Back to Journals » Therapeutics and Clinical Risk Management » Volume 17
Parenteral Nutrition Process Management for Newborn and Preterm Infants – A Preliminary Risk Analysis
Authors Sommer I , Palmero D , Fischer Fumeaux CJ, Bonnabry P, Bouchoud L, Sadeghipour F
Received 8 September 2020
Accepted for publication 13 April 2021
Published 28 May 2021 Volume 2021:17 Pages 497—506
DOI https://doi.org/10.2147/TCRM.S280938
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Garry Walsh
Isabelle Sommer,1– 4 David Palmero,1 Céline Julie Fischer Fumeaux,5 Pascal Bonnabry,3,4,6 Lucie Bouchoud,6 Farshid Sadeghipour1– 4
1Service of Pharmacy, Lausanne University Hospital, Lausanne, Switzerland; 2Center for Research and Innovation in Clinical Pharmaceutical Sciences, Lausanne University Hospital and University of Lausanne, Lausanne, Switzerland; 3School of Pharmaceutical Sciences, University of Geneva, Geneva, Switzerland; 4Institute of Pharmaceutical Sciences of Western Switzerland, University of Geneva, Geneva, Switzerland; 5Clinic of Neonatology, Department Woman Mother Child, Lausanne University Hospital, Lausanne, Switzerland; 6Service of Pharmacy,Geneva University Hospital, Geneva, Switzerland
Correspondence: Isabelle Sommer Service of Pharmacy, Lausanne University Hospital, Lausanne, Switzerland
Tel +41 79 556 63 59
Email [email protected]
Background: There are variable practices in the management of the parenteral nutrition (PN) process in hospitals having a neonatal intensive care unit (NICU). In our hospital, PN is prepared partially on the neonatal ward by nurses but also at the central pharmacy by trained pharmacy technicians. A previous study showed a concentration non-conformity of 34% of on-ward PN preparations potentially resulting in under- or overfeeding of the patients.
Objective: The objectives were to perform preliminary risk analyses (PRA) in preparation for our hospital’s transition to universal central pharmacy PN compounding.
Methods: A working group including pharmacists, neonatologists, nurses, and pharmacy technicians performed two PRA. The risks of 9 management steps of the PN process were identified, evaluated, and quoted. A comparison of the number of risks and their criticality index (CI) was conducted.
Results: A total of 36 and 39 risks were identified for PN preparation in the NICU and the pharmacy, respectively. For the NICU, ten risks (28%) had an “acceptable” CI, 15 risks (42%) were “under control” and eleven (31%) were defined as “non-acceptable”. For the pharmacy, 14 risks (36%) had an “acceptable” CI, 19 risks (49%) were “under control” and six (15%) were defined as “non-acceptable”. Risks directly related to the preparation process, including the steps preparation hood, PN preparation and analytical quality control, represented a cumulated CI of 145 for eleven NICU-risks vs 108 for twelve pharmacy risks (− 26%). The implementation of immediate improvement measures, eg, an electronic prescription form, reduces the total CI by 5.7% and 2.2% for the NICU and the pharmacy, respectively.
Conclusion: This PRA highlighted the safety differences between PN preparation in the NICU vs the pharmacy at our institution, and facilitated our moving forward with a process change that should improve the care of our neonatal patients. Nevertheless, long-term improvement measures have to be implemented to further reduce risks related to the PN management process.
Keywords: parenteral nutrition, drug compounding, risk assessment, standardization, neonatology, preterm infants
Introduction
Parenteral nutrition is a crucial part of the initial nutritional support provided for critical preterm or term neonates. Worldwide, different ways of compounding parenteral nutrition (PN) for neonates are applied.1,2 High-risk PN preparation steps are usually managed by the hospital’s pharmacy in collaboration with the neonatal service. In some cases, the whole process, including the compounding of PN, is organized by the neonatal service. Both strategies include risks and constraints.
In our hospital, PN is either prepared at the central pharmacy by trained pharmacy technicians or on the neonatal ward by nurses without any involvement of the pharmacy staff. The place where PN is prepared depends on the physician’s evaluation concerning the emergency to start or adapt nutrition, which may be urgent in critical situations like very preterm infants, (very) low birth weight, metabolic disorder, or critical illness.
In 2015, the Inspection générale des affaires sociales (IGAS) of France published the report of a nationwide survey on PN treatment.3 This survey was performed following the death of five babies in the hospital of Chambery, France in 2012 caused by the administration of contaminated PN. The IGAS came to the decision to totally prohibit on-ward preparations for PN treatment and to delegate the whole responsibility to pharmacists. Due to this report and the different PN preparation practices at our hospital, our interest was directed on the situation of safety of PN treatment at our site.
As PN preparation is known to be one of the most critical steps within its management4 and a major risk factor for healthcare-associated infections in neonates,2 its centralization at the pharmacy is recommended.5 The planned centralization at our site will include the take-over of PN compounding still performed on-ward during the week (Monday to Friday) in a first step and during weekends by the pharmacy emergency service in a second step.
ISO9001 certified, the hospital pharmacy has a quality management system to assure pharmaceutical services. Conforming to the guidelines Q96 of the International Council for Harmonisation of Technical Requirements for Pharmaceuticals for Human Use (ICH) as well as GMP7 of the European Commission’s EudraLex on quality risk management, a risk assessment of this hybrid model was performed.
This study aims to compare the management processes of the two PN preparing sites (NICU and pharmacy) by means of a preliminary risk analysis (PRA) and describes our center’s evaluation of the risks and benefits associated with transitioning towards universal pharmacy PN preparation for our NICU.
Methods and Materials
Process Description
At our university hospital, PN containing glucose and amino acids with or without electrolytes was prepared at the hospital pharmacy as well as on the ward of the NICU.
During opening hours, for medically stable patients, PN is generally prepared at our hospital pharmacy. The process being time consuming, meaning that the prescription order must be placed at noon at the latest for a delivery of the individual PN at 5:00 pm, nurses have to prepare PN on the ward for emergency situations or unstable patients. Furthermore, as the pharmacy does not prepare PN during the night, weekend or holiday, NICU nurses have also to prepare them for new admissions during these shifts.
The neonatal ward also wished for maintaining the flexibility and knowledge of preparing PN on-ward when a preparation at the pharmacy is too time-critical.
At our hospital, no data is available for infections related to contaminated PN or electrolyte disturbances related to under- or over-concentrated PN. This lack of data is due to the unusual process of analyzing PN treatment as root cause for these cases. What is known, is that 34% of PN prepared on the ward is likely to not conform to the medical prescription in a range from 90% to 110%.8
Pharmacy
At the moment of this study, each prescription was written manually on a PN order form which was edited and validated by neonatologists and pharmacists. This form – only used for PN preparation at the pharmacy – was faxed to the pharmacy where technicians transcribed the PN order in a validated Excel sheet interfaced with the compounding automate BAXA EM 2400.9 Before the PN preparation, each prescription was double-checked and validated by a pharmacist.
The pharmacy, qualified by the national authority Swissmedic, followed Ph. Helv. GMP guidelines and was therefore working with a GMP class A Horizontal Laminar Airflow Hood (HLAH), placed in a GMP class B cleanroom, operating with trained and qualified personnel.10 The high-risk PN preparation was completed by means of an automate (BAXA EM 2400) and analytical controls for quantitative determination of critical components (glucose, Na+, K+, Ca2+, Mg2+) were performed on each final product before pharmaceutical release of the PN preparation.11,12
Neonatal Intensive Care Unit
When PN was prepared by nurses on the ward, another order form was used than the validated one for the pharmacy. This form served as instruction for the preparation as well as for transcription of ingredients on the label to be affixed on the prepared PN syringe or bag. New nurses were trained by reference nurses for PN treatment on the handling, preparation and administration of PN. No regular requalification was mandatory.
PN was prepared manually by nurses following the handwritten medical prescription in a non-classified and non-qualified HLAH placed inside the NICU pharmacy. The transcribed labels as well as the volume withdrawn and raw solution of critical components like potassium (as hydrochloride or phosphate salt) were double-checked by a second nurse or physician. For all non-critical ingredients, the preparation of PN was performed and auto controlled by a single nurse only. No analytical controls were carried out for these on-ward preparations before administration to the vulnerable patients.
Even with a huge staff of nurses, PN preparation represented a time-consuming task and reduced the time for patients’ care.
Preliminary Risk Analysis
Since several years, risk analyses are performed in the field of pharmaceutical science for quality management purposes based on the methods applied initially in the aeronautic and military domains.13 Different kinds of risk assessment methods exist, of which the failure modes and effects analysis (FMEA), the failure modes, effects, and criticality analysis (FMECA), and the preliminary risk analysis (PRA) are the most known and applied.14 The FMEA and FMECA are supposed to assess risks in a current, well-established setting and to define if an action plan to secure this setting must be implemented.15 The PRA is performed where a project is planned and the aim is to prevent risks when carrying out the project and to secure the new setting.16 It is also possible to perform a PRA on several domains of risks as far as they concern the same activity.17
As the project of centralization of PN compounding at the pharmacy is planned, the PRA method was chosen to analyze existing and potential future risks associated to the whole PN management process from prescription based on patients’ laboratory values until administration of the individual PN. To define the urgency for centralizing and the need of an action plan awaiting the completion of this project, the PRA was performed for the two PN preparing sites to compare the risk levels. Results of this risk assessment will help to better conduct and legitimate the project and to implement the planned measures.18
Composition of the Working Group
The working group of nine participants comprised the chief pharmacist, the clinical pharmacist for the neonatology department, the responsible pharmacist for PN preparation, a pharmacy technician, a PhD student (pharmacist) moderating the PRA, the neonatologist responsible for PN, the chief nurse of neonatology department, the chief nurse of the unit, and a clinical nurse.
Definition of the PN Management Process Steps
Following a brainstorming with all members of the working group during the first meeting, nine principal topics have been defined to describe the different steps of the PN process:
1. Medical prescription
2. Transcription of medical prescription
3. Primary material
4. Preparation hood
5. PN preparation
6. Analytical quality control
7. PN administration
8. Documentation and traceability
9. Laboratory values
All nine process steps were discussed separately and one after the other to identify all possible risks related to the tasks composing the concerned process step.
Risk Quotation
All identified risks were quoted separately by consensus of all working group members during the second meeting. This was done once for the risks identified for the neonatal department and once for the pharmacy.
The assessment of each risk was performed by identifying the level of severity (S) as shown in Table 1 and the level of probability (P) as shown in Table 2.17 The effects of severity levels as well as the frequency of probability levels have been defined in advance of the PRA by the working group following internal examples (eg, previous risk assessments) and experiences.
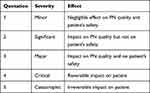 |
Table 1 Level of Severity (S) |
 |
Table 2 Level of Probability (P) |
The evaluation of all risks was done by consensus regarding clinical and pharmaceutical aspects of each risk independent on its nature.
Risk Evaluation
The criticality index (CI) of each risk was calculated by multiplying the quoted severity and probability. The acceptability of risks was defined using the Pareto principle or 80/20 rule,19 meaning that about 20% of most critical risks will need to be focused on to reach the most positive outcome of the whole assessment. Therefore, as shown in Table 3, risks with a CI of 1–6 (green) were defined as “acceptable”, CI of 7–14 (yellow) were risks classified as “under control”, and “non-acceptable” risks had a CI of 15–25 (red).
 |
Table 3 Criticality Index (CI) and Level of Acceptability (Green: “Acceptable”; Yellow: “Under Control”; Red: “Non-Acceptable”) |
Following this risk assessment for the two preparation sites, the third meeting served to focus on all “non-acceptable” risks of CI ≥ 15. For some of these risks, planned measures for improvement already existed. In this instance, a second assessment was performed exactly like the first one including the calculation of a hypothetical CI. The aim still being the identification of residual risks and the need of a corrective and preventive action plan (CAPA plan). For the remaining risks without an already planned improvement project, measures were proposed but the corresponding risks were not quoted again.
Results
1st PRA
In total, 75 risks have been identified, 36 of which were for the whole PN management process at the NICU and 39 risks at the pharmacy.
The number of risks identified for the two preparation sites are listed in Table 4. Several risks were the same for the two sites but sometimes differed in calculated criticality. Risks in common were for example related to the medical prescription what has to be done for both scenarios and what presents the same risks for the final product and the patient. An example for risks not in common are related to the PN preparation as this step is quite different between the two sites.
 |
Table 4 Number of Risks for Each of the 9 Management Steps for Parenteral Nutrition |
The CI distribution of all identified risks is shown in the following Table 5.
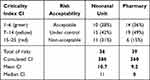 |
Table 5 Distribution of Criticality Index (CI) of Identified Risks |
Comparison of Main Process Differences
The PN management steps that significantly differ between the NICU and the pharmacy include steps n° 4. Preparation hood, n° 5. PN preparation and n° 6. Analytical quality control, for which the differences of CI are shown in Table 6.
 |
Table 6 Comparison of Criticality Index (CI) Sums of Differing Management Process Steps |
Focused Risks
The working group focused on all “non-acceptable” risks (CI = 15–25) following the Pareto principle. Therefore, the attention was brought to 11 vs 6 risks for the NICU and the pharmacy, respectively. Two of the 17 focused risks were identified as equal for both preparation sites (risks related to PN administration), meaning that 15 different risks of CI ≥ 15 were further discussed (Table 7).
 |
Table 7 Details of “Non-Acceptable” Risks with Criticality Index (CI) of 15 and Higher for the Neonatal Unit (NICU) and the Pharmacy (PHA) |
2nd PRA
Table 7 details the risks the working group focused on to define measures to reduce their criticality. The hypothetic risk assessment was also performed on these risks following a brainstorming and an evaluation of the potential influence of the planned and immediately possible measures as detailed hereafter.
Medical Prescription
An improvement measure from the NICU planned to be implemented shortly after the second PRA was a prescription form (Excel sheet) including an extensive calculation base for all kinds of medication (oral, intravenous, subcutaneous, etc.) to be administered to their patients including PN. This quasi-electronic prescription form is the evolution of a preformatted medical order sheet that has been introduced previously for medication prescription except for PN.20 It represents an important step towards a complete electronic prescription, a so-called computerized provider order entry (CPOE) system. This measure hypothetically allows to reduce three risks related to the prescription step as shown in Table 8.
 |
Table 8 Hypothetical Reduction of Criticality Index (CI) After Implementation of Planned Improvement Measures for the Pharmacy (PHARM) and the Neonatal Intensive Care Unit (NICU) |
PN Preparation
Another improvement measure within the preparation step that hypothetically allows to reduce the CI for risk 5.5. “Non-respect of procedures and auto-control” is the revision and application of standard operating procedures (SOP) for the PN preparation on-ward as well as new notices and information for the auto- and double-control.
PN Administration
Finally, the risk 7.1. “False infusion rate” of the administration step might be reduced by sensitizing the nurses to the importance of the correctness of the infusion rate adjustment and to fulfill the requested double-control.
For the NICU, the second PRA reduced the number of “non-acceptable” risks from 11 to 7 and their cumulated CI from 187 to 165.
For the pharmacy, the number of “non-acceptable” risks were reduced from 6 to 5 and the cumulated CI for these risks sank from 102 to 94.
With these short-term improvements, the total CI can be reduced from 386 to 364 (−5.7%) and from 360 to 352 (−2.2%) for the NICU and the pharmacy, respectively.
Long-Term Improvement Measures
Despite the above described as immediately possible and planned improvement measures, the working group defined long-term measures to improve the 15 risks rated with a CI of 15 and higher prior to the centralization of PN preparation at the pharmacy.
In total, six different measures are supposed to have a positive impact on 14 of the 15 risks. Only one risk (6.1.) will probably remain unchanged (CI = 15) as no measure for improvement is envisaged, because the NICU will not be able to perform analytical quality controls on-ward.
Hereafter, the six proposed improvement measures are described:
Computerized Provider Order Entry (CPOE) System
A CPOE system including calculation base and recommendation ranges, interfaced with an automated preparation tool will permit to secure the prescription step and to improve all related risks (1.1.-1.4.). The risk “false labeling” which is related to the preparation step (5.1.) will also be reduced by generating labels automatically and scanning the barcode of these labels to start production.
Training and Standardized Protocols
During our PRA, the working group identified that training and standardized protocols will have an impact on the risks 4.1., 5.4., and 5.5. These measures, already in place for the PN process, need to be revised and harmonized.
High-Visibility Vest
The high-visibility vest, to be worn on the NICU during preparation and administration of PN, might reduce risks related to these two PN management steps (5.3., 5.6. and 7.2.). This will allow neonatal staff handling PN to be easily identifiable and to not be disturbed when wearing this vest.
Standardized Nutritional Solutions
Standardized nutritional solutions like standard glucose dilutions or standardized PN infusion bags will drastically reduce the risk related to the PN preparation on the ward (5.7.).
Backup Preparation Tool
The risk related to defective facilities for automated compounding at the pharmacy (5.2.) will be minimized by acquisition of a backup preparation tool (BAXA EM 2400).
New Infusion Pumps
New infusion pumps precisely programmable and clearly showing the infusion rate will have a huge impact on this risk related to the administration step (7.1.).
Discussion
Even though several risk assessments have been performed on the parenteral nutrition (PN) processes,15,16,21–24 the novelty of our work is the comparison in risks of two sites within the same hospital that are involved in the process of PN for neonatal patients.
The preliminary risk analyses (PRA) performed on the management process of PN for the neonatal intensive care unit (NICU) and the pharmacy showed that most of the risks are related to the medical prescription, the PN preparation and the PN administration. Corresponding statements were recently reported by Palmero et al for our NICU.25 The AMELIORE study conducted by Boulé et al identified the same process steps as principal sources of risks by performing a failure mode, effect, and criticality analysis (FMECA).26 Our results also correlate with those of Villafranca et al who conducted a failure mode and effects analysis (FMEA) on the neonatal PN process from the perspective of the hospital’s pharmacy.27
Bonnabry et al were the first to perform a FMECA on PN order and compounding to compare the handwritten prescription with a computerized provider order entry (CPOE) system as well as the manual with the semi-automatic compounding technique.15 They repeated their risk assessment on the CPOE system some years later to generally improve the high-risk prescription process of all kinds of medications including PN.22 In our study, the implementation of a CPOE system including patient data, nutritional recommendations (ESPGHAN/ESPEN/ESPR guidelines), calculation base and error alerts as well as an interface with the automated preparation tool (BAXA EM 2400)9 will be the most important measure to improve several identified risks.
The NICU who plans to implement a quasi-electronic prescription form (Excel sheet), is already aware of some deficiencies within their process and is facing them actively while awaiting the centralization of PN preparation at the pharmacy. A real CPOE system for PN prescription will be a common tool for NICU and pharmacy and is known to improve the prescription and transcription process.28
Another study described that PN preparation error rates at pharmacies decreased from 37% to 22% when the process was partly automated. Most of these errors included wrong dose (>3%) of components of PN solution or observed omission.4 We also showed in a previous article that 34% of PN prepared manually by nurses on the ward did not conform to their medical prescription (Pharmacopoeia concentration limits for compounded preparations: 90–110%) and concentration of ingredients ranged from 58% to 164% based on their target value (=100%).8
Following our assessments, measures to standardize the PN preparation process were proposed to face these risks as recommended by the American Society for Parenteral and Enteral Nutrition ASPEN.29 As immediate action until the complete take-over of compounding at the pharmacy, standardized PN preparation protocols for the NICU must be reviewed and applied.
At the same time, a standard ready-to-use PN solution is in development to furnish immediate nutritional treatment for newborn term and preterm infants as recommended by the ESPGHAN guidelines30 and practiced all over France.31 The supply with a standardized PN solution for neonatal patients offers a safe, high-quality, and ready-to-use alternative to individually compounded PN and therefore reduces the number of PN needing to be prepared under unsafe conditions.
PN administration safety can principally be influenced by the neonatal caregivers by the simple measure of patient-focused (high-visibility vest) control of correspondence of medication and medical prescription, infusion bag assembly and pump data entry following standard administration procedures as suggested in the ASPEN guidelines. They also recommend to “purchase infusion pumps with capacity to reduce errors due to incorrect programming” which was contemplated at the moment of our risk assessments.32
Most of the risks quoted with a criticality index (CI) of 15 and higher (“non-acceptable”) potentially resulted either in microbial contamination of the product or in a false dose of the different components meaning under- or overfeeding of the patient. The consequences of false doses can be eliminated by analyzing the composition (glucose, Na+, K+, Ca2+, Mg2+) of the compounded PN before administration as already performed on PN prepared by the pharmacy.33,34 Potential contamination might also be analyzed by means of endotoxin testing on pharmacy compounded PN.
Our risk assessments show that the whole process is slightly safer when the pharmacy is involved in the management of parenteral nutrition for patients treated on the neonatal ward (total CI of 386 for the NICU vs 360 for the pharmacy). For the whole process, 36 vs 39 risks have been identified for the NICU and the pharmacy, respectively. The number of risks being higher for the pharmacy can be explained by the multiple steps and interventions on PN before, during and after its preparation process including control of the medical prescription by pharmacists as well as the analytical quality of the final product. The compliance to GMP guidelines being mandatory for the pharmacy but not for the NICU is another reason for the difference in number of risks and their quotation.
When having a look at the management process steps that are independent between the two sites, a clear difference in safety can be observed. The steps concerned are the preparation hood, PN preparation and analytical quality control. The CI of the two sites differ from 145 to 108 for the NICU and the pharmacy, respectively. This means a risk is 26% less likely to occur for the vulnerable patients when PN is prepared at the pharmacy in controlled conditions (class A hood in cleanroom class B) with an automated compounding system by trained pharmacy technicians and with analytical quality controls to prove conformity of the PN preparation with the prescription.
Risks concerning the steps of primary material, documentation and traceability, and laboratory values are more or less the same for both sites, but do not necessarily have the same occurrence (probability) or the same impact on the system or the patients (severity). All these risks were quoted with a CI <15 and therefore not considered as critical but as “acceptable” or risks “under control”. They have not been further discussed.
The residual high-quoted risks, like hygienic issues causing contamination of the final product or of the infusion line and the venous access, might persist even after centralization of PN preparation. These kinds of risks are well known and are difficult to avoid completely,35 but measures to control and minimize their probability are in place (NICU: training of site personnel; pharmacy: in process contamination control, annual control of aseptic working technique, endotoxin testing).
Our study showed the need of standardized computer assisted procedures for the PN management process to secure these high-risk products for vulnerable patients. This standardization is independent of the place of PN preparation. When PN needs to be prepared by nurses on the ward due to an emergency, this PRA demonstrated that the patients are not unnecessarily at risk. Thus the PN preparation at the pharmacy should be preferred as there are more measures in place to guarantee the conformity of PN preparation to its medical prescription as well as the microbial quality.
Still, procedures of both sites (NICU and pharmacy) must be improved to further secure the whole multiple-step PN management process whilst awaiting the centralization of PN preparation at the pharmacy.
All risk assessments are mainly limited by their subjectivity of defining and judging risks related to well-known processes. Therefore, the working group is supposed to represent a wide spectrum of professions and, in consequence, should be sufficiently large. Professionals not knowing the process add important inputs to describe and evaluate possible risks. The lack of this input causes a small limitation of our study since all working group members who participated in our PRAs knew the processes because they work with PN routinely. Nonetheless, the expertise of the working group was of great value to the study.
Another limitation of our study is that we did not distinguish risks where one or the other service does not have influence on, as for example the PN administration which can be influenced by the NICU-staff only. This fact lead to a sort of mix-up of the CI of the two PN preparing sites.
Conclusion
Our PRA demonstrated a potential reduction of 26% in the risk of PN preparation errors when all PN are prepared centrally at the pharmacy, compared to the existing hybrid model of NICU and pharmacy preparation when focusing on the main differing steps (preparation hood, PN preparation, analytical quality control). Although we considered NICU preparation as beneficial for offering a rapid and adequately safe PN preparation process, the potential safety improvements we identified in our PRA outweigh these benefits for this vulnerable population. All working group members as well as the heads of the concerned departments (NICU and pharmacy) agreed that this hybrid model is no longer the state of the art and must be revised rapidly.
Ethics Statements
No review or approval was required for this research by an institutional review board or ethics committee as no intervention on humans was performed and no patient data was analyzed and examined.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Maisonneuve N, Raguso CA, Paoloni-Giacobino A, et al. Parenteral nutrition practices in hospital pharmacies in Switzerland, France, and Belgium. Nutrition. 2004;20:528–535. doi:10.1016/j.nut.2004.03.020
2. Zingg W, Tomaske M, Martin M. Risk of parenteral nutrition in neonates – an overview. Nutrients. 2012;4:1490–1503. doi:10.3390/nu4101490
3. Cecchi-Tenerini RO, Pierrat CH, Vanneste AR. Rapport: evaluation des pratiques en matière de nutrition parentérale pédiatrique. L’igas; 2015.
4. Flynn EA, Pearson RE, Barker KN. Observational study of accuracy in compounding i.v. admixtures at five hospitals. Am J Health-System Pharmacists. 1997;54(8):904–912. doi:10.1093/ajhp/54.8.904
5. Boullata JI. Standardized competencies for parenteral nutrition order review and parenteral nutrition preparation, including compounding: the ASPEN Model. Nutr Clin Practice. 2016;31(4):548–555. doi:10.1177/0884533616653833
6. Committee for Human Medicinal Products. ICH guideline Q9 on quality risk management; 2015. Available from: https://www.ema.europa.eu/en/ich-q9-quality-risk-management.
7. European Commission. EudraLex The Rules Governing Medicinal Products in the European Union Volume 4 EU Guidelines for Good Manufacturing Practice for Medicinal Products for Human and Veterinary Use Chapter 1 Pharmaceutical Quality System; 2013. Available from: https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-4/vol4-chap1_2013-01_en.pdf.
8. Sommer I. Quality and safety of parenteral nutrition for newborn and preterm infants as an on-ward preparation. Eur J Hospital Pharmacy. 2019;01(11):1–5.
9. EXACTAMIX Automated Compounding Systems. Baxter Healthcare Corporation. Available from: https://www.baxtermedicationdeliveryproducts.com/pharmacy-workflow/exactamix.html.
10. European Commission. EudraLex the rules governing medicinal products in the European Union volume 4 EU guidelines to good manufacturing practice medicinal products for human and veterinary use annex 1 manufacture of sterile medicinal products; 2008. Available from: https://ec.europa.eu/health/sites/health/files/files/eudralex/vol-4/2008_11_25_gmp-an1_en.pdf.
11. Kienle PC. ASHP guidelines on compounding sterile preparations. Am J Health-System Pharmacy. 2014;71:145–166.
12. USP General Chapter <797> Pharmaceutical Compounding —Sterile Preparations; 2018. Available from: www.usp.org.
13. Cooke RM. A brief history of quantitative risk assessment. s.l.: resources; 2009.
14. Debray B. Formalisation du savoir et des outils dans le domaine des risques majeurs (DRA-35) Ω-7 Méthodes d’analyse des risques générés par une installation industrielle. Ministère de l’Ecologie et du Développement Durable (MEDD). Verneuil-en-Halatte: INERIS, 2006. INERIS-DRA-2006-P46055-CL47569.
15. Bonnabry P. Use of a systematic risk analysis method to improve safety in the production of paediatric parenteral nutrition solutions. Qual Saf Health Care. 2005;14:93–98. doi:10.1136/qshc.2003.007914
16. Véronique LP, Spiesser-Robelet L, Vrignaud S. Using Preliminary Risk Analysis (PRA) to ensure safety in the preparation process for parenteral nutrition bags in hospital pharmacy. Pharm Technol Hospital Pharm. 2016;06 17.
17. Desroches A, Gatecel C. L’analyse préliminaire des risques: un outil adapté aux établissements de soins. Risques Qualité. 2006;3:3.
18. Desroches A, Baudrin D, Dadoun M. L’analyse préliminaire des risques. Principes et paratiques. Paris: Hermès; 2009.
19. Benjamin HH, Sotardi ST. The Pareto Principle. J Am College Radiol. 2018;15(6):931. doi:10.1016/j.jacr.2018.02.026
20. Palmero D, Di Paolo ER, Beauport L, et al. A bundle with a preformatted medical order sheet and an introductory course to reduce prescription errors in neonates. Eur J Pediatr. 2016;175(1):113–119. doi:10.1007/s00431-015-2607-4
21. Heloury J, Bouguéon G, Deljehier T, et al. Automation of aseptic sterile preparation: risk analysis and productivity comparison with manual process. Pharmeutical Technologie in Hospital Pharmacy. 2019;4(1):15–28. doi:10.1515/pthp-2019-0001
22. Bonnabry P, Despont-Gros C, Grauser D, et al. A risk analysis method to evaluate the impact of a computerized provider order entry system on patient safety. J Am Med Inform Assoc. 2008;15:453–460. doi:10.1197/jamia.M2677
23. Royer M. Controlling risks in the compounding process of individually formulated parenteral nutrition: use of the FMECA method (failure modes, effects, and criticality analysis). Pharm Technol Hospital Pharm. 2019;105–112.
24. Mourkogianni E, Karatza A, Vinni E, et al. Assessment and optimization of the pediatric parenteral nutrition preparation process in a hospital pharmacy. J Parenteral Enteral Nutr. 2020;44(5):928–939. doi:10.1002/jpen.1787
25. Palmero D, Di Paolo ER, Stadelmann C, et al. Incident reports versus direct observation to identify medication errors and risk factors in hospitalised newborns. Eur J Pediatr. 2019;178:259–266. doi:10.1007/s00431-018-3294-8
26. Boulé M, Lachapelle S, Collin-Lévesque L, et al. Failure mode, effect, and criticality analysis of the parenteral nutrition process in a mother-child hospital: the AMELIORE Study. Nutr Clin Practice. 2018;33(5):656–666. doi:10.1002/ncp.10094
27. Villafranca J, Arenas J. Using failure mode and effects analysis to improve the safety of neonatal parenteral nutrition. Am Society Health-System Pharmacists. 2014;71:1210–1218. doi:10.2146/ajhp130640
28. Hermanspann T, Schoberer M, Robel-Tillig E, et al. Incidence and severity of prescribing errors in parenteral nutrition for pediatric inpatients at a neonatal and pediatric intensive care unit. Front Pediatr. 2017;5. doi:10.3389/fped.2017.00149
29. Kochevar M. A.S.P.E.N. Statement on parenteral nutrition standardization. J Parenteral Enteral Nutr. 2007;31:5. doi:10.1177/0148607107031005441
30. Riskin A, Picaud J-C, Shamir R, et al. ESPGHAN/ESPEN/ESPR guidelines on pediatric parenteral nutrition: standard versus individualized parenteral nutrition. Clin Nutr. 2018;37:2409–2417. doi:10.1016/j.clnu.2018.06.955
31. Lapillonne A, Berleur M-P, Brasseur Y, et al. Safety of parenteral nutrition in newborns: results from a nationwide prospective cohort study. Clin Nutr. 2018;37(2):624–629. doi:10.1016/j.clnu.2017.02.002
32. Guenter P, Worthington P, Ayers P, et al. Standardized competencies for parenteral nutrition administration: the ASPEN Model. Nutr Clin Practice. 2018;33(2):295–304. doi:10.1002/ncp.10055
33. Nussbaumer S, Fleury-Souverain S, Bouchoud L, et al. Determination of potassium, sodium, calcium and magnesium in total parenteral nutrition formulations by capillary electrophoresis with contactless conductivity detection. J Pharm Biomed Anal. 2010;53(2):130–136. doi:10.1016/j.jpba.2010.01.042
34. Collins C, Krämer I. Evaluation of a process monitoring method for compounding parenteral nutrition with the Baxter EM2400 in a hospital pharmacy department. Pharm Technologie Hospital Pharmacy. 2017;2(3):107–115.
35. Stucki C, Sautter A-M, Favet J, et al. Microbial contamination of syringes during preparation: the direct influence of environmental cleanliness and risk manipulations on end-product quality. Am J Health-System Pharmacists. 2009;66:2032–2036. doi:10.2146/ajhp070681
 © 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2021 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
