Back to Journals » Cancer Management and Research » Volume 12
Pathologic Response After Weekly Paclitaxel versus Docetaxel in Operable Breast Cancer
Authors Bacinschi XE , Anghel RM, Toma PI, Safta I , Ilie A, Ilie SM
Received 14 October 2019
Accepted for publication 29 January 2020
Published 26 February 2020 Volume 2020:12 Pages 1419—1426
DOI https://doi.org/10.2147/CMAR.S234527
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Antonella D'Anneo
Xenia Elena Bacinschi,1,2 Rodica Maricela Anghel,1,2 Paula Iuliana Toma,3 Inga Safta,4 Alis Ilie,5 Silvia Mihaela Ilie6
1Department of Oncology-Radiotherapy, Institute of Oncology Prof Dr Alexandru Trestioreanu, Bucharest, Romania; 2University of Medicine and Pharmacy Carol Davila, Bucharest, Romania; 3Department of Chemotherapy, OncoFort Clinic, Bucharest, Romania; 4Department of Medical Oncology, Antoine Lacassagne Cancer Center, Nice, France; 5Cancer Biology Transfer Platform, Georges Francois Leclerc Cancer Center, Dijon, France; 6Department of Medical Oncology, Georges Francois Leclerc Cancer Center, Dijon, France
Correspondence: Inga Safta
Department of Medical Oncology, Antoine Lacassagne Cancer Centre, 33 Avenue de Valombrose, Nice 06189, France
Tel +33 4 92031000
Email [email protected]
Introduction: Weekly paclitaxel (Ptx) and q3w docetaxel (Dtx) are equivalent in adjuvant breast cancer treatment. Weekly Ptx is better tolerated than q3w Dtx and became the first choice in daily practice, even preoperatively.
Methods: To compare the efficacy and safety of the two regimens, a retrospective analysis was performed in breast cancer patients (pts) referred for neoadjuvant, sequential, taxane-containing chemotherapy to the Institute of Oncology and Oncofort Clinic, Bucharest, between 2008 and 2017.
Results: Forty-seven cases were eligible, median age was 56 years (34– 73 years), mainly stage IIIA–B (53.2%, 25 pts) and ductal invasive (70.2%, 33 pts) of which 24 pts (51%) received q3w Dtx and 23 pts (48.9%) weekly Ptx. The histological response rates were 62.5% (15 pts) and 73.7% (17 pts) (p=0.47), average dose-intensity was 87.7% and 96.7% (p=0.002) and grade III–IV toxicity rate was 12.5% and 13% (p=0.64), respectively. Pathologic response was correlated with immunophenotype, PgR expression, tumor size and backbone chemotherapy (p< 0.05).
Discussion: Our study showed an improved efficacy of taxane’s weekly administration, probably due to a better tolerance and a lower rate of dose-impairing toxicities.
Keywords: operable breast cancer, neo adjuvant chemotherapy, weekly paclitaxel, taxane regimen, pathologic response, toxicities
Introduction
Around 60% of invasive breast cancers are stage II–III according to the AJCC TNM staging system and approximatively 85% are ductal type and chemosensitive.1,2 In primary non-operable breast cancers, neoadjuvant chemotherapy is recommended either to reduce tumor volume for an R0 conservatory or radical resection or to treat a micro-metastatic presumed disease and to improve survival without relapse.3,4 Moreover, neoadjuvant treatment offers the possibility to evaluate tumor’s biological response.5 Standard neoadjuvant chemotherapy comprises three to four sequential courses of an anthracycline-containing regimen and three to four cycles of q3w docetaxel (Dtx).6 Addition of taxanes to anthracyclines in the neoadjuvant setting improves the response, docetaxelbeing the first level of recommendation.7 Recently, weekly 90 mg/m2 paclitaxel (Ptx) and 100 mg/m2 q3w Dtx were demonstrated to be equivalent in adjuvant setting in breast cancer.8 Chemotherapy efficacy depends on dose intensity, representing the amount of the chemotherapeutic agent (in mg) divided by the size of the patient (in m2 of body surface area) per period of time (in weeks) over which the treatment is given, referenced against a standard regimen and which is correlated to the complete pathologic response rate, a surrogate of relapse-free survival for highly proliferative tumors.9 Several studies have evaluated the benefit of weekly Ptx in neoadjuvant setting. Weekly Ptx is better tolerated than every 3 weeks Dtx administration and the premises for 100% intensity dose are higher.10
In this retrospective analysis, we evaluated the efficacy and toxicity of weekly Ptx compared with Dtx every 3 weeks in preoperatory administration.
Methods
We analyzed the files of adult patients (pts) with stage IIB–IIIB invasive breast cancers referred for neoadjuvant, sequential, taxane-containing chemotherapy to the Institute of Oncology “Prof Dr Alexandru Trestioreanu” and Oncofort Clinic, Bucharest, between January 2008 and January 2017. All patients provided written consent for invasive procedures and for the prospective collection of their data. The study was conducted in accordance with the Declaration of Helsinki and approved by the review boards of both institutions.
Clinical TNM classification was run according to the AJCC 7th edition.11 Tumors were graded according to the Elston and Ellis system, which involves ductoglandular differentiation, nuclear pleomorphism and mitotic index.12 Positivity for the estrogen receptor (ER), progesterone receptor (PgR) and human epidermal growth factor receptor 2 (HER2) were considered according to the ASCO and CAP guidelines through the time.13,14
Systemic treatment followed European guidelines at the time, and the selected files noted the recommended regimen in dose per square meter, the rhythm of administration and the total dose provided and real times of administration.
Among the exclusion criteria were metastatic or inoperable disease, bilateral involvement, antecedents of metachronous breast cancer and wall chest irradiation as well as changes in the type of taxane regimen or split courses of chemotherapy before and after surgery. The measurable outcomes were, as a primary objective, the type and rate of the pathologic response and as secondary objectives, the rate of grade III–IV adverse events according to CTCAE v4.03, median dose intensity and rate of 100% intensity dose, regardless of backbone chemotherapy.15,16
Assessment of histopathological response to chemotherapy was adapted from the Chevallier criteria: complete response if there was an absence of invasive elements, partial response if stromal alterations display signs of regression and no response if there were no changes in histological tumor appearance.17
Statistical analyses were ran using SPSS 22 version (IBM Corporation, Armonk, NY, USA). To run comparisons between the subgroups, nonparametric tests were employed and variables that predicted pathologic response were identified by logistic regression.
All statistical tests were two-sided, and a P-value <0.05 was considered to be significant.
Results
After removing missing data (incomplete immunohistopathological information, partial information about the chemotherapeutical agents, no information about the surgical specimen histopathological and immunohistochemical parameters) of the 68 cases analyzed, 47 were found eligible. Median age was 56 years (34–73 years), cTNM stage was III A-B in 25 pts (53.2%) of which 48.93% (23 pts) had tumors larger than 50 mm and 38 pts (80.8%) displayed clinically significant lymph node involvement. Based on biopsy specimens, the histology was mainly ductal invasive carcinoma (33 pts, 70.21%), tumor grades were split equally as G2 and G3 (40.4%, 19 pts each) and the immune-phenotype distribution was as follows: 25.33% (12 pts) luminal A, 42.55% (20 pts) luminal B, 6% (3 pts) triple negative and 19% (9 pts) displaying HER2 overexpression (Table 1).
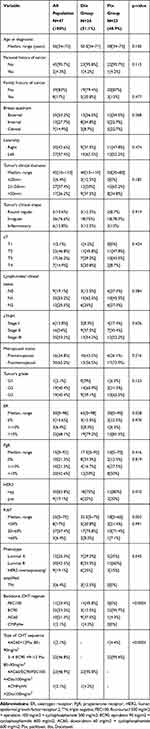 |
Table 1 Tumor and Population Characteristics |
Twenty-four pts (51.01%) received q3w Dtx 75–100 mg/m2 and 23 pts (48.9%) received weekly 80–90 mg/m2 Ptx, and most of these represented the second sequence after a principally anthracycline-containing regimen (45 pts) (Table 1). Significant differences between the two groups were registered only regarding the ER expression (p=0.028), HER2 positivity (p=0.001), medina Ki67 (p=0.003) and backbone chemotherapy (p<0.0001). Even though there was no statistical significance between the two groups in terms of immunophenotypes’ representation (p=0.545), the choice of docetaxelseems to be more frequent than aclitaxel, mostly in triple-negative cases (Figure 1) Ptx regimen was more frequent in ER less-expressing tumors (median ER 30% vs 65% in the Dtx group), while higher HER2 expression (p=0.10) and Ki67 value (median 32.5% Dtx vs 18%Ptx) were found in patients who have received Dtx regimen (Table 1).
The median duration of taxane sequence was 83 days, 65.08 days (21–87) in the Dtx group and 81.87 days (28–105) in the Ptx group. The average intensity doses were 87.71% and 96.74% for Dtx and Ptx, respectively, and the 100% intensity dose rate was 20.8% for Dtx and 87% for Ptx (p=0.001) (Figure 2).
 |
Figure 2 Dose-intensity categories for the Dtx/Ptx subgroups. Abbreviations: Dtx, docetaxel; Ptx, paclitaxel. |
Grade 3–4 toxicity rates were 12.5% (3 pts) in patients treated with Dtx compared with 13% (3 pts) in the Ptx group (p=0.64). Dtx toxicities reported were predominantly digestive events, diarrhea and abdominal pain in 33% of cases (8 pts) and weekly Ptx toxicities reported were predominantly peripheral neuropathy in 34.8% of cases (8 pts). Hematological toxicity of all grades was registered in 25% (6 pts) of the Dtx group and 56% (13 pts) of the Ptx group, of which there was only one case of febrile neutropenia in the first group; 95.83% (23 pts) received primary prophylaxis by GCSF in the Dtx group and 43.47% (10 pts) received the same in the Ptx group (Figure 3).
 |
Figure 3 Taxane regimen received in patients developing hematologic (A), gastrointestinal (B) and neurologic adverse events (C), all grades. Abbreviation: AE, adverse events. |
The type of surgery was radical in 95.8% of cases (45 pts), and the pathologic response rate was 68.08% (32 pts), 15 pts (62.5%) from the Dtx group and 17 pts (73.7%) from the Ptx group (p=0.47), considering both tumors and lymph nodes. The complete pathologic response in tumor and lymph nodes was recorded in eight cases (17.02%), 3 pts (12.5%) belonging to the Dtx group and 5 pts (21.7%) belonging to the Ptx, representing 37.5% and 62.5% of complete response cases, respectively (p=0.06) (Figure 4). Changes in tumor biology in favor of aggressiveness by means of surrogate phenotype classification were identified in 5 pts (10.63%), all were in the Dtx group, with four luminal A subtypes becoming luminal B and one luminal B becoming triple negative.
 |
Figure 4 Taxane regimen representation in the main categories of the pathologic response. Abbreviations: Dtx, docetaxel; Ptx, paclitaxel. |
The pathologic response was found to correlate with cancer phenotype, PgR expression, tumor size and backbone chemotherapy (Table 2), while pCR correlated with the type of taxane regimen, tumor grade and lymph node involvement (Table 3).
 |
Table 2 Correlation Between Pathologic Response and Histologic or Treatment Variables |
 |
Table 3 Correlation Between pCR and Clinical, Histologic and Treatment Variables; p =0.09 |
Discussion
To date, no prospective study has compared paclitaxel and docetaxelin a neoadjuvant setting in breast cancer, and few retrospective studies have addressed this question. Our study revealed a higher tumor pathologic response rate and stromal changes as criteria of pathologic response, in patients receiving paclitaxel regimen. Moreover, the percentage of full dose administration, until the planned final course of chemotherapy, was higher in the same subgroup (87% vs 20.8%, p=0.001), and the pathologic response per se significantly correlated with the type of taxane regimen. The toxicity profile also favored weekly taxane administration, even though grade 3–4 neurological toxicity was non-negligible (34.8%).
Regarding the choice of weekly administration of Ptx, this posology and Dtx every 3 weeks were equivalent and superior to Ptx, every 3 weeks in adjuvant setting, in a prospective study in primary operable breast cancer patients (N=4950), in terms of disease-free survival (weekly Ptx HR=1.27, 95% CI [1.03–1.57], p=0.006; Dtx HR=1.23, 95% CI [1.00–1.52], p=0.02). Overall survival improvement was registered only for weekly Ptx (HR=1.32 95% CI [1.02–1.72], p=0.01).8
A prospective trial comparing Ptx weekly versus every 3 weeks in a neoadjuvant setting showed a clear benefit for weekly in terms of complete pathologic response (28.2% vs 15.7%, p=0.02) and a similar profile of toxicity, with a rate of grade 3 peripheral neuropathy of 13.5% and 14.2%, respectively.18
Concerning the pathologic response to weekly Ptx, a retrospective analysis of 54 locally advanced, operable breast cancer cases that received four cycles of anthracyclines and 12 cycles of weekly Ptx, demonstrated impressive response rates (38.9% of cases). However, the rate of febrile neutropenia was as high as 24% (13 pts),16.7% of whom required hospitalization.19
The benefit and safety of sequential neoadjuvant every 3 weeks Dtx at 100 mg/m2 was compared to anthracyclines only containing chemotherapy, in a randomized trial for operable breast cancer patients. In this large prospective study (N=2411) evaluating the rate of pathologic remission with neoadjuvant Dtx followed by anthracyclines, complete pathologic response was observed in 26% of Dtx cases versus 12.9% of anthracycline-alone receiving patients (p<0.001). Alongside, a higher rate of grade 3–4 toxicities in Dtx cases, with 21% of the pts presenting febrile neutropenia.20
A smaller randomized trial evaluated the efficacy of neoadjuvant sequential Dtx every 3 weeks after four cycles of an anthracycline-containing regimen versus continuing the initial protocol (CVAD, cyclophosphamide, vincristine, doxorubicin, prednisolone) for additional four cycles in responders. The trial found significantly higher pCR rates for sequential Dtx administration, doubling the rate of response (34% vs 16%, p<0.04), but the rates of neutropenia events were higher than the anthracycline-only protocol (69% vs 46%, p<0.001).21
In the present analysis, the pathologic response was associated with immunophenotype (p=0.044).
Regarding the highly aggressive HER2-positive phenotype, a large meta-analysis, which included data from 15 randomized trials, showed superior pathologic complete response rates for neoadjuvant, sequential Ptx compared with Dtx every 3 weeks (43.4%, 95% CI [41.1–45.7] vs 36.6%, 95% CI [34.3–39], p=0.0001), regardless HER2 blockade or chemotherapy backbone used. Significantly fewer grade 3–4 neutropenic adverse events with an absolute difference of 32% (p<0.0001) and a few higher grade 3–4 neuropathic adverse events (absolute difference of 3%, p=0.0001) were registered with Ptx.10
In triple-negative breast cancer, a retrospective analysis of a small sample (N=33) in primary operable patients who underwent sequential neoadjuvant anthracyclines and taxanes—Ptx 80 mg/m2 weekly or Dtx at a dose of 75 mg/m2, q3w chemotherapy for four cycles, a complete pathological response was observed in 36% of cases (13 pts). These results translated into better five-year disease-free survival, although the effect was not statistically significant (p=0.32). Unfortunately, a direct comparison between the two taxane chemotherapy regimens was not run.22
However, no significant difference in pCR response rate was reported in HER 2 (human epidermal growth factor receptor2) overexpressing tumors, in a randomized Phase II study evaluating the efficacy of 12 weekly doses of 80mg/m2 of paclitaxel versus 4 cycles of 75mg/m2 of docetaxel in neoadjuvant setting for stage II or IIIA breast cancer, following 4 courses of anthracycline regimen, concomitant to anti-HER2 antibody. The response rate was 46.9%, 95% CI [33.7%–60.6%] and 42.6% 95% CI [29.5%–56.8%] (p=0.67), respectively, with no difference observed, not even in the hormone receptors negative subgroup 65.4% vs 45.5%, p=0.13.23
Conclusions
In our population, the results showed a better efficacy of weekly taxane administration, even though no statistical significance was found. In general, regarding the residual disease, no differences were observed concerning any pathologic response. The impression of a better complete response after the Ptx regimen is probably due to better tolerance and to a lower rate of dose-impairing toxicities. Still, a high rate of neurologic adverse events remains and its long-term effects should be evaluated. To our knowledge, this is the first time this question is addressed in the neoadjuvant setting, even retrospectively.
Ethical Approval/Informed Consent
This retrospective analysis was approved by the Institute of Oncology “Prof Dr Alexandru Trestioreanu“ and Oncofort Clinic of Bucharest, which determined that informed consent was exempt for a retrospective study.
Acknowledgments
We would like to Mrs Isabel Grégoire, PhD, Centre Georges François Leclerc for providing the medical writing and editorial assistance and Mr Sorin S Opris for graphical assistance in manuscript preparation.
Disclosure
Prof. Dr. Rodica Maricela Anghel reports being an invited speaker for Bayer, Astra Zeneca, and Roche, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Amin MB, Greene FL, Edge SB, et al. The Eighth Edition AJCC cancer staging manual: continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA Cancer J Clin. 2017;67(2):93–99. doi:10.3322/caac.21388
2. Dupont WD, Page DL. Risk factors for breast cancer in women with proliferative breast disease. N Engl J Med. 1985;312(3):146–151. doi:10.1056/NEJM198501173120303
3. Kaufmann M, von Minckwitz G, Mamounas EP, et al. Recommendations from an international consensus conference on the current status and future of neoadjuvant systemic therapy in primary breast cancer. Ann Surg Oncol. 2012;19(5):1508–1516. doi:10.1245/s10434-011-2108-2
4. Rastogi P, Anderson SJ, Bear HD, et al. Preoperative chemotherapy: updates of National surgical adjuvant breast and bowel project protocols B-18 and B-27. J Clin Oncol. 2008;26(5):778–785. doi:10.1200/JCO.2007.15.0235
5. Gentile LF, Plitas G, Zabor EC, Stempel M, Morrow M, Barrio AV. Tumor biology predicts pathologic complete response to neoadjuvant chemotherapy in patients presenting with locally advanced breast cancer. Ann Surg Oncol. 2017;24(13):3896–3902. doi:10.1245/s10434-017-6085-y
6. NCCN. NCCN guidelines. Breast Cancer version 1.2019. Available from: http://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
7. Cuppone F, Bria E, Carlini P, et al. Taxanes as primary chemotherapy for early breast cancer: meta-analysis of randomized trials. Cancer. 2008;113(2):238–246. doi:10.1002/cncr.v113:2
8. Sparano JA, Wang M, Martino S, et al. Weekly paclitaxel in the adjuvant treatment of breast cancer. N Engl J Med. 2008;358(16):1663–1671. doi:10.1056/NEJMoa0707056
9. Cortazar P, Zhang L, Untch M, et al. Pathological complete response and long-term clinical benefit in breast cancer: the CTNeoBC pooled analysis. Lancet. 2014;384(9938):164–172. doi:10.1016/S0140-6736(13)62422-8
10. Carbognin L, Sperduti I, Nortilli R, et al. Balancing activity and tolerability of neoadjuvant paclitaxel- and docetaxel-based chemotherapy for HER2-positive early stage breast cancer: sensitivity analysis of randomized trials. Cancer Treat Rev. 2015;41(3):262–270. doi:10.1016/j.ctrv.2015.02.003
11. Edge SB, Compton CC. The American Joint Committee on Cancer: the 7th edition of the AJCC cancer staging manual and the future of TNM. Ann Surg Oncol. 2010;17(6):1471–1474. doi:10.1245/s10434-010-0985-4
12. Elston CW, Ellis IO. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: experience from a large study with long-term follow-up. Histopathology. 1991;19(5):403–410. doi:10.1111/his.1991.19.issue-5
13. Hammond ME, Hayes DF, Dowsett M, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for immunohistochemical testing of estrogen and progesterone receptors in breast cancer. Arch Pathol Lab Med. 2010;134(6):907–922.
14. Wolff AC, Hammond ME, Schwartz JN, et al. American Society of Clinical Oncology/College of American Pathologists guideline recommendations for human epidermal growth factor receptor 2 testing in breast cancer. J Clin Oncol. 2007;25(1):118–145. doi:10.1200/JCO.2006.09.2775
15. Available from: https://ctep.cancer.gov/protocolDevelopment/electronic_applications/docs/CTCAE_v4_.QuickReference_5x7.
16. Hryniuk WM. The importance of dose intensity in the outcome of chemotherapy. Important Adv Oncol. 1988;121–141.
17. Chevallier B, Roche H, Olivier JP, Chollet P, Hurteloup P. Inflammatory breast cancer. Pilot study of intensive induction chemotherapy (FEC-HD) results in a high histologic response rate. Am J Clin Oncol. 1993;16(3):223–228. doi:10.1097/00000421-199306000-00006
18. Green MC, Buzdar AU, Smith T, et al. Weekly paclitaxel improves pathologic complete remission in operable breast cancer when compared with paclitaxel once every 3 weeks. J Clin Oncol. 2005;23(25):5983–5992. doi:10.1200/JCO.2005.06.232
19. Kawajiri H, Takashima T, Onoda N, et al. Efficacy and feasibility of neoadjuvant chemotherapy with FEC 100 followed by weekly paclitaxel for operable breast cancer. Oncol Lett. 2012;4(4):612–616. doi:10.3892/ol.2012.801
20. Bear HD, Anderson S, Brown A, et al. The effect on tumor response of adding sequential preoperative docetaxel to preoperative doxorubicin and cyclophosphamide: preliminary results from National Surgical Adjuvant Breast and Bowel Project Protocol B-27. J Clin Oncol. 2003;21(22):4165–4174. doi:10.1200/JCO.2003.12.005
21. Smith IC, Heys SD, Hutcheon AW, et al. Neoadjuvant chemotherapy in breast cancer: significantly enhanced response with docetaxel. J Clin Oncol. 2002;20(6):1456–1466. doi:10.1200/JCO.20.6.1456
22. Masuda H, Masuda N, Kodama Y, et al. Predictive factors for the effectiveness of neoadjuvant chemotherapy and prognosis in triple-negative breast cancer patients. Cancer Chemother Pharmacol. 2011;67(4):911–917. doi:10.1007/s00280-010-1371-4
23. Nakamura S, Ando M, Masuda N, et al. Randomized phase II study of primary systemic chemotherapy and trastuzumab for operable HER2 positive breast cancer. Clin Breast Cancer. 2012;12(1):49–56. doi:10.1016/j.clbc.2011.10.002
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

