
This study provided important insights into the preferences of both patients and physicians for the features of biological medications indicated for the treatment of severe asthma. Patients and physicians expressed strong preferences for SC administration, less frequent dosing, and faster onset of efficacy. A slight majority preferred administration in the specialist office, and financial access and convenience were key barriers to use. Increased awareness and understanding of these preferences and barriers may be useful in facilitating conversations between physicians and their patients as they consider available biologic treatment options.
Ethics approval and informed consent
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Consent for publication
Informed consent was obtained from all individual participants included in the study.
Data sharing statement
Data are not publicly available, but may be provided upon request.
Abbreviations list
DCE, discrete choice experiment; HCP, health care practitioner; IV, intravenous; PFT, pulmonary function test; SC, subcutaneous; TENOR, Epidemiology and Natural History of Asthma: Outcomes and Treatment Regimens.
Acknowledgments
The abstract of this paper was presented at the Academy of Managed Care Pharmacy (AMCP) Conference as a poster presentation with interim findings. The poster’s abstract was published in “Meeting Abstracts” as a supplement to Journal of Managed Care & Specialty Pharmacy: https://www.jmcp.org/doi/pdf/10.18553/jmcp.2018.24.4-a.s1. The abstract of this paper was also presented at the American Thoracic Society (ATS) International Conference as a poster presentation with final study results. The poster’s abstract was published in “Conference Abstracts” in American Journal of Respiratory and Critical Care Medicine: https://www.atsjournals.org/doi/abs/10.1164/ajrccm-conference.2019.199.1_MeetingAbstracts.A5928. The authors would like to thank Melissa Ross, Katelyn Cutts, Regina Buachie, Kimmie McLaurin, and Kathleen M Fox for their assistance in executing the study. The authors would also like to thank Fritz Hamme and Emily Sargent for providing editing and production services in the development of the manuscript. This work was conducted by Evidera, an independent research organization and received research study support from AstraZeneca. The study was funded by AstraZeneca.
Author contributions
All authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
Heather L Gelhorn and Zaneta Balantac are employees of Evidera. Christopher S Ambrose and Yen N Chung are employees of and own stock in AstraZeneca. Brian Stone is a member of Allergy Partners, which has received research support from ALK, AstraZeneca, Merck, and Novartis; he also participates in advisory boards and/or consults for ALK, AstraZeneca, Genentech, and Sanofi/Regeneron. He also acted as a consultant to AstraZeneca for another unrelated project. The authors report no other conflicts of interest in this work.
References
1. Espanol T, Prevot J, Drabwell J, Sondhi S, Olding L. Improving current immunoglobulin therapy for patients with primary immunodeficiency: quality of life and views on treatment. Patient Prefer Adherence. 2014;8:621–629. doi:10.2147/PPA.S60771
2. Mohamed AF, Kilambi V, Luo MP, Iyer RG, Li-McLeod JM. Patient and parent preferences for immunoglobulin treatments: a conjoint analysis. J Med Econ. 2012;15(6):1183–1191. doi:10.3111/13696998.2012.716804
3. Chipps BE, Zeiger RS, Borish L, et al. Key findings and clinical implications from the epidemiology and natural history of asthma: outcomes and Treatment Regimens (TENOR) study. J Allergy Clin Immunol. 2012;130(2):332 e310–342 e310. doi:10.1016/j.jaci.2012.04.014
4. Canonica GW, Senna G, Mitchell PD, O’Byrne PM, Passalacqua G, Varricchi G. Therapeutic interventions in severe asthma. World Allergy Organ J. 2016;9(1):40. doi:10.1186/s40413-016-0118-z
5. Global Initiative for Asthma. Global strategy for asthma management and prevention. 2017; Available from: www.ginasthma.org.
6. Lotvall J, Akdis CA, Bacharier LB, et al. Asthma endotypes: a new approach to classification of disease entities within the asthma syndrome. J Allergy Clin Immunol. 2011;127(2):355–360. doi:10.1016/j.jaci.2010.11.037
7. Ayele Y, Engidawork E, Bayisa T. Assessment of inhaled corticosteroids use and associated factors among asthmatic patients attending Tikur anbessa specialized hospital, Ethiopia. BMC Res Notes. 2017;10(1):314. doi:10.1186/s13104-017-2645-2
8. Bousquet J, Winchester C, Papi A, et al. Inhaled corticosteroid/long-acting beta(2)-agonist combination therapy for asthma: attitudes of specialists in Europe. Int Arch Allergy Immunol. 2012;157(3):303–310. doi:10.1159/000329519
9. Augustovski F, Beratarrechea A, Irazola V, et al. Patient preferences for biologic agents in rheumatoid arthritis: a discrete-choice experiment. Value Health. 2013;16(2):385–393. doi:10.1016/j.jval.2012.11.007
10. Bolge SC, Eldridge HM, Lofland JH, Ravin C, Hart PJ, Ingham MP. Patient experience with intravenous biologic therapies for ankylosing spondylitis, Crohn’s disease, psoriatic arthritis, psoriasis, rheumatoid arthritis, and ulcerative colitis. Patient Prefer Adherence. 2017;11:661–669. doi:10.2147/PPA.S121032
11. Bolge SC, Goren A, Brown D, Ginsberg S, Allen I. Openness to and preference for attributes of biologic therapy prior to initiation among patients with rheumatoid arthritis: patient and rheumatologist perspectives and implications for decision making. Patient Prefer Adherence. 2016;10:1079–1090. doi:10.2147/PPA.S107790
12. Bolge SC, Goren A, Tandon N. Reasons for discontinuation of subcutaneous biologic therapy in the treatment of rheumatoid arthritis: a patient perspective. Patient Prefer Adherence. 2015;9:121–131. doi:10.2147/PPA.S70834
13. Cantini F, Niccoli L, Nannini C, et al. Tailored first-line biologic therapy in patients with rheumatoid arthritis, spondyloarthritis, and psoriatic arthritis. Semin Arthritis Rheum. 2016;45(5):519–532. doi:10.1016/j.semarthrit.2015.10.001
14. Desplats M, Pascart T, Jelin G, et al. Are abatacept and tocilizumab intravenous users willing to switch for the subcutaneous route of administration? A questionnaire-based study. Clin Rheumatol. 2017;36(6):1395–1400. doi:10.1007/s10067-017-3587-8
15. Glauser TA, Ruderman EM, Kummerle D, Kelly S. Current practice patterns and educational needs of rheumatologists who manage patients with rheumatoid arthritis. Rheumatol Ther. 2014;1(1):31–44. doi:10.1007/s40744-014-0004-5
16. Harrison M, Marra C, Shojania K, Bansback N. Societal preferences for rheumatoid arthritis treatments: evidence from a discrete choice experiment. Rheumatology (Oxford). 2015;54(10):1816–1825. doi:10.1093/rheumatology/kev113
17. Husni ME, Betts KA, Griffith J, Song Y, Ganguli A. Benefit-risk trade-offs for treatment decisions in moderate-to-severe rheumatoid arthritis: focus on the patient perspective. Rheumatol Int. 2017;37(9):1423–1434. doi:10.1007/s00296-017-3760-z
18. Huynh TK, Ostergaard A, Egsmose C, Madsen OR. Preferences of patients and health professionals for route and frequency of administration of biologic agents in the treatment of rheumatoid arthritis. Patient Prefer Adherence. 2014;8:93–99. doi:10.2147/PPA.S55156
19. Navarro-Millan I, Herrinton LJ, Chen L, Harrold L, Liu L, Curtis JR. Comparative effectiveness of etanercept and adalimumab in patient reported outcomes and injection-related tolerability. PLoS One. 2016;11(3):e0149781. doi:10.1371/journal.pone.0149781
20. Poulos C, Hauber AB, Gonzalez JM, Turpcu A. Patients’ willingness to trade off between the duration and frequency of rheumatoid arthritis treatments. Arthritis Care Res (Hoboken). 2014;66(7):1008–1015. doi:10.1002/acr.22265
21. Striesow F, Brandt A. Preference, satisfaction and usability of subcutaneously administered methotrexate for rheumatoid arthritis or psoriatic arthritis: results of a postmarketing surveillance study with a high-concentration formulation. Ther Adv Musculoskelet Dis. 2012;4(1):3–9. doi:10.1177/1759720X11431004
22. Tłustochowicz M, Tłustochowicz W. Patients’ preferences regarding biological treatment in docros’ and patients’ opinions – the results of the RAISE* questionnaire survey. Reumatologia. 2013;51(2):113–118. doi:10.5114/reum.2013.34819
23. van de Kerkhof PC, Reich K, Kavanaugh A, et al. Physician perspectives in the management of psoriasis and psoriatic arthritis: results from the population-based multinational assessment of psoriasis and psoriatic arthritis survey. J Eur Acad Dermatol Venereol. 2015;29(10):2002–2010. doi:10.1111/jdv.13150
24. Zhang J, Xie F, Delzell E, et al. Trends in the use of biologic agents among rheumatoid arthritis patients enrolled in the US medicare program. Arthritis Care Res (Hoboken). 2013;65(11):1743–1751. doi:10.1002/acr.22055
25. Hoffman MB, Hill D, Feldman SR. Current challenges and emerging drug delivery strategies for the treatment of psoriasis. Expert Opin Drug Deliv. 2016;13(10):1461–1473. doi:10.1080/17425247.2016.1188801
26. Kromer C, Peitsch WK, Herr R, Schmieder A, Sonntag D, Schaarschmidt ML. Treatment preferences for biologicals in psoriasis: experienced patients appreciate sustainability. J Dtsch Dermatol Ges. 2017;15(2):189–200.
27. Kromer C, Schaarschmidt ML, Schmieder A, Herr R, Goerdt S, Peitsch WK. Patient preferences for treatment of psoriasis with biologicals: a discrete choice experiment. PLoS One. 2015;10(6):e0129120. doi:10.1371/journal.pone.0129120
28. Nast A, Mrowietz U, Kragballe K, et al. Barriers to the prescription of systemic therapies for moderate-to-severe psoriasis – a multinational cross-sectional study. Arch Dermatol Res. 2013;305(10):899–907. doi:10.1007/s00403-013-1372-3
29. Puig L, de la Cueva P, Linares M, et al. Expert report on psoriasis: spanish dermatologists’ opinions on the use of biologic agents to manage moderate to severe psoriasis in adults. Actas Dermosifiliogr. 2013;104(5):400–408. doi:10.1016/j.adengl.2013.04.003
30. Schaarschmidt ML, Kromer C, Herr R, et al. Patient preferences for biologicals in psoriasis: top priority of safety for cardiovascular patients. PLoS One. 2015;10(12):e0144335. doi:10.1185/030079905X74862
31. Strohal R, Prinz JC, Girolomoni G, Nast A. A patient-centred approach to biological treatment decision making for psoriasis: an expert consensus. J Eur Acad Dermatol Venereol. 2015;29(12):2390–2398. doi:10.1111/jdv.13248
32. Zhang M, Brenneman SK, Carter CT, et al. Patient-reported treatment satisfaction and choice of dosing frequency with biologic treatment for moderate to severe plaque psoriasis. Patient Prefer Adherence. 2015;9:777–784. doi:10.2147/PPA.S85773
33. Gonzalez JM, Johnson FR, McAteer H, Posner J, Mughal F. Comparing preferences for outcomes of psoriasis treatments among patients and dermatologists in the U.K.: results from a discrete-choice experiment. Br J Dermatol. 2017;176(3):777–785. doi:10.1111/bjd.14798
34. Danese S, Vuitton L, Peyrin-Biroulet L. Biologic agents for IBD: practical insights. Nat Rev Gastroenterol Hepatol. 2015;12(9):537–545. doi:10.1038/nrgastro.2015.135
35. Kim ES, Kim KO, Jang BI, et al. Factors contributing to the preference of Korean patients with Crohn’s disease when selecting an anti-tumor necrosis factor agent (CHOICE Study). Gut Liver. 2016;10(3):391–398. doi:10.5009/gnl15126
36. Sullivan E, Piercy J, Waller J, Black CM, Kachroo S. Assessing gastroenterologist and patient acceptance of biosimilars in ulcerative colitis and Crohn’s disease across Germany. PLoS One. 2017;12(4):e0175826. doi:10.1371/journal.pone.0175826
37. Cebas AL, Cascajares SC, Bravo SP, et al. Subcutaneous versus intravenous administration of trastuzumab: preference of HER2+ breast cancer patients and financial impact of its use. JBUON. 2016;22(2):334–339.
38. Fallowfield L, Osborne S, Langridge C, Monson K, Kilkerr J, Jenkins V. Implications of subcutaneous or intravenous delivery of trastuzumab; further insight from patient interviews in the PrefHer study. Breast. 2015;24(2):166–170. doi:10.1016/j.breast.2015.01.002
39. Hechmati G, Hauber AB, Arellano J, et al. Patients’ preferences for bone metastases treatments in France, Germany and the United Kingdom. Support Care Cancer. 2015;23(1):21–28. doi:10.1007/s00520-014-2309-x
40. Jackisch C, Muller V, Dall P, et al. Subcutaneous trastuzumab for HER2-positive breast cancer – evidence and practical experience in 7 German centers. Geburtshilfe Frauenheilkd. 2015;75(6):566–573. doi:10.1055/s-0035-1546172
41. MacDonald D, Crosbie T, Christofides A, Assaily W, Wiernikowski J. A Canadian perspective on the subcutaneous administration of rituximab in non-Hodgkin lymphoma. Curr Oncol. 2017;24(1):33–39. doi:10.3747/co.24.3470
42. Pivot X, Gligorov J, Muller V, et al. Preference for subcutaneous or intravenous administration of trastuzumab in patients with HER2-positive early breast cancer (PrefHer): an open-label randomised study. Lancet Oncol. 2013;14(10):962–970. doi:10.1016/S1470-2045(13)70383-8
43. Pivot X, Gligorov J, Muller V, et al. Patients’ preferences for subcutaneous trastuzumab versus conventional intravenous infusion for the adjuvant treatment of HER2-positive early breast cancer: final analysis of 488 patients in the international, randomized, two-cohort PrefHer study. Ann Oncol. 2014;25(10):1979–1987. doi:10.1093/annonc/mdu364
44. Emkey R, Koltun W, Beusterien K, et al. Patient preference for once-monthly ibandronate versus once-weekly alendronate in a randomized, open-label, cross-over trial: the Boniva Alendronate Trial in Osteoporosis (BALTO). Curr Med Res Opin. 2005;21(12):1895–1903. doi:10.1185/030079905X74862
45. Sylwestrzak G, Liu J, Stephenson JJ, Ruggieri AP, DeVries A. Considering patient preferences when selecting anti-tumor necrosis factor therapeutic options. Am Health Drug Benefits. 2014;7(2):71–81.
46. Poulos C, Kinter E, Yang JC, et al. A discrete-choice experiment to determine patient preferences for injectable multiple sclerosis treatments in Germany. Ther Adv Neurol Disord. 2016;9(2):95–104.
47. Zhang M, Carter C, Olson WH, et al. Patient preference for dosing frequency based on prior biologic experience. J Drugs Dermatol. 2017;16(3):220–226.
48. Gelhorn H, Ross M, Balantac Z, et al. Analysis of social media data using qualitative methods: understanding preferences and perceptions of biologic medications among patients with severe asthma.
49. Friese S, Ringmayr TG. User’s Manual for ATLAS.ti 7. Berlin: ATLAS.ti Scientific Software Development GmbH; 2014.
50. Hsieh HF, Shannon SE. Three approaches to qualitative content analysis. Qual Health Res. 2005;15(9):1277–1288. doi:10.1177/1049732305276687
51. Boeije H. A purposeful approach to the constant comparative method in the analysis of qualitative interviews. Qual Quant. 2002;36(4):391–409. doi:10.1023/A:1020909529486
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


