Back to Journals » Cancer Management and Research » Volume 11
Pathological risk factors for lymph node metastasis in patients with submucosal invasive colorectal carcinoma
Authors Zhang Q , Wang L, Huang D, Xu M , Weng W, Ni S , Tan C, Sheng W
Received 28 July 2018
Accepted for publication 21 December 2018
Published 30 January 2019 Volume 2019:11 Pages 1107—1114
DOI https://doi.org/10.2147/CMAR.S181740
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Rituraj Purohit
Qiongyan Zhang,1–3,* Lei Wang,1,2,* Dan Huang,1,2 Midie Xu,1,2 Weiwei Weng,1,2 Shujuan Ni,1,2 Cong Tan,1,2 Weiqi Sheng1,2
1Department of Pathology, Fudan University Shanghai Cancer Center, Shanghai 200032, China; 2Department of Oncology, Shanghai Medical College, Fudan University, Shanghai 200032, China; 3Department of Pathology, Zhongshan Hospital, Fudan University, Shanghai 200032, China
*These authors contributed equally to this work
Background: Risk grade assessment determines therapy in patients with submucosal invasive colorectal carcinoma (CRC). However, treatment decisions are often difficult due to a lack of consensus on which risk factors should be considered. We aimed to identify predictive risk factors for lymph node metastasis (LNM) in a large cohort of submucosal invasive CRC patients from China.
Patients and methods: Following collection of clinicopathological data and disease-free survival (DFS) rates from 290 patients who underwent radical intestinal resection with regional lymphadenectomy, we immunohistochemically assessed expression of DNA mismatch repair (MMR) proteins and p53. The correlation between clinicopathological parameters, MMR expression, p53 status, and LNM status was determined using chi-squared tests and logistic analysis. Receiver operator characteristic curve analysis was used to compare the predictive values. The DFS curves were plotted using the Kaplan–Meier method.
Results: LNM was detected in 15.5% of the cases (45/290 patients). Three pathological characteristics, high tumor differentiation grade, lymphovascular invasion (LVI), and tumor budding, were all positively related to LNM in univariate and multivariate analyses (P<0.05). MMR status did not correlate with either LNM or the pathological characteristics (P>0.05). Overexpression of p53 was associated with tumor budding status (P=0.036). With a negative predicative value of 0.92 and area under the curve of 0.76 (95% CI: 0.68–0.85), the combination of these three factors provided optimal predictive ability. Patients with all three risk factors had poorer DFS (P<0.001).
Conclusion: High tumor grade, LVI, and positive tumor budding serve as useful LNM predictors in submucosal invasive CRC.
Keywords: CRC, LNM, risk factor, MMR, p53
Introduction
Colorectal cancer (CRC) is the fourth leading cause of cancer-related death, with >1.2 million patients diagnosed worldwide each year.1 Compared with advanced-stage CRC, early-stage CRC exhibits a far better prognosis. Currently, low-risk submucosal carcinomas tend to be managed using endoscopic treatment. Endoscopic mucosal resection, endoscopic submucosal dissection, conventional snare resection, and hybrid endoscopic submucosal dissection are effective and oncologically safe.2–5 However, high-risk patients should receive additional radical surgery, which is intestinal resection with regional lymphadenectomy. The appropriate treatment choice depends on the risk of lymph node metastasis (LNM) and local recurrence. LNM risk may be inferred upon rigorous assessment of pathological parameters, and is a key factor to consider in the setting of complete endoscopic resection.
Pathologists have conducted various studies from different perspectives to identify predictive factors for LNM.3,6–11 However, considerable discrepancies exist concerning their conclusions.12–14 Moreover, guidelines and practices also differ among various countries. In the USA, surgical intervention is proposed for tumors with high-grade morphology, lymphovascular invasion (LVI), and a close or positive margin.8 In Japan, the indications for surgical intervention also include submucosal invasion depth ≥1,000 µm and high tumor budding.15 We supposed that the conclusions might vary due to geographic disparities, and differing levels of endoscopic technique and operative skills. So we sought to clarify the risk factors related to LNM in a cohort of Chinese T1CRC patients.
Data on the molecular characteristics of submucosal invasive carcinoma are scarce. In the current study, we evaluated the expression levels of DNA mismatch repair (MMR) proteins and p53, which are among the most frequent alterations associated with CRC oncogenesis.16 The maintenance of MMR function is essential for genetic fidelity. The mismatch repair protein deficient (dMMR) phenotype leads to replication errors in DNA sequences. This may cause accumulation of frameshift mutations in repetitive nucleotide regions, a condition termed microsatellite instability (MSI), that results in cancer development.17 As one important carcinogenetic pathway in CRC, MSI is present in ~15% of CRCs. Tumors with MSI show distinctive features, such as a lower incidence of LNM, a better prognosis, and no benefit from fluorouracil-based chemotherapy.16,18 Accordingly, it has been reported that MSI status might serve as a negative predictive factor in estimating LNM in T1 CRC.19 The TP53 (p53) tumor suppressor gene functions in the cell cycle checkpoint system, triggering cell cycle arrest and apoptosis in response to oncogenic stress signals. Disruption of p53 function causes unchecked growth of potentially malignant cells, leading to carcinogenesis.20 p53 mutations are among the most notable driver events in CRC and have reportedly been detected in 40%–50% of colorectal tumors.21 However, the prognostic role of p53 in CRC remains under debate.
MSI and p53 status have been previously addressed mainly in advanced CRC. Here, we assessed these two variables, readily detected by immunohistochemistry (IHC) in our daily diagnostic routine, to identify potential molecular characteristics of T1 CRC and to evaluate their association with LNM.
Patients and methods
Patients
A total of 290 patients who underwent radical surgical resection for submucosal invasive colorectal carcinoma at Fudan University Shanghai Cancer Center from 2008 to 2014 were evaluated retrospectively. The Clinical Research Ethics Committee of Fudan University Shanghai Cancer Center approved this study. The study was conducted in accordance with the Declaration of Helsinki. Written informed consent was obtained from patients. Patients diagnosed with Lynch syndrome, inflammatory bowel disease, and those with synchronous or previous advanced CRC were excluded from the study. All cases were pathologically confirmed as submucosal invasive colorectal adenocarcinoma. Clinical data, including patients’ age and gender, tumor location, tumor size, and plasma carcinoembryonic antigen (CEA) level before surgery, were collected from the medical record system. The tumor size was measured in the gross examination process. Patient follow-up was performed every 3 months during the first year after surgery and every 3–6 months thereafter until December 31, 2016.
Evaluation of pathological features
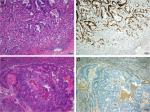
Two experienced pathologists specialized in gastrointestinal diseases reviewed the H&E-stained slides to evaluate the pathological features. All histological diagnoses were made according to the WHO Classification of Tumors of the Digestive System (4th Edition, 2010). The following pathological parameters were assessed: tumor differentiation grade, LVI, status of the muscularis mucosae, tumor border configuration, and tumor growth type. In the process of LVI status evaluation, we first carefully reviewed the slides and picked the suspicious areas, then performed the IHC staining of D2-40 and special staining of elastic fiber to facilitate the evaluation. The tumor border was defined according to the contour of the tumor invasive front. When the tumor expanded by pushing the surrounding normal tissue, it was assumed as expanding growth pattern. And an infiltrative margin referred to tumor dispersedly infiltrated into the normal tissues. The depth of submucosal invasion was evaluated using the method recommended by the Japanese Society for Cancer of the Colon and Rectum (JSCCR).15 The depth was measured from the lower border of the muscularis mucosae to the invasive front of the tumor when the muscularis mucosae were identifiable. When the muscularis mucosae were unidentifiable, the depth was measured from the surface to the deepest of the lesion. For pedunculated tumors, the distance between the deepest invasion and the reference line (the boundary between the tumor head and the stalk) was measured. And 1,000 µm was used as cutoff value. The budding status was evaluated at the invasive front of the tumor. We counted the numbers of budding in a hotspot area with the densest budding. The presence of five or more budding foci under a 20× objective lens (magnification 200×, measuring 0.785 mm2) was defined as a positive state22,23 (Figure 1A).
Immunohistochemistry
We evaluated the expression of four MMR proteins and of p53 by IHC using an automated stainer (Ventana, Tucson, AZ, USA). Primary antibodies included anti-MLH1 (Clone G168-728), anti-MSH2 (Clone G219-1129), anti-MSH6 (Clone 44), and anti-PMS2 (Clone EPR3947), all purchased from Roche (Basel, Switzerland). These antibodies were selected based on a previous study that recommended their use in IHC as a sensitive method to detect tumors with MSI.24 The anti-p53 (Clone DO-7) and anti-D2-40 antibodies (Item number M3619, Clone D2-40) were purchased from DAKO (Carpinteria, CA, USA). Omission of the primary antibody was used as negative controls. For MMR proteins, normal colonic mucosa and lymphocytes were used as positive internal controls. Complete loss of nuclear staining in tumor cells was considered loss of expression. The absence of expression of any of the four MMR proteins was defined as dMMR. Tumors with preserved expression of all MMR proteins were considered mismatch repair protein proficient (pMMR). Known p53-positive cases were used as positive external controls for p53 immunostaining. Cases with at least 10% of the tumor cells exhibiting nuclear p53 staining were considered positive.25
Statistical analysis
All statistical analyses were performed using SPSS 20.0 (IBM, Chicago, IL, USA). Correlations between clinicopathological features and LNM status were examined using chi-squared or Fisher’s exact probability tests. Variables with P<0.05 in the univariate analysis were included in the multivariate logistic analysis to identify independent predictive factors of LNM. Receiver operator characteristic (ROC) curve analysis was used to compare the predictive values. All P-values were two-sided, and statistical significance was established at P<0.05. Disease-free survival (DFS) curves were plotted using the Kaplan–Meier method. The DFS rates were calculated from the date of surgery to the date of disease progression (local or distal tumor recurrence).
Results
Patient characteristics
In total, 290 CRC patients were included in the study. The mean and median ages were 59.7 and 60 years, respectively (range: 27–86 years). The mean and median tumor size was 22.3 mm and 20.0 mm (range 7–93 mm). The mean number of lymph nodes removed with each surgical specimen was 13.3 (range 1–31). LNM was present in 45/290 (15.5%) patients. Among the 45 patients with LNM, 41 were staged as pN1 and 4 were staged as pN2. Notably, positive lymph node status was present in 11 of 39 (28.2%) right colon carcinomas, 8 of 57 (14.0%) left colon carcinomas, and 26 of 194 (13.4%) rectal carcinomas, difference existed in occurrence rate of LNM according to the position of tumors (P=0.005).
Association of LNM with tumor differentiation grade, LVI, and tumor budding status
We analyzed the association between LNM and several clinicopathological features (Table 1). Tumor differentiation grade (P<0.001), LVI (P<0.001), tumor budding status (P<0.001), and tumor border configuration (P=0.036) all correlated with LNM. Other parameters, such as plasma CEA, tumor size, muscularis mucosae state, submucosal invasion depth, precursor adenoma, and tumor growth pattern, showed no association with nodal status (P>0.05). The invasion depth did not correlate with LNM in the whole group analysis (P>0.05). When we analyzed the pedunculated and non-pedunculated tumors separately, it turned out in the non-pedunculated tumors, the deeper invasion showed significant association with higher LNM incidence in the univariate analysis (P=0.043). But we did not find this correlation in the group of pedunculated cases (P>0.05).
Based on the results of univariate analysis, we performed multivariate logistic regression analysis to identify independent risk factors for LNM. The results revealed that three factors, tumor differentiation grade (P<0.001), LVI (P=0.021), and tumor budding status (P=0.036), were significantly associated with LNM (Table 2).
  | Table 2 Univariate and multivariate risk analysis of lymph node metastasis in submucosal invasive colorectal carcinoma Note: Bold value indicates P<0.05. |
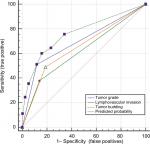
In addition, the incidence of LNM rose as the number of those three risk factors increased. The incidence of LNM was 6.4%, 18.7%, 40.5%, and 83.3% in patients with zero, one, two, and three risk factors, respectively. The ROC analysis showed that the combination of these three factors provided optimal predictive ability, with an area under the curve of 0.76 (95% CI: 0.678–0.848, Figure 2). The sensitivity was 0.60 and the specificity was 0.84. Since the negative predicative value (0.92) was much higher than the positive predictive value (0.33), it would be better to provide optimal predictive ability for negative LNM status rather than positive LNM. It indicates that patients without the three adverse parameters probably have negative LNM status.
Correlation of molecular features and adverse pathological factors
IHC analysis of MMR protein expression status showed that most cases (239/290, 82%) were pMMR. The 51 dMMR cases tended to show a proximal tumor location (P=0.777) and expanding border configuration (P=0.002). There was no correlation between MMR status and those tumoral features such as lymph node status, tumor differentiation grade, and LVI, nor with tumor budding (P>0.05, Table 3).
IHC evaluation of p53 revealed that 195/290 cases (67.24%) exhibited positive p53 staining, and this was associated with tumor budding (P=0.036). As shown in Figure 1, the rate of p53 positivity was higher in budding tumors (50/64, 76.13%) compared with tumors without budding (145/226, 64.16%). There was no correlation between p53 status and other histological characteristics, including lymph node status, tumor differentiation grade, and LVI (P>0.05, Table 3).
Clinical outcomes
The median DFS time for all the patients was 47.3 months. Seven patients developed tumor recurrence 3–54 months after the initial surgery. One patient had an anastomotic adenocarcinoma. Three patients progressed with bone and lung metastases, two patients developed liver metastasis, and one patient developed lung metastasis. Kaplan–Meier analysis showed that patients with LNM had significantly shorter DFS than patients without lymph node involvement (P=0.03, Figure 3A). Each of the three LNM risk factors hereby identified, as well as the MMR and p53 expression phenotypes, had no independent impact on survival (P>0.05). However, patients with all three adverse risk factors (n=7) had significantly poorer survival (P<0.001, Figure 3B).
Discussion
Our study focused on a large group of Chinese pT1 CRC patients to assess the risk factors associated with LNM, and identified three tumoral characteristics (high tumor differentiation grade, LVI, and tumor budding) that were significantly related to nodal involvement in CRC.
Currently, the principal treatment for early-stage CRC is intestinal resection with regional lymphadenectomy. However, the metastatic risk of submucosal invasive carcinomas is quite low, with a reported rate of nodal involvement of 6.8%–13.8%.9,12 For most low-risk patients, local resection is appropriate. Thus, the challenge is to predict the probability of LNM according to preoperative imaging results and histological findings. Multiple studies have been conducted to assess the histopathological factors influencing the risk of LNM.2,10,11 Thus far, these parameters have not been well evaluated in Chinese cohorts. Our study therefore provides value because it is the first to assess the pathological risk factors associated with LNM in a large cohort of Chinese patients with pT1 carcinomas.
We identified tumor differentiation grade, LVI, and tumor budding as predictive risk factors of LNM. The incidence of LNM rose as the number of those characteristics increased. With a high negative predicative value, the three parameters serve as valuable predictive risk factors for negative LNM status. Patients without the three adverse parameters probably have a high probability of normal lymph nodes. Since LNM is related to tumor recurrence and poor survival, the necessity of additional treatment is mainly based on the risk of LNM. Patients without the three risk factors in the setting of complete endoscopic resection with negative margins can probably skip the additional intestinal resection with lymph node dissection.
Whereas high tumor grade and LVI are well-established adverse prognostic indicators of CRC, tumor budding was also regarded as such by literatures.26 Tumor budding is considered a snapshot of the dynamic epithelial–mesenchymal transition process, which is a vital step in tumor migration and distant metastasis.27 Increasing attention has been paid to tumor budding because accumulating evidence has demonstrated that it correlated with tumor metastasis, recurrence, and poor survival.28 However, debates concerning the evaluation criteria have restricted its application in routine diagnostic practice.29,30 Ueno’s method that we have used in this study was an easy quantitative method.22 Recently, the International Tumor Budding Consensus Conference recommended a three-tier scoring system used by JSCCR. After counting the buds at medium power (20× objective, 0.785 mm2) in the hotspot field at the invasive front, it is classified as low budding (grade 1, 0–4 buds), intermediate budding (grade 2, 5–9 buds), and high budding (grade 3, 10 or more buds).23 The grade 2/3 is defined as risk predictor of LNM.15 We suppose this method can be recommended in locally resected submucosal invasive CRC patients for risk stratification.
In our research, tumor budding was significantly associated with p53 overexpression. p53 is a tumor suppressor with comprehensive functions. Currently, its prognostic role in CRC remains uncertain. Makino and others reported that p53 overexpression is a useful biological marker of LNM in T1 CRC,31 whereas other studies mainly focused on advanced CRC. In the current study, p53 did not correlate with DFS rates, adverse histological features, or LNM. Indeed, p53 was reported to possibly have a different prognostic impact depending on the specific mutation type, tumor site, and adjuvant treatment status,16,21,32 so we suppose the biological role of p53 in T1 CRC requires further research.
We found that tumor border configuration was related to LNM in univariate analysis. The tumor margin was shaped by tumor–host interactions. Compared with an expanding margin, an infiltrative margin reflects the increasing capability of tumor cells to invade into host tissue. Previous studies reported that an invasive tumor border was a highly significant predictor of regional LNM and recurrence; however, the results are controversial due to insufficient data and lack of standard evaluation criteria.33 Thus, the clinical impact of tumor border morphology should be further confirmed in a larger population of submucosal CRC patients.
Previous literatures had conflicting views regards the prognostic role of submucosal invasion depth.13,15,34 The depth of submucosal invasion has been shown to be significant with LNM in some studies.15,35 However, some study reported that submucosal invasion depth was not a risk factor for LNM.13 Also in our cohort, we did not find the correlation between deep invasion and LNM in the whole group analysis. But when analyzed the pedunculated and non-pedunculated cases separately, we observed the positive correlation between deep invasion and LNM in the non-pedunculated cases. In our practice, the measurement of the submucosal depth was more feasible in sessile lesions. For pedunculated carcinomas, the assessment was highly dependent on tissue handling and processing. It was difficult to accurately evaluate the depth when the specimen was fragmented or poorly orientated. We suppose the refinement of this pathological factor needs further evaluation according to the different tumor morphology types.
MMR-deficient CRC exhibits specific characteristics, such as high tumor grade and favorable prognosis. Here, we sought to identify any relationships between MMR and early-stage CRC. We did not identify any correlation between MMR expression and LNM, which was consistent with the literature. For example, the study of Pai et al did not identify any significant molecular differences between lymph node-positive and lymph node-negative pT1 CRC carcinomas; however, some adverse histological features were associated with molecular alterations.8 Further studies are needed to thoroughly evaluate these histological factors and the molecular characteristics of submucosal CRC.
Conclusion
Our study confirmed that tumor differentiation grade, LVI, and tumor budding status were risk factors for LNM in T1 CRC. With a high negative predicative value, the three characteristics serve as valuable predictive risk factors for negative LNM status. Because LNM is related to tumor recurrence and poor survival, the three risk factors can be applied in clinic routine to help evaluate the necessity of radical intestinal resection with lymph node dissection after endoscopic treatment.
Data sharing statement
The data supporting the results in the manuscript can be obtained from the corresponding author based on reasonable request.
Acknowledgments
This study was supported by the National Natural Science Foundation of China (81201898), the Natural Science Foundation of Shanghai (17ZR1406500), Shanghai Hospital Development Center Emerging advanced technology joint research project (HDC12014105), and Shanghai Key Developing Disciplines (2015ZB0201).
Disclosure
The authors report no conflicts of interest in this work.
References
Torre LA, Bray F, Siegel RL, Ferlay J, Lortet-Tieulent J, Jemal A. Global cancer statistics, 2012. CA Cancer J Clin. 2015;65(2):87–108. | ||
Nam MJ, Han KS, Kim BC, et al. Long-term outcomes of locally or radically resected T1 colorectal cancer. Colorectal Dis. 2016;18(9):852–860. | ||
Asayama N, Oka S, Tanaka S, et al. Long-term outcomes after treatment for pedunculated-type T1 colorectal carcinoma: a multicenter retrospective cohort study. J Gastroenterol. 2016;51(7):702–710. | ||
Overwater A, Kessels K, Elias SG, et al. Endoscopic resection of high-risk T1 colorectal carcinoma prior to surgical resection has no adverse effect on long-term outcomes. Gut. 2018;67(2):284–290. | ||
Saitoh Y, Inaba Y, Sasaki T, Sugiyama R, Sukegawa R, Fujiya M. Management of colorectal T1 carcinoma treated by endoscopic resection. Dig Endosc. 2016;28(3):324–329. | ||
Yoshii S, Nojima M, Nosho K, et al. Factors associated with risk for colorectal cancer recurrence after endoscopic resection of T1 tumors. Clin Gastroenterol Hepatol. 2014;12(2):292–302.e3. | ||
Caputo D, Caricato M, La Vaccara V, Taffon C, Capolupo GT, Coppola R. T1 colorectal cancer: poor histological grading is predictive of lymph-node metastases. Int J Surg. 2014;12(3):209–212. | ||
Pai RK, Cheng YW, Jakubowski MA, Shadrach BL, Plesec TP, Pai RK. Colorectal carcinomas with submucosal invasion (pT1): analysis of histopathological and molecular factors predicting lymph node metastasis. Mod Pathol. 2017;30(1):113–122. | ||
Tateishi Y, Nakanishi Y, Taniguchi H, Shimoda T, Umemura S. Pathological prognostic factors predicting lymph node metastasis in submucosal invasive (T1) colorectal carcinoma. Mod Pathol. 2010;23(8):1068–1072. | ||
Suh JH, Han KS, Kim BC, et al. Predictors for lymph node metastasis in T1 colorectal cancer. Endoscopy. 2012;44(6):590–595. | ||
Macias-Garcia F, Celeiro-Muñoz C, Lesquereux-Martinez L, et al. A clinical model for predicting lymph node metastasis in submucosal invasive (T1) colorectal cancer. Int J Colorectal Dis. 2015;30(6):761–768. | ||
Horcic M, Koelzer VH, Karamitopoulou E, et al. Tumor budding score based on 10 high-power fields is a promising basis for a standardized prognostic scoring system in stage II colorectal cancer. Hum Pathol. 2013;44(5):697–705. | ||
Kouyama Y, Kudo SE, Miyachi H, et al. Practical problems of measuring depth of submucosal invasion in T1 colorectal carcinomas. Int J Colorectal Dis. 2016;31(1):137–146. | ||
Mitrovic B, Schaeffer DF, Riddell RH, Kirsch R. Tumor budding in colorectal carcinoma: time to take notice. Mod Pathol. 2012;25(10):1315–1325. | ||
Watanabe T, Muro K, Ajioka Y, et al. Japanese Society for Cancer of the Colon and Rectum (JSCCR) guidelines 2016 for the treatment of colorectal cancer. Int J Clin Oncol. 2018;23(1):1–34. | ||
Zaanan A, Cuilliere-Dartigues P, Guilloux A, et al. Impact of p53 expression and microsatellite instability on stage III colon cancer disease-free survival in patients treated by 5-fluorouracil and leucovorin with or without oxaliplatin. Ann Oncol. 2010;21(4):772–780. | ||
Yamamoto H, Imai K. Microsatellite instability: an update. Arch Toxicol. 2015;89(6):899–921. | ||
Gryfe R, Kim H, Hsieh ET, et al. Tumor microsatellite instability and clinical outcome in young patients with colorectal cancer. N Engl J Med. 2000;342(2):69–77. | ||
Kang J, Lee HW, Kim IK, Kim NK, Sohn SK, Lee KY. Clinical implications of microsatellite instability in T1 colorectal cancer. Yonsei Med J. 2015;56(1):175–181. | ||
Munro AJ, Lain S, Lane DP. P53 abnormalities and outcomes in colorectal cancer: a systematic review. Br J Cancer. 2005;92(3):434–444. | ||
Iacopetta B. TP53 mutation in colorectal cancer. Hum Mutat. 2003;21(3):271–276. | ||
Ueno H, Mochizuki H, Hashiguchi Y, et al. Risk factors for an adverse outcome in early invasive colorectal carcinoma. Gastroenterology. 2004;127(2):385–394. | ||
Lugli A, Kirsch R, Ajioka Y, et al. Recommendations for reporting tumor budding in colorectal cancer based on the International Tumor Budding Consensus Conference (ITBCC) 2016. Mod Pathol. 2017;30(9):1299–1311. | ||
Southey MC, Jenkins MA, Mead L, et al. Use of molecular tumor characteristics to prioritize mismatch repair gene testing in early-onset colorectal cancer. J Clin Oncol. 2005;23(27):6524–6532. | ||
Noda M, Okayama H, Kofunato Y, et al. Prognostic role of FUT8 expression in relation to p53 status in stage II and III colorectal cancer. PLoS One. 2018;13(7):e0200315. | ||
Rogers AC, Winter DC, Heeney A, et al. Systematic review and meta-analysis of the impact of tumour budding in colorectal cancer. Br J Cancer. 2016;115(7):831–840. | ||
Kevans D, Wang LM, Sheahan K, et al. Epithelial-mesenchymal transition (EMT) protein expression in a cohort of stage II colorectal cancer patients with characterized tumor budding and mismatch repair protein status. Int J Surg Pathol. 2011;19(6):751–760. | ||
Jayasinghe C, Simiantonaki N, Kirkpatrick CJ. Histopathological features predict metastatic potential in locally advanced colon carcinomas. BMC Cancer. 2015;15(1):14. | ||
Graham RP, Vierkant RA, Tillmans LS, et al. Tumor budding in colorectal carcinoma: confirmation of prognostic significance and histologic cutoff in a population-based cohort. Am J Surg Pathol. 2015;39(10):1340–1346. | ||
Karamitopoulou E, Zlobec I, Kölzer V, et al. Proposal for a 10-high-power-fields scoring method for the assessment of tumor budding in colorectal cancer. Mod Pathol. 2013;26(2):295–301. | ||
Makino M, Yamane N, Taniguchi T, Honboh T, Kurayoshi K, Kaibara N. p53 as an indicator of lymph node metastases in invasive early colorectal cancer. Anticancer Res. 2000;20(3B):2055–2059. | ||
Russo A, Bazan V, Iacopetta B, et al. The TP53 colorectal cancer international collaborative study on the prognostic and predictive significance of p53 mutation: influence of tumor site, type of mutation, and adjuvant treatment. J Clin Oncol. 2005;23(30):7518–7528. | ||
Koelzer VH, Lugli A. The tumor border configuration of colorectal cancer as a histomorphological prognostic indicator. Front Oncol. 2014;4:29. | ||
Lee S, Kim A, Kim Y. The significance of tumor budding in T1 colorectal carcinoma: the most reliable predictor of lymph node metastasis especially in endoscopically resected T1 colorectal carcinoma. Hum Pathol. 2018;78:8–17. | ||
Kawachi H, Eishi Y, Ueno H, et al. A three-tier classification system based on the depth of submucosal invasion and budding/sprouting can improve the treatment strategy for T1 colorectal cancer: a retrospective multicenter study. Mod Pathol. 2015;28(6):872–879. |
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.