Back to Journals » Clinical Ophthalmology » Volume 15
Pars Plana Vitrectomy with Internal Limiting Membrane Peeling for Treatment-Naïve Diabetic Macular Edema: A Prospective, Uncontrolled Pilot Study
Received 19 May 2021
Accepted for publication 11 June 2021
Published 21 June 2021 Volume 2021:15 Pages 2619—2624
DOI https://doi.org/10.2147/OPTH.S320214
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 4
Editor who approved publication: Dr Scott Fraser
Ryan B Rush,1– 4 Sloan W Rush2,3
1Instituto de la Visión– Hospital La Carlota, Montemorelos, Nuevo León, 67530, México; 2Panhandle Eye Group, Amarillo, TX, 79106, USA; 3Department of Surgery, Texas Tech University Health Science Center, Amarillo, TX, 79106, USA; 4Southwest Retina Specialists, Amarillo, TX, 79106, USA
Correspondence: Ryan B Rush
Southwest Retina Specialists, 7411 Wallace Blvd, Amarillo, TX, 79106, USA
Tel +1 806 351-1870
Email [email protected]
Purpose: To report the outcomes in subjects undergoing pars plana vitrectomy (PPV) with internal limiting membrane (ILM) peeling for the management of treatment-naïve diabetic macular edema (DME).
Methods: Ten treatment-naïve subjects with non-proliferative diabetic retinopathy prospectively underwent PPV with ILM peeling for the treatment of DME at a single university-affiliated institution. The preoperative features, intraoperative details and postoperative outcomes were collected and analyzed.
Results: All 10 subjects underwent PPV with ILM peeling without significant intraoperative or postoperative complications at 6 months follow-up. Visual acuity improved from a baseline of 0.74 (95% CI: 0.48– 1.0) logMAR (Snellen 20/110) to 0.46 (95% CI: 0.3– 0.62) logMAR (Snellen 20/58) at 6 months follow-up (p=0.045). Optical coherence tomography central macular thickness reduced from a baseline of 456 (95% CI: 394.7– 516.4) microns to 316.8 (95% CI: 275.9– 357.7) microns at 6 months follow-up (p < 0.001).
Conclusion: This pilot study suggests that PPV with ILM peeling may be a viable treatment option for the management of treatment naïve DME in subjects with non-proliferative diabetic retinopathy. Development of a randomized controlled trial may be justified to validate the results of this study.
Clinicaltrials.gov Identifier #: NCT03660345.
Keywords: vitrectomy, internal limiting membrane peeling, diabetic macular edema
Introduction
Diabetic retinopathy is the number one cause of vision loss in working-age adults, and macular edema is the most frequent cause of visual impairment in diabetic patients.1 Diabetic macular edema (DME) has been treated by a number of different modalities including focal and grid laser,2 intravitreal corticosteroids,3 intravitreal anti-vascular endothelial growth factor (VEGF) medications,4 and pars plana vitrectomy (PPV) with or without internal limiting membrane peeling.5,6
PPV for the treatment of DME was first described in 1992 by Lewis et al,7 and since then has been studied by numerous investigators under a variety of different clinical settings including the presence of an epiretinal membrane (ERM),8 vitreomacular traction (VMT),9 and diffuse DME.10 The postulated mechanisms by which PPV may improve DME have included a reduction in macular tangential and anterior-posterior traction,11 improved oxygenation of the vitreous cavity,12 and enhanced diffusion of vasogenic growth factors.13 Other factors that may modulate the response to PPV comprise the patient’s lens status and the presence of macular ischemia.
PPV for DME has generally been considered only in patients that responded poorly to other interventions such as laser and/or intravitreal therapy.5,6 Typically, such patients have chronic and diffuse DME with or without concomitant VMT or an ERM. Several small prospective, controlled trials have been performed to assess the merits of PPV as a treatment option for such recalcitrant cases with mostly disappointing functional outcomes despite having structural improvements.6,14 However, since PPV was reserved as a last-ditch effort following a long ordeal with what included multiple lasers and/or intravitreal injections, it should not be surprising that visual outcomes were poor under such circumstances. Presumably, most of these patients already would have had irreversible damage to the retina with little or no potential for visual acuity improvement no matter what the intervention might have been. Currently, there are no reports in the literature evaluating PPV as an initial treatment for DME. In this pilot study, the authors prospectively performed PPV with internal limiting membrane (ILM) peeling on treatment-naïve subjects with DME in order to assess the potential role and merits of PPV with ILM peeling as an initial treatment option for the management of DME.
Methods
Design
This interventional case series was conducted as a prospective, uncontrolled pilot study of treatment-naïve patients with DME and non-proliferative diabetic retinopathy undergoing PPV with ILM peeling from September 2018 through October 2020 at a university-allied teaching facility in Montemorelos, Nuevo Leon, Mexico. The rules of the Declaration of Helsinki were followed and the acknowledged standards in research involving humans were obeyed during the trial. The University of Montemorelos Institutional Review Board (IRB0009239) endorsed the patient consent forms and the trial’s established protocol. Each study participant provided written informed consent. The trial is registered at ClinicalTrials. gov (NCT03660345, last accessed May 15, 2021).
Participants
The retinal unit was referred treatment-naive subjects with DME in order to determine each patient’s suitability for the study. The criteria for inclusion and exclusion are displayed in Table 1. One eye per participant was permitted into the study. If both eyes were found to meet criteria for enrollment, the eye with the lowest best-corrected visual acuity (BCVA) was selected.
 |
Table 1 Vitrectomy for Diabetic Macular Edema. Inclusion and Exclusion Criteria |
Assessments and Interventions
Enrolled participants underwent a baseline examination within 28 days from PPV, which consisted of a medical and ocular history, Snellen BCVA, an intraocular pressure (IOP) measurement, and an evaluation of the anterior and posterior segments. Phakic subjects underwent an intraocular lens power calculation. Optical coherence tomography (OCT) (Zeiss Cirrus HD-OCT) was performed on all participants at baseline.
All phakic subjects underwent phacoemulsification with intraocular lens implantation prior to PPV during the same operating session. All participants underwent small-gauge (23-gauge or 25-gauge) three port PPV under retrobulbar anesthesia with the Constellation Vision System (Alcon, TX, USA) and the Resight 500 (Carl Zeiss Meditec, Inc., Dublin, USA) by one surgeon (RBR). Indocyanine green-assisted ILM peeling was performed until at least one disc diameter of ILM was removed from the center of the macula in all directions. Vitreous substitution was at the judgment of the operating surgeon. Subtenons 20 mg triamcinolone was administered at the conclusion of each case. Topical moxifloxacin 0.5% QID and prednisolone acetate 1% QID were prescribed for 1 and 3 weeks, respectively, following the operation.
Postoperatively participants underwent data collection at three visits: 1) 15 ± 5 days, 2) 40 ± 10 days, and 3) 185 ± 15 days following surgery. Participants were examined at non-study evaluations at the discretion of the managing specialist. Central macular thickness (CMT) on OCT was conducted at 6 months on all subjects. The occurrence of any postoperative complication attributable to surgery such as vitreous hemorrhage, retinal detachment, high IOP, etc. was recorded. Treatment of DME with anti-VEGF therapy, laser or any other therapy was not permissible during the 6-month postoperative study period.
Outcome Measures
The primary outcome was change in BCVA during the 6-month (185±15 days) study period. The secondary outcome was change in CMT on OCT during the 6-month study period.
Data Analysis
It was pre-determined that a sample of 10 treatment-naïve subjects undergoing PPV with ILM peeling for the indication of DME would be analyzed in order to determine if a larger, controlled trial would be warranted in the future. BCVA is displayed in logMAR with Snellen equals recorded parenthetically. Comparative analysis employing one-way analysis of the variance was utilized to create numerical outcomes. The JMP 11 statistical program (SAS Institute, USA) conducted the data analysis. A p-value less than 0.05 was considered significant.
Results
Ten participants were enrolled into the pilot study. All 10 enrolled participants underwent PPV and completed 6 month follow-up.
Baseline and Intraoperative Details
Displayed in Table 2 are the baseline and intraoperative details of the study population. There were no notable intraoperative complications occurring during surgery for any of the subjects.
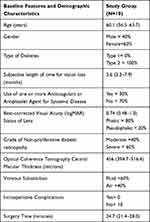 |
Table 2 Vitrectomy for Diabetic Macular Edema. Baseline Features and Intraoperative Details. Means with (95% Confidence Intervals) |
Main Outcome
BCVA improved from a baseline of 0.74 (95% CI: 0.48–1.0) logMAR (Snellen 20/110) to 0.46 (95% CI: 0.3–0.62) logMAR (Snellen 20/58) at 6 months follow-up (p=0.045). There were no subjects who lost ≥1 line(s) of BCVA at 6 months follow-up. There were 5 of 10 subjects (50%) who gained ≥3 lines of BCVA at 6 months follow-up.
Secondary Outcomes
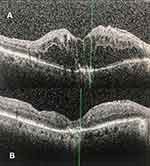
Displayed in Table 3 are the postoperative outcomes of the study population at 6 months follow-up. Optical coherence tomography central macular thickness reduced from a baseline of 456 (95% CI: 394.7–516.4) microns to 316.8 (95% CI: 275.9–357.7) microns at 6 months follow-up (p < 0.001). There were no notable postoperative complications occurring during surgery for any of the subjects. There were no unexpected returns to the operating room during the 6-month trial period. There were no occurrences of endophthalmitis or suprachoroidal/vitreous hemorrhaging during the study interval. The two subjects who developed increased IOP during the postoperative period were successfully managed with topical IOP-lowering medications. A case example has been presented in Figure 1.
 |
Table 3 Vitrectomy for Diabetic Macular Edema. Postoperative Outcomes. Means with (95% Confidence Intervals) |
Discussion
There exists a great need for longer lasting and cheaper therapies in the management of DME, especially in the developing world where it is cost prohibitive and unrealistic from a transportation and workforce standpoint to repetitively administer expensive treatments such as anti-VEGF medications to the afflicted patient population. As discussed above in the Introduction, there has been interest in PPV as a possible modality for DME treatment because of its potential to reduce cost (relative to continuous anti-VEGF therapy) and require fewer future follow-ups and additional treatments.7–10 Unfortunately, the current literature lacks any robust data on the subject at hand as patients with DME have not been considered for PPV until all other interventions were exhausted and the patient no longer had good visual potential, having sustained irreversible damage to the retina from the chronic, unresolved DME.5,6,14 The authors have had the idea that perhaps offering PPV as an initial treatment option for the management of DME (not as a last-ditch effort) may provide a cost-effective alternative to repetitive and incessant anti-VEGF therapy, which has been the standard of care in this patient population for the past 15 years. Prior to undertaking a larger trial with an anti-VEGF control group, the authors tested in this study the feasibility of PPV with ILM peeling as an initial treatment modality in a small sample of patients with treatment naïve DME.
The results of this pilot study demonstrate anatomic and functional benefits to the overall study population, and PPV with ILM peeling was safe and well tolerated by the study population. Although our study reports outcomes of just 10 patients, there were no intraoperative or postoperative complications occurring and no unexpected returns to the operating room during the 6-month trial period. The improvements in both visual acuity and CMT on OCT compared favorably to the 6-month data reported in the landmark anti-VEGF trials15–17 in patients with treatment naïve DME. However, the authors advise caution against such comparisons given that our patient population was principally indigent from a developing nation, whilst the landmark anti-VEGF trials15–17 involved patients from developed nations with good access to care. Although no subjects lost any lines of visual acuity and half of the subjects gained ≥3 lines of visual acuity at 6 months follow-up, presumably the visual acuity outcomes might have been even better if our trial permitted subjects to undergo further DME treatment (such as anti-VEGF therapy) during the trial period under certain prespecified conditions. The authors recognize that PPV with ILM peeling is quite unlikely to be a one-time treatment for DME over the course of a patient’s lifetime and prespecified retreatment protocols will need to be established prior to conducting a randomized controlled trial with follow-up ≥1 year. The authors would be interested to know how many fewer anti-VEGF treatments patients might need after undergoing PPV with ILM peeling compared to a control group receiving initial anti-VEGF therapy during a time period such as 2 years.
Weaknesses of our study include the small number of patients involved, its relatively short postoperative follow-up interval (6 months), and its lack of a control group receiving anti-VEGF therapy. However, given that this study was set up to be a pilot study, these weaknesses were expected. Strengths of our study include its prospective design and its 100% patient completion rate to 6 months follow-up once enrolled. The authors are encouraged by our study’s results and believe this pilot study demonstrates that PPV with ILM peeling may be a potentially safe, effective, and viable alternative to repetitive anti-VEGF therapy for the treatment of DME as an initial treatment and intend to move forward with conducting a randomized controlled trial. Ultimately, well-designed randomized controlled trials in this patient population will be needed to establish PPV with ILM peeling as a feasible and cost-effective alternative to anti-VEGF therapy for treatment naïve DME.
Abbreviations
OCT, optical coherence tomography; PPV, pars plana vitrectomy; BCVA, corrected visual acuity; ILM, internal limiting peeling; DME, diabetic macular edema; VEGF, vascular endothelial growth factor.
Declarations
The study was approved by the University of Montemorelos Institutional Review Board (IRB0009239) in accordance with the Ethical Standards laid down in the Declaration of Helsinki.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Author Contributions
All authors made substantial contributions to conception and design, acquisition of data, or analysis and interpretation of data; took part in drafting the article or revising it critically for important intellectual content; agreed to submit to the current journal; gave final approval of the version to be published; and agreed to be accountable for all aspects of the work.
Funding
There is no funding to report.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Varma R, Bressler NM, Doan QV, et al. Prevalence of and risk factors for diabetic macular edema in the United States. JAMA Ophthalmol. 2014;132(11):1334–1340. doi:10.1001/jamaophthalmol.2014.2854
2. Early Treatment Diabetic Retinopathy Study report number 1. Early Treatment Diabetic Retinopathy Study research group. Photocoagulation for diabetic macular edema. Arch Ophthalmol. 1985;103(12):1796–1806. doi:10.1001/archopht.1985.01050120030015
3. Lam DS, Chan CK, Mohamed S, et al. Intravitreal triamcinolone plus sequential grid laser versus triamcinolone or laser alone for treating diabetic macular edema: six-month outcomes. Ophthalmology. 2007;114(12):2162–2167. doi:10.1016/j.ophtha.2007.02.006
4. Wells JA, Glassman AR, Ayala AR, et al.; Diabetic Retinopathy Clinical Research Network. Aflibercept, bevacizumab, or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–1203.
5. Nakajima T, Roggia MF, Noda Y, et al. Effect of internal limiting membrane peeling during vitrectomy for diabetic macular edema: systematic Review and meta-analysis. Retina. 2015;35(9):1719–1725. doi:10.1097/IAE.0000000000000622
6. Simunovic MP, Hunyor AP, Ho IV. Vitrectomy for diabetic macular edema: a systematic review and meta-analysis. Can J Ophthalmol. 2014;49(2):188–195. doi:10.1016/j.jcjo.2013.11.012
7. Lewis H, Abrams GW, Blumenkranz MS, et al. Vitrectomy for diabetic macular traction and edema associated with posterior hyaloidal traction. Ophthalmology. 1992;99(5):753–759. doi:10.1016/S0161-6420(92)31901-3
8. Yamamoto T, Akabane N, Takeuchi S. Vitrectomy for diabetic macular edema: the role of posterior vitreous detachment and epimacular membrane. Am J Ophthalmol. 2001;132(3):369–377. doi:10.1016/S0002-9394(01)01050-9
9. Haller JA, Qin H, Flaxel CJ, et al. Diabetic retinopathy clinical research network writing committee. Vitrectomy outcomes in eyes with diabetic macular edema and vitreomacular traction. Ophthalmology. 2010;117:1087–1093.
10. Kumagai K, Furukawa M, Ogino N, et al. Long-term follow-up of vitrectomy for diffuse nontractional diabetic macular edema. Retina. 2009;29(4):464–472. doi:10.1097/IAE.0b013e31819c632f
11. Harbour JW, Smiddy WE, Flynn HW
12. Nasrallah FP, Jalkh AE, Van Coppenolle F, et al. The role of the vitreous in diabetic macular edema. Ophthalmology. 1988;95(10):1335–1339. doi:10.1016/S0161-6420(88)33004-6
13. Laidlaw DA. Vitrectomy for diabetic macular oedema. Eye (Lond). 2008;22(10):1337–1341. doi:10.1038/eye.2008.84
14. Hoerauf H, Bruggemann A, Muecke M, et al. Pars plana vitrectomy for diabetic macular edema. Internal limiting membrane delamination vs posterior hyaloid removal. A prospective randomized trial. Graefes Arch Clin Exp Ophthalmol. 2011;249(7):997–1008. doi:10.1007/s00417-010-1610-8
15. Nguyen QD, Brown DM, Marcus DM, et al. Ranibizumab for diabetic macular edema: results from 2 Phase III randomized trials: RISE and RIDE. Ophthalmology. 2012;119(4):789–801. doi:10.1016/j.ophtha.2011.12.039
16. Korobelnik JF, Do DV, Schmidt-Erfurth U, et al. Intravitreal aflibercept for diabetic macular edema. Ophthalmology. 2014;121(11):2247–2254. doi:10.1016/j.ophtha.2014.05.006
17. Wells JA, Glassman AR, Ayala AR, et al. Diabetic retinopathy clinical research network. Aflibercept, bevacizumab or ranibizumab for diabetic macular edema. N Engl J Med. 2015;372:1193–1203.
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.