Back to Journals » Clinical Interventions in Aging » Volume 18
Pathophysiological Association of Alzheimer’s Disease and Hypertension: A Clinical Concern for Elderly Population
Authors Yao Q, Jiang K, Lin F, Zhu T, Khan NH, Jiang E
Received 29 December 2022
Accepted for publication 22 April 2023
Published 5 May 2023 Volume 2023:18 Pages 713—728
DOI https://doi.org/10.2147/CIA.S400527
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Prof. Dr. Nandu Goswami
Qianqian Yao,1,* Kexin Jiang,1,* Fei Lin,2 Tao Zhu,3 Nazeer Hussain Khan,1,4 Enshe Jiang1,4
1Institute of Nursing and Health, Henan University, Kaifeng, People’s Republic of China; 2School of Medicine, Shangqiu Institute of Technology, Shangqiu, People’s Republic of China; 3Department of Geriatrics, Kaifeng Traditional Chinese Medicine Hospital, Kaifeng, People’s Republic of China; 4Henan International Joint Laboratory for Nuclear Protein Regulation, Henan University, Kaifeng, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Enshe Jiang, Email [email protected]
Abstract: Alzheimer’s disease (AD), the most common cause of dementia and the fifth leading cause of death in the adult population has a complex pathophysiological link with hypertension (HTN). A growing volume of published literature on a parallel elevation of blood pressure (BP), amyloid plaques, and neurofibrillary tangles formation in post-middle of human brain cells has developed new, widely accepting foundations on this association. In particular, HTN in elderly life mediates cerebral blood flow dysfunction, neuronal dysfunction, and significant decline in cognitive impairment, primarily in the late-life populace, governing the onset of AD. Thus, HTN is an established risk factor for AD. Considering the impact of AD, 1.89 million deaths annually, and the failure of palliative therapies to cure AD, the scientific research community is looking to adopt integrated approaches to target early modified risk factors like HTN to reduce AD burden. The current review highlights the significance and impact of HTN-based prevention in lowering the AD burden in the elderly by providing a comprehensive overview of the physiological relationship between AD and HTN with an in-detail explanation of the role and applications of pathological biomarkers in this clinical association. The review will gain worth in presenting new insights and providing inclusive discussion on the correlation between HTN and cognitive impairment. It will increase across a wider scientific audience to expand understanding of this pathophysiological association.
Keywords: Alzheimer’s disease, hypertension, elderly population, clinical biomarkers
Introduction
Alzheimer’s disease (AD) is one of the leading age-related brain diseases in older adults and imposes a high impact. There are more than 1.89 million deaths annually, with estimated healthcare costs of $305 billion.1,2 The high prevalence of this chronic age-related disease is raising clinical concerns in the elderly population. For example, 6.5 million Americans over 65 lived with AD and other dementia-related illnesses, which caused 121,499 deaths in 2019.3 Unfortunately, this disease has no definitive cure, and the available drugs only relieve the patients from symptoms.4 This lack of specific treatment and the failure of available palliative therapies for AD have increased the mortality rate among older people, especially in low-income countries.5 In addition, clinically, there is no obvious mechanism for mid-life AD development.
Despite the many physio-clinical strides made in the past two decades, there is still enough to investigate responsible factors and their mechanism of action in AD development.6 For most of history, researchers have classified the driving forces in the onset of AD pathology into two main groups: non-modifiable risk factors (aging, sex and genetics) and modifiable risk factors.7,8 In addition, the educational-learning level, smoking, high body weight, diabetes, and hypertension (HTN) are interlinked with the pathology of the disease.9
It has been observed that intervention of modifiable risk factors could prevent the up to 35% of AD-related dementia cases.10 In addition, positive lifestyle changes like diet and exercise can prevent cognitive functioning deterioration.11 With some limitations, many studies have concluded that strategic preclinical intervention may prevent the onset and development of AD if the risk factor is the cause and slow down the progression of the disease if the risk factor is a symptom.12,13
In particular, HTN is the most decisive factor in all modifiable risk factors mediating AD development.14,15 Targeting HTN has a considerable impact on lowering AD in the elderly population. Figure 1 explains the modifiable risk factors-treatable medical conditions and lifestyle choices that play a role in AD onset.
 |
Figure 1 Figurative description of a modified risk factor for AD developments. |
Today’s population aged 65 and over is expected to grow rapidly, and older people live worldwide. Unfortunately, this rapid population growth has birthed several socioeconomic and psychological issues which impose adverse severe health outcomes on the elderly population.16–18 With these HTN depression-related risk factors, late–age exacerbates AD and dementia risk in this population segment effectively.19,20 Furthermore, these conditions develop complex, positively correlated clinical associations between HTN and AD development.21,22 The relationship between HTN and AD development in older people is an understood research topic. Therefore, this review concentrates on and summarizes the studies on AD development and the mediating role of HTN in this disease with associated risk factors. We aimed to highlight the significance and impact of HTN-based prevention in lowering the AD burden in the elderly. Furthermore, this literature review might encourage and assist researchers and clinicians in collaborating in designing various experimental approaches to explore the clinical links between AD development and HTN.
Pathological and Molecular Considerations for the Brain in the Alzheimer’s Disease State
As previously said, AD is a clinically diagnosed disorder followed by amyloid plaques and neurofibrillary tangles in neurons, ultimately leading to the loss of neurons in patients with AD, presenting various clinical symptoms that change over time.23,24 Signs and progression from mild to moderate and moderate to severe vary depending on the damage to neurons across multiple brain areas.25 A healthy adult brain contains 100 trillion synapses.26 They let impulses traverse swiftly across the brain’s neuronal circuits, establishing the cellular basis of memories, thoughts, feelings, emotions, movements, and talents.27 Clinically, AD is characterized as the accumulation of the proteins fragment, beta-amyloid (referred to as beta-amyloid plaques) outside neurons and forming an aberrant version of the protein tau (referred to as tau tangles) inside neurons.28 These brain alterations halt the communication process of brain machinery.29 Beta-amyloid plaques cause cell death by interfering with neuron-to-neuron transmission at synapses, whereas tau tangles prevent nutrition and other critical chemicals from entering neurons. When beta-amyloid levels reach a threshold level, aberrant tau spreads throughout the brain.30 These alternations ultimately induce cognitive impairment. Therefore, they are considered the gold standard for pathological AD diagnosis.
The amyloid hypothesis best describes the molecular profile of AD development. It explains that the cleaving of an enzyme called beta-secretase (BACE-1) or amyloid initiates the production of the toxic amyloid β (Aβ) of AD-related pathologies.31,32 In the beginning, the C-terminus of BACE-1 contributes to the breakage of amyloid precursor protein(APP) that lead to the amyloid genic-APP processes to form soluble amyloid precursor protein (sAPP).33,34 This soluble amyloid penetrates the neuronal membrane and eventually binds with sAPP death receptor-6 (DC-6), further activating caspase (caspase-6) in the cell.35 Caspases that have been activated then launch apoptotic pathways and cause neuronal death.36,37
In this amylogenic pathway, after the breakage of remaining membrane-bound APP, four monomer fragments ranging in length from 40 to 42 amino acids (A40/A42) formed, in which A40 dominates the formation of produced monomers.38 The aggregation of monomers outside the neuron membrane forms thick, insoluble oligomers or senile plaques.39 Misfolded peptides are created in a variety of conformations. They are released into the extracellular environment by donor neurons as naked proteins or vesicles called exosomes, which are then picked up by receptor-mediated endocytosis by receiver neurons.40 A40/A42 binds to several receptors on the neuron’s membrane. It influences the synaptic transmission via inhibiting ion channels and leads to a disorder in the tau protein function (a predominant protein of brain cells working as a stabilizer of the internal skeleton of nerve cells). Tau pathology may be produced by independent regulators such as apolipoprotein-E (ApoE), cholesterol metabolism, receptor-mediated endocytosis, and microglial activation.41–43 The creation of the toxic Aβ plaques in neurons drives the release of chemokines and cytokines involved in generating reactive oxygen species (ROS).44,45 This causes mitochondrial oxidative stress and triggers a cascade of apoptotic caspases via the synthesis of p53, Bad, and Bax, resulting in lipid peroxidation, membrane damage, and neuronal death.46,47
Moreover, amyloid formation stimulates protein kinase C (PKC), protein kinase A (PKA), and Extracellular Signal-Regulated Kinases 2 (ERK2), leading to tau hyperphosphorylation and neurofibrillary tangle development.48,49 The activation of protein kinase B (PKB) or Akt to activate glycogen synthase kinase-3 (GSK3) causes tau hyperphosphorylation.50,51 The activation of cyclin-dependent kinases 5 (CDK5) and P25 by P35-calpain increases tau hyperphosphorylation and neuronal death (Figure 2).51
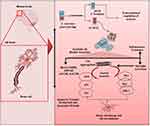 |
Figure 2 Molecular levels factors involved in neural death—from β-secretase processing to synaptic dysfunction and neuronal cell death in AD development. |
Apart from the amyloid hypothesis, other molecular pathways contribute to AD development. Memory and learning are governed by cholinergic neurotransmitters like acetylcholine. It is an essential neurotransmitter for proper synaptic transmission. An enzyme known as acetylcholinesterase (AchE) located in neuromuscular junctions, degrades acetylcholine into choline and acetate, leading to the end of synaptic transmission.52,53 Cholinergic system deterioration has been observed in AD when AchE activity significantly reduces acetylcholine levels.54 Anatomically, the limbic lobe regions in the AD brain frequently show mild atrophy.55 In addition, most AD patients’ frontal and temporal cortices display ventricular enlargement and gyri atrophy, although the main motor and somatosensory cortices are intact.56 Altogether, these molecular and anatomical events are highly relevant in AD-related pathologies. However, we still lack the exact mechanism and driving forces behind the initiation of these molecular cascades.
Aging-Dementia and AD
Human aging is known to decrease brain weight. It is connected with gyri atrophy, a reduction in the number of neurons and the amount of white matter. In addition, AD-related drivers like the development of amyloid deposits, granulovacuolar degeneration (GVD), and Hirano bodies (Hb) in the hippocampus change with age.57,58 Furthermore, many amyloid plaques and neurofibrillary tangles, the two most well-known pathological markers of AD, are detected in the aging human brain, even those without dementia.59
Clinical observations have shown that in the aging brain, argyrophilic grain disease (AGD) and dementia diseases reflect elderly- AD-like signs.60 Although depending on many factors, including the patient history of visiting neurology or psychiatry clinics, the clinical patterns of AGD vary, the clinical outcomes at the neuropsychiatric clinic tend to appear in a front temporal dementia pattern, similar to AD.61,62 Similarly, memory studies in clinics on elderly GVD brain and primary age-related tauopathy (PART) revealed a clinical characteristic similar to moderate cognitive impairment or AD.63,64
These studies on the association of this aging-related dementia (AGD and PART) and AD show enough resemblance to tau protein accumulation and rise with age. However, the pathological results and clinical symptoms are not always correlated with age, dementia, and AD onset. Therefore, new approaches are required to investigate this AD association regarding later-age dementia-related neuropathological issues and the disease itself.
Hypertension and Its Prevalence
Clinically, HTN is a condition of high systolic blood pressure (BP) ≥140 mmHg that has affected 1 billion individuals worldwide.65 HTN prevalence increases with age, and if untreated, it leads to serious health risks, including heart disease and stroke.17,66 Symptoms of HTN include early morning headache, nose bleeds, irregular rhythms, vision changes and buzzing in the ears.67 However, lifestyle interventions like a healthier diet with less salt, routine exercise, and taking medication on advice effectively reduce the risk of HTN.68
According to current global statistics, approximately 1.13 billion adults had HTN in 2015, predicted to increase to 1.56 billion in 2025.69,70 The prevalence of HTN is high in low and middle-income countries.71 Comparative data shows that there were 333 Million adults with HTN in high-income countries in 2000, while 654 Million were in low-and middle-income countries (LMIC).71,72 The senior population suffers a disproportionate share of the burden of HTN due to its increasing prevalence and associated morbidity and mortality, which raise a serious concern.73,74
In today’s society, systemic HTN is a growing public health risk. It is a well-known cause of several potentially deadly outcomes, such as cerebrovascular accidents, coronary artery disease, heart failure, peripheral atrial problems, renal failure, and AD development in the elderly.75–77 Furthermore, HTN is recognized as a major modifiable risk factor for cardiovascular disease (CVD), accounting for about 45% of global CVD morbidity and mortality in 2010, with 9.4 million deaths documented globally.78,79
In addition, HTN is associated with a substantial financial burden. This burden comprises direct healthcare costs related to HTN management, such as medications, laboratory tests, clinical visits, and other expenses. The global financial burden of HTN was projected to be roughly $ 370 billion, accounting for around 10% of global healthcare expenditure.80,81 According to the US national database, the average yearly adjusted extra cost for patients with HTN was $1920 more than those without HTN.82–84 The American Heart Association estimates that the direct cost of HTN in the United States will exceed $200 billion by 2030.85 The rising trends of HTN worldwide and its impact on human healthcare expenditure are challenges for policymakers. They emphasize implementing evidence-based clinical recommendations and public health strategies to lessen HTN’s worldwide impact.
Chronic Hypertension and Cerebrovasculature Disease
Over the life span, BP and age are connected in somewhat distinct ways: systolic BP tends to grow with age, but diastolic BP peaks around age 50 and then drops. As increasing age causes an elevation in BP through psychological/behavioral interventions of the brain,86,87 it also determines the HTN.88 Extending the given viewpoint of growing age and HTN development, it is highly significant to investigate how HTN relates to cerebrovasculature illness and find common risk factors mediating cognition decline in the elderly population.
Chronic HTN positively correlates with a cerebrovasculature state, stroke, cognitive dysfunction and dementia. Given the premise that natural aging raises BP, it has been observed that HTN and aging have comparable effects on the vasculature, including cerebrovasculature and vascular structural alterations.89,90 Clinical findings reveal that HTN thickens the vascular wall and lowers the number of vessels in the brain. Furthermore, HTN progress leads to the narrowing of pial and intracerebral capillaries in the latter stages of HTN.91 Chronic exposure to these conditions eventually overwhelms brain defenses and interferes with brain function, leading to primary dementia and cerebrovasculature disease, predominantly stroke.92,93 With the coexistence of our perspective and described findings, Figure 3 clearly shows that brain functional and structural changes with age are also connected with high BP. These findings suggest that the progression of HTN causes specific apparent aging effects over time and that the condition affects the brain far before consequences such as stroke.
Chronic Hypertension and Alzheimer’s Disease
As mentioned above, HTN is the most decisive modifiable risk factor for cerebrovascular disease, leading to stroke and dementia. Available knowledge on the association between HTN and high BP strongly linked HTN to stroke, vascular dementia and increased risk of AD.94,95 Pathogenic pathways, including atherosclerosis and arteriolosclerosis with stroke and cerebral ischemia, occur in HTN patients, leading to a significant decline in cognitive function.96 Based on the clinical outcomes of high BP and cognitive decline, large-scale clinical studies demonstrated a complex link between cognitive function and progressive levels of HTN.97,98 The severity of HTN to the brain depends on both stage of the disease and the age of the patient; higher risk of dementia at an elderly age as compared to young ones (65–75), and it is not effective at the age of 75–85 or > 85.99,100 Furthermore, clinical data of AD patients also support this association as a directional cue. Patients in the early stage of AD show high BP values, while late-life AD patients have more severe HTN complications with substantial cognitive decline.101 These findings further support that HTN-based cognitive decline is lower and slow in late life than young-old age.102,103 Furthermore, evidence from female reproductive time and HTN studies show females are more prone to HTN-based cognitive decline and AD onset than men.104–106
Although some well-established correlations between vascular dementia and HTN are highly relevant to AD development, they have not been thoroughly studied to answer the ambiguous factors, such as severity, type (systolic or diastolic), duration, and age, and these need additional large-scale clinical data validation.
Pathogenesis of Hypertension and Alzheimer’s Disease: Experimental Findings
Given the fast accumulating evidence supporting the vascular hypothesis regarding AD onset, it has been well characterized that the early stage of AD is predominantly a microvascular condition, narrowing of brain arteries.107,108 Furthermore, according to this theory, cerebrovascular dysfunction may be the first and most aberrant indicator of AD development.109,110 In line with these findings, several population-based cross-sectional and longitudinal studies have been published depicting the relationships between vascular risk factors, incidence, and AD progression in older people.111–113 In particular, among all these vascular risk factors, HTN is the most important leading Factor in AD development. Furthermore, it is recognized as a critical factor for doubling the risk rate of AD in older adults.114,115
Through advancements in experimental procedures evaluating HTN as a risk factor, several modifications and expansions to the original vascular hypothesis of AD have been added. First, it accelerated the HTN-induced microvascular injury in different pathological manifestations of AD ranging from cerebral microhemorrhages to blood-brain barrier disruption and subsequent neuroinflammation.116,117 It is noted that neuroinflammation plays an essential role in the development of both HTN and AD. For example, chronic neuroinflammation in the paraventricular nucleus of the hypothalamus induced by long-term high salt intake could lead to HTN in the Dahl salt-sensitive rat model.118,119 On another side, HTN could also induce microvascular inflammation in the brain. So, cerebral microvascular inflammation likely accelerated cognitive impairment in the elderly with AD under HTN.120
According to the amyloid cascade hypothesis concerning AD, higher levels of Aβ cause progressive, multidimensional cerebromicrovascular damage, which plays a role in forming early-stage pre-plaque cognitive dysfunctions and the disease’s later progression.121,122 In particular, Aβ production, processing, and deposition in neurons and cerebral microvessels play a critical role in AD development. A large amount of genetic and biochemical evidence supports this idea.123,124
Recapitulating the human clinical data on AD pathy from both the vascular theory and the amyloid hypothesis of AD, it articulately suggests that HTN exacerbates Aβ-induced cerebromicrovascular damage in AD, worsening the disease and accelerates its progression.125,126 Recently, critical insights into the pathophysiological mechanism have been provided to explain the links between Aβ deposition, HTN, and AD development.15,127 According to experimental research in transgenic animal models with angiotensin II infusion, long-term HTN consistently enhances microvascular amyloid deposition in Tg2576 mice and accelerates beta-secretase APP cleavage.128,129
Transverse aortic-coarctation mediates the Aβ deposition in the brain. In the mouse model, it has been evaluated that HTN is associated with transverse aortic coarctation in the brain and enhances the Aβ deposition, further promoting cognitive decline. It has also been observed that Aβ deposition was manifested within four weeks after induction of HTN to the brain, suggesting that triggering of the molecular process contributes to the pathogenesis of AD.130,131 Thus, HTN is enough to trigger cerebromicrovascular impairment.132,133 In another novel study, the amyloid genic gene is overexpressed in the brain of aging and HTN-induced mouse.134 Furthermore, activating the receptor for advanced glycation end-products (RAGE) in cerebral microvessels is also thought to be a route to the processes of HTN-induced AD development. This idea is similarly important in elucidating how HTN exacerbates AD pathology,68,69 concluding that blocking one or more of these biological targets might delay the emergence of microvascular-related AD impairments.
Tau pathology, in addition to Aβ pathology, is regarded as a substantial risk factor for AD.135 Despite significant research still lacking, new studies have revealed vital insights into the molecular link between HTN and tau hyperphosphorylation and miss-folding in AD136,137 (Figure 4). The Aβ levels in cerebrospinal fluid (CSF) in a cohort of AD Neuroimaging Initiative patients were investigated. Researchers discovered that lobar microbleeds caused by HTN were associated with increased longitudinal cognitive decline and a higher likelihood of having defective CSF levels of phosphorylated tau proteins.138,139
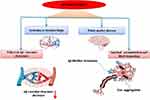 |
Figure 4 A detailed description of the pathophysiological association between Alzheimer’s Disease and hypertension. |
It has been observed that Aβ deposition produced intraneuronal tau hyperphosphorylation in hypertensive, non-transgenic, and spontaneously hypertensive stroke-prone mice and rat models. In addition, findings revealed that HTN induces cerebral small vessel disease (CSVD), meaning that CSVD is associated with HTN and causes a rise in brain Aβ.140 Kurata et al observed that telmisartan treatment decreased the number of Aβ and phospho-tau-positive neurons and neuro-inflammation markers.141 Clinical observations in cerebrovascular AD and progressive supranuclear palsy (PSP) patients highlight that aberrantly misfolded tau may accumulate in neurons in AD and tau pathology.142
In line with our current review, the cited studies above have provided fundamental key aspects on the association of AD pathy and HTN severity. These notable findings can be considered fingerprints in designing the new integrated approach to recapitulate the clinical and ex vivo data. Given these findings, it could be rightly postulated that HTN in the elderly population works as the progenitor for the onset of AD pathy.
Clinical Pathological Biomarkers of AD in HTN
Despite the evidence on the fairly established association of HTN and AD, there are limitations. Therefore, biologists are working to understand the processes behind this relationship to determine if this link directly causes AD-related neuropathy or contributes to cognitive impairment.143
To explore this association, approaches based on clinical tests (positron emission tomography, PET; Magnetic Resonance Imaging, MRI) and physiological examinations based on BP, systolic BP, and CSF are adopted to understand better the active biological markers involved in HTN-AD associations. Clinical observations from elderly participants with known vascular risk factors like HTN showed higher levels of amyloid bodies in their brains. Authors proposed that HTN in late life might be a possible direct factor for elevated brain amyloid. However, it needs further exploration.144 In another study, clinical evidence from PET (focusing on both tau and amyloid) and MRI testing of people over 60 years old revealed that increased vascular risk, particularly HTN, is linked to brain cell shrinkage and neurodegeneration rather than brain amyloid formation.145 Despite the importance of BP monitoring, BP was not associated with brain amyloid in a late-life neuropathological sample; however, systolic BP has been associated with neurofibrillary tangles.146
Similarly, CSF biomarkers suggested HTN was not associated with amyloid; however, it has an association with tau in APOE-4 homozygotes and showed a putative relationship between BP and APOE genotype.147 Furthermore, the associations between HTN and regional brain shrinkage suggest that high BP may play a role in AD’s neurodegeneration diagnosis. In addition, MRI has revealed a link between midlife BP and hippocampal atrophy, with the strongest associations reported in untreated HTN patients.148
Antihypertensive Drugs and AD
To overcome the effects of HTN, several anti-hypertension drugs (AHDs) prevent, control and treat HTN.149 The clinical findings of the study on the relationship between HTN and late-life dementia have provided a rationale for using AHDs to control HTN and reduce the risk of dementia.150,151 In addition, a large-scale clinical study on the use of AHDs demonstrates that lowering the systolic BP to less than 120 mmHg compared to 140 mmHg exhibits excellent results by reducing the risk of the secondary outcome of mild cognitive impairment and dementia.152 Similarly, in another study, it has been concluded that antihypertensive medications (AHM) have significant results in neuroprotective treatments.153 Although studies differ in source populations and the prevalence of confounding factors, the observational data endorsed the potential role of different AHM as the most potent candidate drugs to reduce AD-related pathologies and their prevention.154,155
Clinical trials are ongoing using AHM to cure dementia and AD-related pathologies. In addition, investigations are underway regarding the AHM drug’s efficacy in reducing AD risks. Table 1. Demonstrates studies on the relationship between AHM drug use and AD therapy. Calcium channel blockers (CCBs), angiotensin-converting enzyme inhibitors (ACEIs), angiotensin II receptor blockers (ARBs), and beta-blockers are the four major groups of AHM drugs.
 |
Table 1 The Studies on the Relationship Between AHM Drug Use and AD Therapy |
Potential Limitations to Using Antihypertensive Drugs for AD
Despite the evidence supporting the effectiveness of AHM in controlling AD, numerous physiological and pharmacological restrictions limit the case for further clinical benefits of this treatment. Age dependence is the biggest obstacle to AHD-based AD therapeutic success. According to neural clinicians, only 40 years and older AD patients showed better cognitive performance.156,157 The findings of Dalen et al also restricted the efficacy of this treatment. They explained that stopping AHDs in old AD patients between 70 and 80 did not protect cognition and may increase the risk of dementia.158 The effectiveness of this therapy is seriously hampered by these restrictions, which need to be thoroughly examined.
Prevention and Awareness of HTN: Public Health Nursing
Low public awareness about HTN also causes mayhem. Despite the significant prevalence of HTN, most people are unaware of its symptoms or presence, which raises the risk of related problems, especially in the elderly population.159–161 In contrast to its prevalence, awareness of HTN at the time of diagnosis is higher in developed countries (73% among adult Americans) than in developing countries (30% among adult Nigerians).162,163 Patients’ commitment to dietary changes and medication depends on their understanding of the diagnosis and the risk factors leading to the onset of HTN.164 Studies have shown that increasing HTN knowledge can reduce the risk of developing HTN. Practical actions are needed to lessen the burden of HTN in the population through collaborative patient care coordination of different domains as a single HTN team, including primary care physicians, pharmacists, behavioral scientists, internationalists, HTN specialists, nutritionists, and exercise specialists (Figure 5).
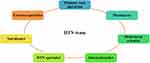 |
Figure 5 A coordination of different domains as HTN-team to knowledge the general public about HTN. |
Conclusions, Remarks and Future Prospective
Available knowledge supports the link between amyloid deposition in developing AD patients’ brain cells and cerebrovascular impairment as a marker of HTN. Older adults are more likely to have HTN, which is linked to higher AD-related morbidity and mortality rates. The summarized literature on this physiological association is substantial. This review will encourage the preclinical trials to accumulate data on administering antihypertensive drugs to treat AD. Future studies need integrated strides for novel methodological strategies and disease models to recapitulate the association accurately. Doubtlessly, the exploration of this association holds great promise in identifying predictive biomarkers and applied therapeutic targets. Furthermore, the clinicians must also work at the management level to continuously advise policymakers to develop long-term programs that address the incidence of HTN patients and insert awareness about this public health concern, eventually reducing the AD burden in the elderly population.
Abbreviations
AD, Alzheimer’s disease; HTN., hypertension; AHDs, Antihypertensive drugs; CVD, Cardiovascular disease; BP, Blood Pressure; Aβ, β-peptide; CSF, Cerebral spinal fluid; sAPP, soluble Amyloid Precursor Protein beta; CDK5, Cyclin-Dependent Kinases 5; ERK2, Extracellular Signal-Regulated Kinase 2; ApoE, Apolipoprotein-E; ROS., Reactive Oxygen Species; AHMs, Antihypertensive medications; PSP, Progressive supranuclear palsy; CSVD, Cerebral small vessel disease; ACEIs, angiotensin-converting enzyme inhibitors; ARBs, angiotensin II receptor blockers; BBs, beta-blockers (BBs); CCBs, calcium channel blockers; AT1R, angiotensin receptor subtype 1.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
This work was supported by the Postgraduate Education Reform and Quality Improvement Project of Henan Province (No.YJS2022KC30), Postgraduate Cultivating Innovation and Quality Improvement Action Plan of Henan University (No.YJSJG2022XJ059), and Henan Provincial Science and Technology Research Project (No.222102310251).
Disclosure
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
References
1. Association A. Alzheimer’s disease facts and figures. Alzheimers Dement. 2019;15(3):321–387.
2. Gustavsson A, Norton N, Fast T, et al. Global estimates on the number of persons across the Alzheimer’s disease continuum. Alzheimers Dement. 2023;19(2):658–670. doi:10.1002/alz.12694
3. Wong W. Economic burden of Alzheimer disease and managed care considerations. Am J Manag Care. 2020;26(8 Suppl):S177–S183.
4. Vaz M, Silvestre S. Alzheimer’s disease: recent treatment strategies. Eur J Pharmacol. 2020;887:173554. doi:10.1016/j.ejphar.2020.173554
5. Stephan BC, Pakpahan E, Siervo M, et al. Prediction of dementia risk in low-income and middle-income countries (the 10/66 Study): an independent external validation of existing models. Lancet Global Health. 2020;8(4):e524–e535. doi:10.1016/S2214-109X(20)30062-0
6. Ju Y, Tam KY. Pathological mechanisms and therapeutic strategies for Alzheimer’s disease. Neural Regener Res. 2022;17(3):543. doi:10.4103/1673-5374.320970
7. Silva MVF, Loures CDMG, Alves LCV, et al. Alzheimer’s disease: risk factors and potentially protective measures. J Biomed Sci. 2019;26(1):1–11. doi:10.1186/s12929-019-0524-y
8. Zhang -X-X, Tian Y, Wang Z-T, et al. The epidemiology of Alzheimer’s disease modifiable risk factors and prevention. J Prevent Alzheimer’s Dis. 2021;8:313–321. doi:10.14283/jpad.2021.15
9. Lennon MJ, Makkar SR, Crawford JD, et al. Midlife hypertension and Alzheimer’s disease: a systematic review and meta-analysis. J Alzheimer’s Dis. 2019;71(1):307–316. doi:10.3233/JAD-190474
10. Livingston G, Sommerlad A, Orgeta V, et al. Dementia prevention, intervention, and care. Lancet. 2017;390(10113):2673–2734.
11. Baranowski BJ, Marko DM, Fenech RK, et al. Healthy brain, healthy life: a review of diet and exercise interventions to promote brain health and reduce Alzheimer’s disease risk. Appl Physiol Nutr Metabol. 2020;45(10):1055–1065. doi:10.1139/apnm-2019-0910
12. Sagud M, Tudor L, Pivac N. Personalized treatment interventions: nonpharmacological and natural treatment strategies in Alzheimer’s disease. Expert Rev Neurother. 2021;21(5):571–589. doi:10.1080/14737175.2021.1906223
13. Cordone S, Scarpelli S, Alfonsi V, et al. Sleep-based interventions in Alzheimer’s disease: promising approaches from prevention to treatment along the disease trajectory. Pharmaceuticals. 2021;14(4):383. doi:10.3390/ph14040383
14. Lennon MJ, Koncz R, Sachdev PS. Hypertension and Alzheimer’s disease: is the picture any clearer? Curr Opin Psychiatry. 2021;34(2):142–148. doi:10.1097/YCO.0000000000000684
15. Nyúl-Tóth Á, Tarantini S, Kiss T, et al. Increases in hypertension-induced cerebral microhemorrhages exacerbate gait dysfunction in a mouse model of Alzheimer’s disease. Geroscience. 2020;42(6):1685–1698. doi:10.1007/s11357-020-00256-3
16. de Oliveira LDSSCB, Souza EC, Rodrigues RAS, et al. The effects of physical activity on anxiety, depression, and quality of life in elderly people living in the community. Trends Psychiatry Psychother. 2019;41(p):36–42. doi:10.1590/2237-6089-2017-0129
17. Diao D, Diao F, Xiao B, et al. Bayes conditional probability-based causation analysis between gestational diabetes mellitus (gdm) and pregnancy-induced hypertension (PIH): a statistic case study in Harbin, China. J Diabetes Res. 2022;2022. doi:10.1155/2022/2590415
18. Pan Z-Y, Zhong HJ, Huang DN, Wu LH, He XX. Beneficial effects of repeated washed microbiota transplantation in children with autism. Front Pediatr. 2022;971. doi:10.3389/fped.2022.928785
19. Martins G, Corrêa L, Caparrol AJDS, et al. Sociodemographic and health characteristics of formal and informal caregivers of elderly people with Alzheimer’s Disease. Escola Anna Nery. 2019;23(2). doi:10.1590/2177-9465-ean-2018-0327
20. Wang H, Wang K, Xue Q, et al. Transcranial alternating current stimulation for treating depression: a randomized controlled trial. Brain. 2022;145(1):83–91. doi:10.1093/brain/awab252
21. Dafsari FS, Jessen F. Depression—an underrecognized target for prevention of dementia in Alzheimer’s disease. Transl Psychiatry. 2020;10(1):160. doi:10.1038/s41398-020-0839-1
22. Jeon SY, Byun MS, Yi D, et al. Influence of hypertension on brain amyloid deposition and Alzheimer’s disease signature neurodegeneration. Neurobiol Aging. 2019;75:62–70. doi:10.1016/j.neurobiolaging.2018.11.001
23. Scheltens P, De Strooper B, Kivipelto M, et al. Alzheimer’s disease. Lancet. 2021;397(10284):1577–1590. doi:10.1016/S0140-6736(20)32205-4
24. Matej R, Tesar A, Rusina R. Alzheimer’s disease and other neurodegenerative dementias in comorbidity: a clinical and neuropathological overview. Clin Biochem. 2019;73:26–31. doi:10.1016/j.clinbiochem.2019.08.005
25. Porsteinsson A, Isaacson RS, Knox S, et al. Diagnosis of early Alzheimer’s disease: clinical practice in 2021. J Prevent Alzheimer’s Dis. 2021;8:371–386. doi:10.14283/jpad.2021.23
26. Ayre K, Krishnamoorthy G. 3.1 How the brain develops. In: Trauma Informed Behaviour Support: A Practical Guide to Developing Resilient Learners. University of Southern Queensland; 2020.
27. Ismail Z, Black SE, Camicioli R, et al. Recommendations of the 5th Canadian Consensus Conference on the diagnosis and treatment of dementia. Alzheimers Dement. 2020;16(8):1182–1195. doi:10.1002/alz.12105
28. Ashrafian H, Zadeh EH, Khan RH. Review on Alzheimer’s disease: inhibition of amyloid beta and tau tangle formation. Int J Biol Macromol. 2021;167:382–394. doi:10.1016/j.ijbiomac.2020.11.192
29. Vogel JW, Iturria-Medina Y, Strandberg OT, et al. Spread of pathological tau proteins through communicating neurons in human Alzheimer’s disease. Nat Commun. 2020;11(1):2612. doi:10.1038/s41467-020-15701-2
30. Volicer L. Physiological and pathological functions of beta-amyloid in the brain and Alzheimer’s disease: a review. Chin J Physiol. 2020;63(3):95. doi:10.4103/CJP.CJP_10_20
31. Karran E, De Strooper B. The amyloid hypothesis in Alzheimer disease: new insights from new therapeutics. Nat Rev Drug Discov. 2022;21(4):306–318. doi:10.1038/s41573-022-00391-w
32. Hampel H, Vassar R, De Strooper B, et al. The β-secretase BACE1 in Alzheimer’s disease. Biol Psychiatry. 2021;89(8):745–756. doi:10.1016/j.biopsych.2020.02.001
33. Babusikova E, Dobrota D, Turner AJ, et al. Effect of global brain ischemia on amyloid precursor protein metabolism and expression of amyloid-degrading enzymes in rat cortex: role in pathogenesis of Alzheimer’s disease. Biochemistry. 2021;86(6):680–692. doi:10.1134/S0006297921060067
34. Guo Y, Wang Q, Chen S, et al. Functions of amyloid precursor protein in metabolic diseases. Metabolism. 2021;115:154454. doi:10.1016/j.metabol.2020.154454
35. Leong YQ, Ng KY, Chye SM, et al. Mechanisms of action of amyloid-beta and its precursor protein in neuronal cell death. Metab Brain Dis. 2020;35(1):11–30. doi:10.1007/s11011-019-00516-y
36. Flores J, Noël A, Fillion M-L, et al. Therapeutic potential of Nlrp1 inflammasome, Caspase-1, or Caspase-6 against Alzheimer disease cognitive impairment. Cell Death Differ. 2022;29(3):657–669. doi:10.1038/s41418-021-00881-1
37. Sharma VK, Singh TG, Singh S, et al. Apoptotic pathways and Alzheimer’s disease: probing therapeutic potential. Neurochem Res. 2021;46(12):3103–3122. doi:10.1007/s11064-021-03418-7
38. Seubert P, Oltersdorf T, Lee MG, et al. Secretion of β-amyloid precursor protein cleaved at the amino terminus of the β-amyloid peptide. Nature. 1993;361(6409):260–263. doi:10.1038/361260a0
39. Hashimoto M, Rockenstein E, Crews L, et al. Role of protein aggregation in mitochondrial dysfunction and neurodegeneration in Alzheimer’s and Parkinson’s diseases. Neuromolecular Med. 2003;4:21–35. doi:10.1385/NMM:4:1-2:21
40. Peng C, Trojanowski JQ, Lee VM-Y. Protein transmission in neurodegenerative disease. Nat Rev Neurol. 2020;16(4):199–212. doi:10.1038/s41582-020-0333-7
41. Holtzman DM, Herz J, Bu G. Apolipoprotein E and apolipoprotein E receptors: normal biology and roles in Alzheimer disease. Cold Spring Harb Perspect Med. 2012;2(3):a006312. doi:10.1101/cshperspect.a006312
42. Bu G. Apolipoprotein E and its receptors in Alzheimer’s disease: pathways, pathogenesis and therapy. Nat Rev Neurosci. 2009;10(5):333–344. doi:10.1038/nrn2620
43. Zhang X, Qu -Y-Y, Liu L, et al. Homocysteine inhibits pro-insulin receptor cleavage and causes insulin resistance via protein cysteine-homocysteinylation. Cell Rep. 2021;37(2):109821. doi:10.1016/j.celrep.2021.109821
44. Johnstone M, Gearing AJ, Miller KM. A central role for astrocytes in the inflammatory response to β-amyloid; chemokines, cytokines and reactive oxygen species are produced. J Neuroimmunol. 1999;93(1–2):182–193. doi:10.1016/S0165-5728(98)00226-4
45. Ramesh G, MacLean AG, Philipp MT. Cytokines and chemokines at the crossroads of neuroinflammation, neurodegeneration, and neuropathic pain. Mediators Inflamm. 2013;2013:1–20. doi:10.1155/2013/480739
46. Alberghina L, Colangelo AM. The modular systems biology approach to investigate the control of apoptosis in Alzheimer’s disease neurodegeneration. BMC Neurosci. 2006;7:1–26. doi:10.1186/1471-2202-7-S1-S2
47. Gunn AP, Wong BX, Johanssen T, et al. Amyloid-β peptide Aβ3pE-42 induces lipid peroxidation, membrane permeabilization, and calcium influx in neurons. J Biol Chem. 2016;291(12):6134–6145. doi:10.1074/jbc.M115.655183
48. Gan X, Huang S, Wu L, et al. Inhibition of ERK-DLP1 signaling and mitochondrial division alleviates mitochondrial dysfunction in Alzheimer’s disease cybrid cell. Biochim Biophys Acta. 2014;1842(2):220–231. doi:10.1016/j.bbadis.2013.11.009
49. Zeng Q, Bie B, Guo Q, et al. Hyperpolarized Xe NMR signal advancement by metal-organic framework entrapment in aqueous solution. Proce Natl Acad Sci. 2020;117(30):17558–17563. doi:10.1073/pnas.2004121117
50. Hooper C, Killick R, Lovestone S. The GSK3 hypothesis of Alzheimer’s disease. J Neurochem. 2008;104(6):1433–1439. doi:10.1111/j.1471-4159.2007.05194.x
51. Town T, Zolton J, Shaffner R, et al. p35/Cdk5 pathway mediates soluble amyloid‐β peptide‐induced tau phosphorylation in vitro. J Neurosci Res. 2002;69(3):362–372. doi:10.1002/jnr.10299
52. Das A, Dikshit M, Nath C. Role of molecular isoforms of acetylcholinesterase in learning and memory functions. Pharmacol Biochem Behav. 2005;81(1):89–99. doi:10.1016/j.pbb.2005.02.006
53. Tiwari P, Dwivedi S, Singh MP, et al. Basic and modern concepts on cholinergic receptor: a review. Asian Pacific J Trop Dis. 2013;3(5):413–420. doi:10.1016/S2222-1808(13)60094-8
54. Hampel H, Mesulam -M-M, Cuello AC, et al. Khachaturian. Brain: a Journal of Neurology. 2018;141(7):1917–1933. doi:10.1093/brain/awy132
55. Plant C, Teipel SJ, Oswald A, et al. Automated detection of brain atrophy patterns based on MRI for the prediction of Alzheimer’s disease. Neuroimage. 2010;50(1):162–174. doi:10.1016/j.neuroimage.2009.11.046
56. Rami L, Sala-Llonch R, Solé-Padullés C, et al. Distinct functional activity of the precuneus and posterior cingulate cortex during encoding in the preclinical stage of Alzheimer’s disease. J Alzheimer’s Dis. 2012;31(3):517–526. doi:10.3233/JAD-2012-120223
57. Rodriguez RD, Grinberg LT. Argyrophilic grain disease: an underestimated tauopathy. Dement Neuropsychol. 2015;9(1):2–8. doi:10.1590/S1980-57642015DN91000002
58. Wang F, Wang H, Zhou X, et al. A driving fatigue feature detection method based on multifractal theory. IEEE Sens J. 2022;22(19):19046–19059. doi:10.1109/JSEN.2022.3201015
59. Abner EL, Neltner JH, Jicha GA, et al. Diffuse amyloid-β plaques, neurofibrillary tangles, and the impact of APOE in elderly persons’ brains lacking neuritic amyloid plaques. J Alzheimer’s Dis. 2018;64(4):1307–1324. doi:10.3233/JAD-180514
60. Ferrer I, Santpere G, van Leeuwen FW. Argyrophilic grain disease. Brain. 2008;131(6):1416–1432. doi:10.1093/brain/awm305
61. Togo T, Isojima D, Akatsu H, et al. Clinical features of argyrophilic grain disease: a retrospective survey of cases with neuropsychiatric symptoms. Am J Geriatric Psychiatry. 2005;13(12):1083–1091. doi:10.1097/00019442-200512000-00008
62. Zhang K, Yang Y, Ge H, et al. Neurogenesis and Proliferation of neural stem/progenitor cells conferred by artesunate via FOXO3a/p27Kip1 Axis in mouse stroke model. Mol Neurobiol. 2022;59(8):4718–4729. doi:10.1007/s12035-021-02710-5
63. Petersen RC, Parisi JE, Dickson DW, et al. Neuropathologic features of amnestic mild cognitive impairment. Arch Neurol. 2006;63(5):665–672. doi:10.1001/archneur.63.5.665
64. Saito Y, Murayama S. Neuropathology of mild cognitive impairment. Neuropathology. 2007;27(6):578–584. doi:10.1111/j.1440-1789.2007.00806.x
65. Brouwers S, Sudano I, Kokubo Y, et al. Arterial hypertension. Lancet. 2021;398(10296):249–261. doi:10.1016/S0140-6736(21)00221-X
66. Twagirumukiza M, De Bacquer D, Kips JG, et al. Current and projected prevalence of arterial hypertension in sub-Saharan Africa by sex, age and habitat: an estimate from population studies. J Hypertens. 2011;29(7):1243–1252. doi:10.1097/HJH.0b013e328346995d
67. Snarska K, Chorąży M, Szczepański M, et al. Quality of life of patients with arterial hypertension. Medicina. 2020;56(9):459. doi:10.3390/medicina56090459
68. Valenzuela PL, Carrera-Bastos P, Gálvez BG, et al. Lifestyle interventions for the prevention and treatment of hypertension. Nat Rev Cardiol. 2021;18(4):251–275. doi:10.1038/s41569-020-00437-9
69. Noubiap JJ, Nansseu JR, Nyaga UF, et al. Global prevalence of resistant hypertension: a meta-analysis of data from 3.2 million patients. Heart. 2019;105(2):98–105. doi:10.1136/heartjnl-2018-313599
70. Zeng Z, Chen J, Xiao C, Chen W. A global view on prevalence of hypertension and human develop index. Ann Global Health. 2020;86(1):67.
71. Schutte AE, Srinivasapura Venkateshmurthy N, Mohan S, et al. Hypertension in low-and middle-income countries. Circ Res. 2021;128(7):808–826. doi:10.1161/CIRCRESAHA.120.318729
72. Macquart de Terline D, Kane A, Kramoh KE, et al. Factors associated with poor adherence to medication among hypertensive patients in twelve low and middle income Sub-Saharan countries. PLoS One. 2019;14(7):e0219266. doi:10.1371/journal.pone.0219266
73. Uchmanowicz I, Markiewicz K, Uchmanowicz B, et al. The relationship between sleep disturbances and quality of life in elderly patients with hypertension. Clin Interv Aging. 2019;Volume 14:155–165. doi:10.2147/CIA.S188499
74. Liu P, Shi J, Wang Z-A. Pattern formation of the attraction-repulsion Keller-Segel system. Discrete Continuous Dyn Syst Ser B. 2013;18(10):2597. doi:10.3934/dcdsb.2013.18.2597
75. Yamazaki D, Hitomi H, Nishiyama A. Hypertension with diabetes mellitus complications. Hyperten Res. 2018;41(3):147–156. doi:10.1038/s41440-017-0008-y
76. Vidal-Petiot E, Greenlaw N, Ford I, et al. Relationships between components of blood pressure and cardiovascular events in patients with stable coronary artery disease and hypertension. Hypertension. 2018;71(1):168–176. doi:10.1161/HYPERTENSIONAHA.117.10204
77. Jin H-Y, Wang Z-A. Global stabilization of the full attraction-repulsion Keller-Segel system. arXiv preprint arXiv:1905 05990; 2019.
78. Jamerson KA, Nasser SA, Ferdinand KC. Cardiovascular disease in minorities: unique considerations: hypertension in African and Hispanic Americans. Cardiovascular Disease in Racial and Ethnic Minority Populations. Springer. 2021:159–166.
79. Costa SDM, Lima CDA, Nobre ALCSD, et al. Hypertension bearers with high risk/big risk of cardiovascular diseases and socioeconomic and health indicators. Revista da Associação Médica Brasileira. 2018;64(p):601–610. doi:10.1590/1806-9282.64.07.601
80. Mills KT, Stefanescu A, He J. The global epidemiology of hypertension. Nat Rev Nephrol. 2020;16(4):223–237. doi:10.1038/s41581-019-0244-2
81. Mollan SP, Aguiar M, Evison F, et al. The expanding burden of idiopathic intracranial hypertension. Eye. 2019;33(3):478–485. doi:10.1038/s41433-018-0238-5
82. Jin H-Y, Wang Z-A. Boundedness, blowup and critical mass phenomenon in competing chemotaxis. J Differ Equ. 2016;260(1):162–196. doi:10.1016/j.jde.2015.08.040
83. Kirkland EB, Heincelman M, Bishu KG, et al. Trends in healthcare expenditures among US adults with hypertension: national estimates, 2003–2014. J Am Heart Assoc. 2018;7(11):e008731. doi:10.1161/JAHA.118.008731
84. Jin HY, Wang ZA. Asymptotic dynamics of the one‐dimensional attraction–repulsion Keller–Segel model. Math Methods Appl Sci. 2015;38(3):444–457. doi:10.1002/mma.3080
85. Foy AJ, Mandrola JM. Heavy heart: the economic burden of heart disease in the United States Now and in the future. Prim Care. 2018;45(1):17–24. doi:10.1016/j.pop.2017.11.002
86. Wiinberg N, Høegholm A, Christensen HR, et al. 24-h ambulatory blood pressure in 352 normal Danish subjects, related to age and gender. Am J Hypertens. 1995;8(10):978–986. doi:10.1016/0895-7061(95)00216-2
87. Jennings JR, Zanstra Y. Is the brain the essential in hypertension? Neuroimage. 2009;47(3):914–921. doi:10.1016/j.neuroimage.2009.04.072
88. Korner PI. Essential Hypertension and Its Causes: Neural and Non-Neural Mechanisms. Oxford University Press; 2007.
89. Harvey A, Montezano AC, Lopes RA, et al. Vascular fibrosis in aging and hypertension: molecular mechanisms and clinical implications. Can J Cardiol. 2016;32(5):659–668. doi:10.1016/j.cjca.2016.02.070
90. Meissner A. Hypertension and the brain: a risk factor for more than heart disease. Cerebrovasc Sis. 2016;42(3–4):255–262. doi:10.1159/000446082
91. Wong TY, Klein R, Sharrett AR, et al. Retinal arteriolar diameter and risk for hypertension. Ann Intern Med. 2004;140(4):248–255. doi:10.7326/0003-4819-140-4-200402170-00006
92. Gorelick PB, Scuteri A, Black SE, et al. Vascular contributions to cognitive impairment and dementia: a statement for healthcare professionals from the American Heart Association/American Stroke Association. stroke. 2011;42(9):2672–2713. doi:10.1161/STR.0b013e3182299496
93. Petrou P, Gothelf Y, Argov Z, et al. Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral sclerosis: results of Phase 1/2 and 2a clinical trials. JAMA Neurol. 2016;73(3):337–344. doi:10.1001/jamaneurol.2015.4321
94. Shang X, Hill E, Zhu Z, et al. The association of age at diagnosis of hypertension with brain structure and incident dementia in the UK Biobank. Hypertension. 2021;78(5):1463–1474. doi:10.1161/HYPERTENSIONAHA.121.17608
95. Ungvari Z, Toth P, Tarantini S, et al. Hypertension-induced cognitive impairment: from pathophysiology to public health. Nat Rev Nephrol. 2021;17(10):639–654. doi:10.1038/s41581-021-00430-6
96. Aam S, Gynnild MN., Munthe-Kaas R, et al. The impact of vascular risk factors on post-stroke cognitive impairment: the Nor-COAST study. Front Neurol. 2021;1083. doi:10.3389/fneur.2021.678794
97. Sierra C. Hypertension and the risk of dementia. Front Cardiovasc Med. 2020;7:5. doi:10.3389/fcvm.2020.00005
98. Abell JG, Kivimäki M, Dugravot A, et al. Association between systolic blood pressure and dementia in the Whitehall II cohort study: role of age, duration, and threshold used to define hypertension. Eur Heart J. 2018;39(33):3119–3125. doi:10.1093/eurheartj/ehy288
99. Gottesman RF, Seshadri S. Risk factors, lifestyle behaviors, and vascular brain health. Stroke. 2022;53(2):394–403. doi:10.1161/STROKEAHA.121.032610
100. Li G, Rhew IC, Shofer JB, et al. Age‐varying association between blood pressure and risk of dementia in those aged 65 and older: a community‐based prospective cohort study. J Am Geriatr Soc. 2007;55(8):1161–1167. doi:10.1111/j.1532-5415.2007.01233.x
101. Wetterberg H, Najar J, Rydén L, et al. Blood pressure at the age of 70 as a predictor of incident dementia: a 15‐year longitudinal study: epidemiology/Risk and protective factors in MCI and dementia. Alzheimers Dement. 2020;16:e045841. doi:10.1002/alz.045841
102. Rutherford BR, Brewster K, Golub JS, et al. Sensation and psychiatry: linking age-related hearing loss to late-life depression and cognitive decline. Am J Psychiatry. 2018;175(3):215–224. doi:10.1176/appi.ajp.2017.17040423
103. Thorin-Trescases N, de Montgolfier O, Pinçon A, et al. Impact of pulse pressure on cerebrovascular events leading to age-related cognitive decline. Am J Physiol. 2018;314(6):H1214–H1224. doi:10.1152/ajpheart.00637.2017
104. Johnson EE, Alexander C, Lee GJ, et al. Examination of race and gender differences in predictors of neuropsychological decline and development of Alzheimer’s disease. Clin Neuropsychol. 2022;36(2):327–352. doi:10.1080/13854046.2021.1940299
105. Joo SH, Yun SH, Kang DW, et al. Body mass index in mild cognitive impairment according to age, sex, cognitive intervention, and hypertension and risk of progression to Alzheimer’s disease. Frontiers in Psychiatry. 2018;9:142. doi:10.3389/fpsyt.2018.00142
106. Gilsanz P, Mayeda ER, Glymour MM, et al. Female sex, early-onset hypertension, and risk of dementia. Neurology. 2017;89(18):1886–1893. doi:10.1212/WNL.0000000000004602
107. Forcaia G, Formicola B, Terribile G, et al. Multifunctional liposomes modulate purinergic receptor-induced calcium wave in cerebral microvascular endothelial cells and astrocytes: new insights for Alzheimer’s disease. Mol Neurobiol. 2021;58(6):2824–2835. doi:10.1007/s12035-021-02299-9
108. Myers DR, Lam WA. Vascularized microfluidics and their untapped potential for discovery in diseases of the microvasculature. Annu Rev Biomed Eng. 2021;23(1):407–432. doi:10.1146/annurev-bioeng-091520-025358
109. Uddin MS, Rahman MA, Kabir MT, et al. Multifarious roles of mTOR signaling in cognitive aging and cerebrovascular dysfunction of AlzheimerAlzheimer’s disease. Iubmb Life. 2020;72(9):1843–1855. doi:10.1002/iub.2324
110. Ramos-Cejudo J, Wisniewski T, Marmar C, et al. Traumatic brain injury and Alzheimer’s disease: the cerebrovascular link. EBioMedicine. 2018;28:21–30. doi:10.1016/j.ebiom.2018.01.021
111. Cheng Y-W, Chiu MJ, Chen YF, Cheng TW, Lai YM, Chen TF. The contribution of vascular risk factors in neurodegenerative disorders: from mild cognitive impairment to Alzheimer’s disease. Alzheimer’s Res Ther. 2020;12(1):1–10.
112. Tariq S, Barber PA. Dementia risk and prevention by targeting modifiable vascular risk factors. J Neurochem. 2018;144(5):565–581. doi:10.1111/jnc.14132
113. He J-T, Zhao X, Xu L, et al. Vascular risk factors and Alzheimer’s disease: blood-brain barrier disruption, metabolic syndromes, and molecular links. J Alzheimer’s Dis. 2020;73(1):39–58. doi:10.3233/JAD-190764
114. Mosconi L, Walters M, Sterling J, et al. Lifestyle and vascular risk effects on MRI-based biomarkers of Alzheimer’s disease: a cross-sectional study of middle-aged adults from the broader New York City area. BMJ open. 2018;8(3):e019362. doi:10.1136/bmjopen-2017-019362
115. Cheng G, He S, He Q, et al. Trajectory patterns of blood pressure change up to six years and the risk of dementia: a nationwide cohort study. Aging. 2021;13(13):17380. doi:10.18632/aging.203228
116. Tarantini S, Tucsek Z, Valcarcel-Ares MN, et al. Circulating IGF-1 deficiency exacerbates hypertension-induced microvascular rarefaction in the mouse hippocampus and retrosplenial cortex: implications for cerebromicrovascular and brain aging. Age. 2016;38(4):273–289. doi:10.1007/s11357-016-9931-0
117. Bowman GL, Dayon L, Kirkland R, et al. Blood-brain barrier breakdown, neuroinflammation, and cognitive decline in older adults. Alzheimers Dement. 2018;14(12):1640–1650. doi:10.1016/j.jalz.2018.06.2857
118. Liao Y, Fan Y, He Q, et al. Exogenous H2S ameliorates high salt-induced hypertension by alleviating oxidative stress and inflammation in the paraventricular nucleus in Dahl S rats. Cardiovasc Toxicol. 2022;22(5):477–491. doi:10.1007/s12012-022-09729-7
119. Jiang E, Chapp AD, Fan Y, et al. Expression of proinflammatory cytokines is upregulated in the hypothalamic paraventricular nucleus of dahl salt-sensitive hypertensive rats. Front Physiol. 2018;9:104. doi:10.3389/fphys.2018.00104
120. Fang X, Crumpler RF, Thomas KN, et al. Contribution of cerebral microvascular mechanisms to age-related cognitive impairment and dementia. Physiol Int. 2022;109(1):20–30. doi:10.1556/2060.2022.00020
121. Park L, Zhou P, Koizumi K, et al. Brain and circulating levels of Aβ1–40 differentially contribute to vasomotor dysfunction in the mouse brain. Stroke. 2013;44(1):198–204. doi:10.1161/STROKEAHA.112.670976
122. Park L, Koizumi K, El Jamal S, et al. Age-dependent neurovascular dysfunction and damage in a mouse model of cerebral amyloid angiopathy. Stroke. 2014;45(6):1815–1821. doi:10.1161/STROKEAHA.114.005179
123. Bertsch M, Franchi B, Meacci L, et al. The amyloid cascade hypothesis and Alzheimer’s disease: a mathematical model. Eur J Appl Math. 2021;32(5):749–768. doi:10.1017/S0956792520000339
124. Tolar M, Abushakra S, Sabbagh M. The path forward in Alzheimer’s disease therapeutics: reevaluating the amyloid cascade hypothesis. Alzheimers Dement. 2019. doi:10.1016/j.jalz.2019.09.075
125. De La Torre J, Mecocci P. The vascular hypothesis of Alzheimer’s disease: a key to preclinical prediction of dementia using neuroimaging. J Alzheimer’s Dis. 2018;63(1):35–52. doi:10.3233/JAD-180004
126. Stakos DA, Stamatelopoulos K, Bampatsias D, et al. The Alzheimer’s disease amyloid-beta hypothesis in cardiovascular aging and disease: JACC focus seminar. J Am Coll Cardiol. 2020;75(8):952–967. doi:10.1016/j.jacc.2019.12.033
127. Woods C, Marques-Lopes J, Contoreggi NH, et al. Tumor necrosis factor α receptor type 1 activation in the hypothalamic paraventricular nucleus contributes to glutamate signaling and angiotensin II-dependent hypertension. J Neurosci. 2021;41(6):1349–1362. doi:10.1523/JNEUROSCI.2360-19.2020
128. Oscanoa TJ, Amado J, Vidal X, Romero-Ortuno R. Angiotensin-receptor blockers and the risk of Alzheimer s disease: a meta-analysis. Curr Rev. 2021;16(1):73–78.
129. Cosarderelioglu C, George CJ, Xue Q-L, et al. Angiotensin receptor blockers upregulate angiotensin type 4 receptor in brains of cognitively intact individuals. Innovat Aging. 2021;5(Supplement_1):634. doi:10.1093/geroni/igab046.2411
130. Giardini A, Piva T, Picchio FM, et al. Impact of transverse aortic arch hypoplasia after surgical repair of aortic coarctation: an exercise echo and magnetic resonance imaging study. Int J Cardiol. 2007;119(1):21–27. doi:10.1016/j.ijcard.2006.07.036
131. Tran S, Kuruppu S, Rajapakse NW. Chronic renin-angiotensin System Activation induced neuroinflammation: common mechanisms underlying hypertension and dementia? J Alzheimer’s Dis. 2022;85(3):943–955. doi:10.3233/JAD-215231
132. Zhang D, Wang YG, Liu CY, et al. Aminoguanidine ameliorates ovariectomy‑induced neuronal deficits in rats by inhibiting AGE‑mediated Aβ production. Acta Neurobiol Exp. 2021;81(1):10–20. doi:10.21307/ane-2021-002
133. Wang Y, Zhang R, Tao C, et al. Blood-brain barrier disruption and perivascular beta-amyloid accumulation in the brain of aged rats with spontaneous hypertension: evaluation with dynamic contrast-enhanced magnetic resonance imaging. Korean J Radiol. 2018;19(3):498–507. doi:10.3348/kjr.2018.19.3.498
134. Csiszar A, Tucsek Z, Toth P, et al. Synergistic effects of hypertension and aging on cognitive function and hippocampal expression of genes involved in β-amyloid generation and Alzheimer’s disease. Am J Physiol. 2013;305(8):H1120–H1130. doi:10.1152/ajpheart.00288.2013
135. van der Kant R, Goldstein LS, Ossenkoppele R. Amyloid-β-independent regulators of tau pathology in Alzheimer disease. Nat Rev Neurosci. 2020;21(1):21–35. doi:10.1038/s41583-019-0240-3
136. Hu H, Meng L, Bi Y-L, et al. Tau pathologies mediate the association of blood pressure with cognitive impairment in adults without dementia: the CABLE study. Alzheimers Dement. 2022;18(1):53–64. doi:10.1002/alz.12377
137. Laing KK, Simoes S, Baena-Caldas GP, et al. Cerebrovascular disease promotes tau pathology in Alzheimer’s disease. Brain commun. 2020;2(2):fcaa132. doi:10.1093/braincomms/fcaa132
138. Chiang GC, Mao X, Kang G, et al. Relationships among cortical glutathione levels, brain amyloidosis, and memory in healthy older adults investigated in vivo with 1 H-MRS and Pittsburgh compound-B PET. Am J Neuroradiol. 2017;38(6):1130–1137. doi:10.3174/ajnr.A5143
139. Raskin J, Cummings J, Hardy J, et al. Neurobiology of Alzheimer’s disease: integrated molecular, physiological, anatomical, biomarker, and cognitive dimensions. Curr Alzheimer Res. 2015;12(8):712–722. doi:10.2174/1567205012666150701103107
140. Schreiber S, Drukarch B, Garz C, et al. Interplay between age, cerebral small vessel disease, parenchymal amyloid-β, and tau pathology: longitudinal studies in hypertensive stroke-prone rats. J Alzheimer’s Dis. 2014;42(s3):S205–S215. doi:10.3233/JAD-132618
141. Kurata T, Lukic V, Kozuki M, et al. Telmisartan reduces progressive accumulation of cellular amyloid beta and phosphorylated tau with inflammatory responses in aged spontaneously hypertensive stroke resistant rat. J Stroke Cerebrovasc Dis. 2014;23(10):2580–2590. doi:10.1016/j.jstrokecerebrovasdis.2014.05.023
142. Castillo-Carranza DL, Nilson AN, Van Skike CE, et al. Cerebral microvascular accumulation of tau oligomers in Alzheimer’s disease and related tauopathies. Aging Dis. 2017;8(3):257. doi:10.14336/AD.2017.0112
143. Iadecola C, Gottesman RF. Cerebrovascular alterations in Alzheimer disease: incidental or pathogenic? Circ Res. 2018;123(4):406–408. doi:10.1161/CIRCRESAHA.118.313400
144. Watase H, Sun J, Hippe DS, et al. Carotid artery remodeling is segment specific: an in vivo study by vessel wall magnetic resonance imaging. Arterioscler Thromb Vasc Biol. 2018;38(4):927–934. doi:10.1161/ATVBAHA.117.310296
145. Kaess BM, Rong J, Larson MG, et al. Aortic stiffness, blood pressure progression, and incident hypertension. JAMA. 2012;308(9):875–881. doi:10.1001/2012.jama.10503
146. Jefferson AL, Cambronero FE, Liu D, et al. Higher aortic stiffness is related to lower cerebral blood flow and preserved cerebrovascular reactivity in older adults. Circulation. 2018;138(18):1951–1962. doi:10.1161/CIRCULATIONAHA.118.032410
147. DuBose LE, Boles Ponto LL, Moser DJ, et al. Higher aortic stiffness is associated with lower global cerebrovascular reserve among older humans. Hypertension. 2018;72(2):476–482. doi:10.1161/HYPERTENSIONAHA.118.11143
148. Schnerr RS, Jansen JFA, Uludag K, et al. Pulsatility of lenticulostriate arteries assessed by 7 Tesla flow MRI—Measurement, reproducibility, and applicability to aging effect. Front Physiol. 2017;8:961. doi:10.3389/fphys.2017.00961
149. Jackson R, Bellamy M. Antihypertensive drugs. Contin Educ Anaesth Crit Care Pain. 2015;15(6):280–285.
150. Brunström M, Carlberg B. Association of blood pressure lowering with mortality and cardiovascular disease across blood pressure levels: a systematic review and meta-analysis. JAMA Intern Med. 2018;178(1):28–36. doi:10.1001/jamainternmed.2017.6015
151. Iadecola C, Yaffe K, Biller J, et al. Impact of hypertension on cognitive function: a scientific statement from the American Heart Association. Hypertension. 2016;68(6):e67–e94. doi:10.1161/HYP.0000000000000053
152. Williamson JD, Pajewski NM, Auchus AP, et al. Effect of intensive vs standard blood pressure control on probable dementia: a randomized clinical trial. JAMA. 2019;321(6):553–561. doi:10.1001/jama.2018.21442
153. Hernandorena I, Duron E, Vidal J-S, et al. Treatment options and considerations for hypertensive patients to prevent dementia. Expert Opin Pharmacother. 2017;18(10):989–1000. doi:10.1080/14656566.2017.1333599
154. Li N-C, Lee A, Whitmer RA, et al. Use of angiotensin receptor blockers and risk of dementia in a predominantly male population: prospective cohort analysis. BMJ. 2010;340(1):b5465. doi:10.1136/bmj.b5465
155. Khachaturian AS, Zandi PP, Lyketsos CG, et al. Antihypertensive medication use and incident Alzheimer disease: the Cache County Study. Arch Neurol. 2006;63(5):686–692. doi:10.1001/archneur.63.5.noc60013
156. Vazirinejad R, Mirmotalebi M, Bageri M, et al. Age-related effect of antihypertensive treatment on cognitive performance: is it better preventing dementia in older age? Am J Alzheimer’s Dis Other Dement. 2019;34(7–8):486–491. doi:10.1177/1533317519859197
157. McGeer EG, McGeer PL. Clinically tested drugs for Alzheimer’s disease. Expert Opin Investig Drugs. 2003;12(7):1143–1151. doi:10.1517/13543784.12.7.1143
158. van Dalen JW, Moll van Charante EP, van Gool WA, et al. Discontinuation of antihypertensive medication, cognitive complaints, and incident dementia. J Am Med Dir Assoc. 2019;20(9):1091–1097. e3. doi:10.1016/j.jamda.2018.12.006
159. Desormais I, Amidou SA, Houehanou YC, et al. The prevalence, awareness, management and control of hypertension in men and women in Benin, West Africa: the TAHES study. BMC Cardiovasc Disord. 2019;19(1):1–12. doi:10.1186/s12872-019-01273-7
160. Gelaw S, Yenit MK, Nigatu SG, Moreira TMM. Self-care practice and associated factors among hypertensive patients in Debre Tabor Referral Hospital, Northwest Ethiopia, 2020. Int J Hypertens. 2021;2021:1–9. doi:10.1155/2021/3570050
161. Watts P, Rance S, McGowan V, et al. The long-term health and wellbeing impacts of Healthy New Towns (HNTs): protocol for a baseline and feasibility study of HNT demonstrator sites in England. Pilot Feasibil Stud. 2020;6(1):1–13. doi:10.1186/s40814-020-0550-2
162. Cifkova R, Fodor G, Wohlfahrt P. Changes in hypertension prevalence, awareness, treatment, and control in high-, middle-, and low-income countries: an update. Curr Hypertens Rep. 2016;18(8):1–6. doi:10.1007/s11906-016-0669-y
163. Souza FJ, Santos AG, de Morais KA, et al. Análise do Perfi l dos Praticantes de Mountain Bike (MTB) da Cidade de Trindade (GO). Vita et Sanitas. 2016;10(1):22–37.
164. Jorge C, Cetó M, Arias A, et al. level of understanding of Alzheimer disease among caregivers and the general population. Neurología. 2021;36(6):426–432. doi:10.1016/j.nrl.2018.03.004
165. Danta CC. Calcium channel blockers: a possible potential therapeutic strategy for the treatment of Alzheimer’s dementia patients with SARS-CoV-2 infection. ACS Chem Neurosci. 2020;11(15):2145–2148. doi:10.1021/acschemneuro.0c00391
166. Lebouvier T, Chen Y, Duriez P, et al. Antihypertensive agents in Alzheimer’s disease: beyond vascular protection. Expert Rev Neurother. 2020;20(2):175–187. doi:10.1080/14737175.2020.1708195
167. Schmukler E, Pinkas-Kramarski R. Autophagy induction in the treatment of Alzheimer’s disease. Drug Dev Res. 2020;81(2):184–193. doi:10.1002/ddr.21605
168. Gupta GL, Samant NP. Current druggable targets for therapeutic control of Alzheimer’s disease. Contemp Clin Trials. 2021;109:106549. doi:10.1016/j.cct.2021.106549
169. Ribeiro VT, de Souza LC, Simoes ESAC. Renin-angiotensin system and Alzheimer’s disease pathophysiology: from the potential interactions to therapeutic perspectives. Protein Pept Lett. 2020;27(6):484–511.
170. Chung MK, Karnik S, Saef J, et al. SARS-CoV-2 and ACE2: the biology and clinical data settling the ARB and ACEI controversy. EBioMedicine. 2020;58:102907. doi:10.1016/j.ebiom.2020.102907
171. Thomas J, Smith H, Smith CA, et al. The angiotensin-converting enzyme inhibitor lisinopril mitigates memory and motor deficits in a drosophila model of Alzheimer’s disease. Pathophysiology. 2021;28(2):307–319. doi:10.3390/pathophysiology28020020
172. Ballard C, Aarsland D, Cummings J, et al. Drug repositioning and repurposing for Alzheimer disease. Nat Rev Neurol. 2020;16(12):661–673. doi:10.1038/s41582-020-0397-4
173. Barthold D, Joyce G, Diaz Brinton R, et al. Association of combination statin and antihypertensive therapy with reduced Alzheimer’s disease and related dementia risk. PLoS One. 2020;15(3):e0229541. doi:10.1371/journal.pone.0229541
174. Royea J, Lacalle-Aurioles M, Trigiani LJ, et al. AT2R’s (Angiotensin II Type 2 Receptor’s) role in cognitive and cerebrovascular deficits in a mouse model of Alzheimer disease. Hypertension. 2020;75(6):1464–1474. doi:10.1161/HYPERTENSIONAHA.119.14431
175. Royea J, Hamel E. Brain angiotensin II and angiotensin IV receptors as potential Alzheimer’s disease therapeutic targets. Geroscience. 2020;42(5):1237–1256. doi:10.1007/s11357-020-00231-y
176. Evans AK, Ardestani PM, Yi B, et al. Beta-adrenergic receptor antagonism is proinflammatory and exacerbates neuroinflammation in a mouse model of Alzheimer’s disease. Neurobiol Dis. 2020;146:105089. doi:10.1016/j.nbd.2020.105089
177. Yiannopoulou KG, Papageorgiou SG. Current and future treatments in Alzheimer disease: an update. J Cent Nerv Syst Dis. 2020;12:1179573520907397. doi:10.1177/1179573520907397
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

