Back to Journals » International Journal of Chronic Obstructive Pulmonary Disease » Volume 17
Parental COPD as a Risk Factor for the Development of COPD and Disease Severity in Offspring: A Systematic Scoping Review
Authors Sikjær MG, Klitgaard A , Hilberg O , Løkke A
Received 3 March 2022
Accepted for publication 30 April 2022
Published 8 June 2022 Volume 2022:17 Pages 1323—1338
DOI https://doi.org/10.2147/COPD.S364899
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Richard Russell
Melina Gade Sikjær,1,2 Allan Klitgaard,1,2 Ole Hilberg,1,2 Anders Løkke1,2
1Department of Medicine, Lillebaelt Hospital, Vejle, Denmark; 2Department of Regional Health Research, University of Southern Denmark, Odense, Denmark
Correspondence: Melina Gade Sikjær, Department of Regional Health Research, University of Southern Denmark, J.B.Winsløws vej 19, 3, Odense, 5000, Denmark, Email [email protected]
Background: There is sparse literature on parental chronic obstructive pulmonary disease (COPD) as a risk factor for the development of COPD in adult offspring, and the impact on disease severity. We aimed to map the literature reporting on the prevalence of and/or association between parental COPD and COPD in offspring, and to evaluate whether or not the literature reports on the severity of COPD or other health-related outcomes in offspring with parental COPD.
Methods: A systematic literature search in Embase and Ovid MEDLINE was performed in June 2021. Search terms revolved around COPD and predisposition.
Results: Thirteen studies were identified: 10 case–control studies, two cross-sectional studies and one cohort study. Population size varied from 44 to 2668 offspring cases; the distribution of female cases varied from 5% to 80% and mean age ranged from 27 to 65. Nine studies used an antecedents approach and evaluated the prevalence of parental COPD in patients with COPD, which ranged from 19% to 58%. Four studies used a descendants approach, by identifying patients with COPD and subsequently evaluated prevalence of COPD in their offspring, and found a prevalence of 0% to 17%. Apart from one, all the studies found an increased odds ratio for COPD in individuals with parental COPD. Four studies reported on parental smoking history and nine studies reported on smoking history in offspring. Three studies evaluated the association between parental COPD and COPD-related outcomes in patients with COPD.
Conclusion: This review indicates that parental COPD is associated with a higher risk of COPD in offspring. The literature is sparse, and we identified a knowledge gap on whether parental COPD is a risk factor for severe COPD and other health conditions in offspring.
Keywords: COPD severity, disease predisposition, familial predisposition, family history
Introduction
Chronic obstructive pulmonary disease (COPD) is one of the leading causes of morbidity and mortality worldwide, with an estimated prevalence of 10%.1–3 Although the etiology of COPD is not yet fully understood, there seems to be consensus that several environmental and genetic factors and their interactions are involved in its development.4 Tobacco smoking is the single most important risk factor for developing COPD, yet more than half of smokers do not develop COPD. This indicates that the susceptibility for developing COPD also depends on other environmental factors and genetic factors.5–7 Alpha-1-antitrypsin deficiency is the only well-established hereditary defect known to cause emphysema (among smokers).4 Several studies have investigated other possible genes that might alter the susceptibility and thereby increase the risk of developing COPD, but no consensus has been reached.
Recent research on lung function trajectories shows that low peak lung function in early adulthood increases the risk of COPD later in life and is associated with a higher prevalence of respiratory, cardiovascular, and metabolic comorbidities and premature death.8,9 A recently published meta-analysis10 found an association between risk of COPD in adulthood and early life factors, such as maternal smoking, low birth weight, childhood respiratory infections and childhood asthma.
Family history of COPD is often cited in the literature as a known risk factor for developing COPD,11–14 and can reflect both shared environmental exposures and genetics. The definition of family history, however, differs between studies and can include all first-degree relatives (parents, children and siblings). Parental and sibling COPD are often combined as the family history exposure,11,13 but it is important to distinguish between these. Parental and sibling COPD exposure both reflect genetics and shared environmental factors from the time of pregnancy and during childhood and adolescence, but siblingship also reflects different environmental exposures from the time siblings leave home. A systematic review from 201714 addressed parental COPD as a risk factor for developing COPD in adulthood and included eight studies, of which five were eligible for meta-analysis. They found a pooled prevalence of 29% for parental COPD in individuals with COPD, and an odds ratio (OR) for COPD in individuals with parental COPD of 1.57 (95% confidence interval (CI) 1.29–1.93), compared with individuals without COPD.
We hypothesize that parental COPD is a predisposing factor for the development of COPD in offspring and for more severe COPD and poor health outcome in adult offspring. Given the emerging interest in the origin of COPD and early life risk factors15 we expected new literature had been published since the review from 2017.14 A scoping review was found most suitable for an identification of the types of available evidence and possible knowledge gaps,16 given that there is a lack of studies identified in the review from 2017 designed to systematically assess parental COPD as a predisposing factor for developing COPD in offspring.14 The aim was to chart the literature on parental COPD as a predisposing factor for COPD in offspring and, secondly, to evaluate whether or not the literature reports on the severity of COPD or other health-related outcomes in offspring with parental COPD.
Methods
A research protocol was developed in accordance with the guidelines outlined in the Joanna Briggs Institute Manual for Evidence Synthesis17 and is available upon request from the corresponding author. Reporting followed the Preferred reporting items for systematic reviews and meta-analyses extension for scoping reviews (PRISMA-ScR) guidelines.18 The inclusion criteria for both parental and offspring COPD were broad, in order to also capture studies using definitions other than COPD. COPD was introduced in the International Classification of Diseases coding version 10 (ICD-10) in 1992, and definitions such as emphysema and chronic bronchitis (CB) were formerly used. We defined COPD as COPD, emphysema, CB, “chronic respiratory symptoms” or “chronic respiratory diseases”. No criteria on how COPD was diagnosed were applied; hence, the diagnosis could be based on spirometry, self-reported information or medical records. Studies that did not specify exposure as parental, maternal or paternal were excluded (eg, studies only reporting first-degree relatives). Studies evaluating genetics as an outcome or looking at specific familiar genetics (eg, alpha-1-antitrypsin deficiency) and studies investigating specific exposure groups (eg, occupational) were excluded. The search was not restricted by any time period, age range or geographical limitations. Studies written in languages other than English or Nordic languages were excluded. Only original reports were included.
An initial, limited search was conducted to identify relevant articles and extract key words related to these, to build the final search strings. A second search was then conducted, using the identified keywords and index terms. The search strategy was built up around 1) COPD and 2) predisposition, within Embase and Ovid MEDLINE, assisted by a research librarian. The final search was performed on 16 June 2021. (Tables S1 and S2 display the detailed search strategy.)
A two-stage search strategy was used to identify eligible studies. Duplicates were removed using a systematic review software program (Covidence),19 and two authors (MGS and AKS) reviewed the title and abstracts from all studies resulting from the search. The articles eligible for full-text review were then reviewed by MGS and AKS. Disagreements were solved by discussion and, if necessary, a third author (AL) made the final decision. Finally, the reference section in each included article was searched for relevant articles.
Study characteristics and results for each study included in the review were charted in predefined tables by MGS, with input from AKS and AL. The tables were structured so that the studies using an antecedents approach (ie, evaluating parental COPD in offspring with COPD) come first and the studies using a descendants approach (ie, identifying patients with COPD and subsequently evaluated prevalence of COPD in their offspring) come last. A crude OR was estimated in case–control studies, if OR was not presented in the study and data were available.
Results
Study Overview
After removing duplicates, 3780 studies were screened and 105 studies were found eligible for full-text review. In total, 13 studies were included in the review (Figure 1). The systematic search resulted in 11 publications; subsequently, two additional studies were included, following perusal of references. Moll et al20 used data from the COPDGene study and then replicated the study by using data from the ECLIPSE cohort. Results from the two analyses are displayed as two separate studies in the Results section (Tables 1–3).
 |  |  |
Table 1 Study Characteristics |
 |  |  |
Table 2 Study Outcomes and Results |
 |
Table 3 COPD-Related Outcomes in Offspring |
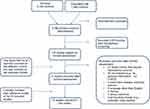 |
Figure 1 PRISMA flowchart of the search and inclusion process. Notes: PRISMA figure adapted from Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71. Creative Commons.45 |
Table 1 displays the characteristics of the included studies. The studies were conducted in USA,20–26 Europe,27–29 Asia30,31 and one was a multicentre study (ECLIPSE study).20 Six studies were published before 2010.21,23–25,29,30
Three studies used cross-sectional data from the COPDGene study.20,22,26 However, because these three studies had different aims and outcomes and, importantly, given that no synthesizing of estimates was conducted in the present scoping review, all three studies were included, as each contributes with different knowledge regarding parental COPD.
Study Methodology
All studies included in the review were observational (Table 1). The majority of studies used a case–control study design with cross-sectional data and only one cohort study27 and two cross-sectional studies25,29 were found. Assessing parental COPD as a predisposing factor for developing COPD in offspring was part of the aim in five studies.21–23,26,27 Nine studies used an antecedents approach and evaluated parental COPD in patients with COPD. Four studies used a descendant approach, in which the index-person was individuals with COPD (ie, parents with COPD) and cases were defined as offspring of the index-persons.24,25,30,31
COPD Diagnostic Criteria
We included studies with a broad range of COPD definitions (Table 1). Spirometry was included as part of the COPD diagnosis in offspring (cases) in all studies using the antecedents approach, aside from the cohort study. Parental COPD was identified by offspring reporting, and this was verified by, eg, spirometry or medical records in a few studies.21,29 The cohort study used ICD 7–10 coding in the national databases for both cases and parents.27
The case–control studies using a descendant approach firstly identified patients who were either hospitalized with COPD or identified in medical records, and then identified their offspring. Offspring were then invited to participate. Three studies24,30,31 used spirometry to identify offspring with abnormal lung function. The cross-sectional study used data from a cohort study where family members were interviewed regarding symptoms of CB.25
Population Characteristics
Study sizes varied considerably between studies (Table 2). The largest study was Moll et al,20 which included 2668 non-Hispanic white cases and 2506 non-Hispanic white controls from the COPDGene study. The smallest sample size was Silverman et al, with 44 cases and 20 controls.23 Mean age of cases were >45 years in all the studies using an antecedents approach (age not available in one study21) and 27–41 years in the studies using a descendant approach. The distribution of genders also varied greatly between studies; Lu et al30 included 95% male cases, whereas Silverman et al23 included 80% female cases (Table 2).
Parental COPD – Prevalence, Incidence and OR
Except for one,20 all studies with a reported or calculated OR found an increased risk of COPD in individuals with parental COPD (Table 2). The prevalence in case–control studies using the antecedents approach ranged from 19.3% to 58%. Three studies found highest prevalence among cases with paternal exposure,21,26,28 and one study among cases with maternal exposure.22 The prevalence in studies using the descendant approach ranged from 0% to 17%.
One study found a higher prevalence of parental COPD among non-Hispanic white cases (37.1%) compared to African-American cases (19.3%), but the ORs did not differ substantially; adjusted OR (non-Hispanic white) = 1.77 (1.55 to 2.03) and adjusted OR (African-American) = 1.71 (1.35–2.17).20 Two studies reported on the prevalence of parental COPD in cases with early-onset COPD, of which Foreman et al22 found almost the same prevalence in cases with maternal exposure (23%) and paternal exposure (21%). Cosio et al,28 however, found the highest prevalence among cases with paternal exposure (46%), compared to maternal exposure (21%).
One study reported a standard incidence ratio (SIR) of COPD in offspring with parental COPD in biological and adoptive parents, using data from the Swedish national registries.27 They found a higher SIR of COPD in offspring with parental COPD in biological parents (SIR = 1.98; 95% CI 1.69–2.31) than in offspring with parental COPD in adoptive parents (SIR = 1.12; 95% CI 0.92–1.35), with the latter SIR being statistically insignificant.
Four studies reported on parental smoking history22,26,29,30 and nine studies reported on smoking history in offspring.20,22,23,26,28–31 In most of these studies, cases were older and had more cumulative smoking exposure pack-years than controls. Hersh et al compared smoking COPD cases with smoking non-COPD controls and found parental COPD to be a significant risk factor for COPD in offspring (OR = 1.73; 95% CI 1.36–2.20) when adjusting for demographic variables, parental COPD, parental smoking, and childhood environmental tobacco smoke. They found an OR = 1.76 (95% CI 1.40–2.23) when only adjusting for demographic variables and parental COPD.
Severity of COPD and Other Health Outcomes in Offspring with Parental COPD
Three studies evaluated the association between parental COPD and COPD-related outcomes in patients with COPD. Table 3 summarizes the most assessed COPD-related outcomes between the three studies. Hersh et al26 found that cases with parental COPD had lower forced expiratory volume (FEV1), more dyspnea (mMRC), lower quality of life – based on St. George’s Respiratory Questionnaire (SGRQ) score, and higher number of severe exacerbations than COPD cases without parental COPD. Moll et al20 assessed the association of parental COPD with various COPD-related outcomes in offspring with COPD, using a prediction regression model. An association between the total SGRQ score and parental COPD (OR=3.6; 95% CI 1.4–5.8) was found in the ECLIPSE data. The COPDGene data showed an association between frequent exacerbations, decreased 6-minute walking distance (6MWD), increased SGRQ score, the body mass index, obstruction, dyspnoea, exercise capacity index (BODE index), and paraseptal emphysema and parental COPD in non-white Hispanic cases, whereas an association was only seen for SGRQ score, BODE and paraseptal emphysema in the African-American cases (Table 3).
No other studies evaluated the impact of parental COPD on COPD severity or other health outcomes. The studies using a descendant approach were not suitable to an assessment of the impact of parental COPD on COPD severity and health-related outcomes, because none of the controls had COPD or because information on parental status was missing.
Discussion
We conducted a systematic scoping review that included 13 studies assessing parental COPD exposure in adults with COPD. We found parental COPD to be associated with developing COPD in adulthood. Two studies found the association for early-onset COPD. Zoller et al, who used the Swedish national registries, found an association between COPD in adopted offspring and their biological parents, whereas no association was found between COPD in adopted offspring and their adoptee parents. This suggests that genetics and/or in utero exposures play an important role in the susceptibility for developing COPD in adulthood. Only one study, based on the ECLIPSE data, did not find an association between parental COPD and COPD in adulthood. This may be explained by the imbalance of controls (n = 147) compared with cases (n = 1713); however, sensitivity analyses did not alter the lack of association.20
We used a broad definition of COPD in order to map any studies that included parental COPD history. This resulted in heterogeneous studies with vast differences in the definition of COPD and obstructive airways. The methodologies, and particularly the characteristics of cases and controls, such as age, gender distribution and smoking history, varied greatly between studies.
We identified six studies that were not included in the review from 2017.14 Three of the studies were published later than the search conducted in 2015 by Li et al20,28 and three studies were published prior to 2015.21,22,30 The inclusion of the latter three studies in our review is likely due to broader inclusion criteria. One study published in 1988 was not included in our review because of Russian language.32 Li et al included one study evaluating early onset COPD.23 The study found parental COPD to be highly associated with early onset COPD. The present review adds to this knowledge by including two more studies that found increased risk of early onset COPD in offspring with parental COPD.22,28
Although smoking is the most common environmental exposure leading to COPD, adjustment for parental and own smoking history was absent in most studies assessing measures of association. One study adjusted for both parental smoking and childhood environmental tobacco smoke, and found significantly higher OR for parental COPD in smoking offspring with COPD compared to smoking offspring without COPD. These findings suggest an increased risk of COPD in offspring with parental COPD, independent of parental and personal smoking history, which supports genetic susceptibility as an important factor.26
All studies using an antecedent approach, apart from one,27 identified parental COPD history by offspring reporting and only one study used spirometry to confirm the diagnosis.29 Research shows that knowledge about COPD is limited in patients with COPD, and in particular in individuals without COPD.33 Therefore, the risk of recall bias and misclassification of parental COPD status by offspring reporting is highly likely. It is also likely that cases with COPD are more aware of whether their parents suffered the same disease as themselves in contrast to controls without COPD. This would lead to underreporting of exposure in the control group. Because COPD is vastly underdiagnosed worldwide and parents are not assessed with spirometry, it is also likely that a significant share of parents have undiagnosed COPD, which will contribute to an underestimation of parental COPD.34–36
Assessing Family History
COPD develops gradually and is often diagnosed late, when lung function impairment is substantial and damage to the lungs irreversible. Individuals with undiagnosed COPD have increased mortality and worse prognosis than individuals with normal lung function.34,37 The identification of high-risk populations and early diagnosis are imperative in order to prevent the development and progression of COPD.
Physicians seldom take into account family history of COPD when assessing individuals at risk of developing COPD. Our present review highlights a scarcity of longitudinal follow-up studies assessing parental COPD as a predisposing factor for developing COPD. We did not include other cohort studies, apart from Zoller et al, who evaluated the incidence rate of adult COPD using biological and adoptee parents as the exposure.
In contrast to COPD, family history assessment is an essential element in preventing other common chronic diseases, such as asthma, diabetes, heart disease and cancer, can aid identification of high-risk individuals and early diagnosis, and can motivate behavioral change.38–40 Life-course studies of common preventable chronic diseases, such as diabetes and cardiovascular disease, have for instance found that, in addition to preventable risk factors (eg, obesity and smoking), heredity is a significant factor in the development of disease. For that reason, heredity is an important element in the detection of individuals in high risk of diabetes and heart disease.41–43
Parental COPD and Early Signs of COPD in Offspring
The four studies using a descendent approach were not designed to identify an association between parental COPD and COPD in offspring, but rather familial aggregation of COPD. The studies identified offspring with a low mean age, and they were therefore not likely to have developed spirometry-identifiable airway obstruction, defined as FEV1/FVC <68–70% in the three studies using spirometry. Hence, two studies (of which one only included non-smoking offspring) found no airway obstruction in offspring. An older study (Khoury, 1985) did, however, find indications of a familial clustering of CB (OR >2.1) and airway obstruction (OR=6.4) among offspring with parental CB. These cross-sectional studies did not evaluate the development of lung function over time. This has been done in a 27-year follow-up study in individuals with a mean age of 41 and FEV1/FVC of 70–75%.44 Cases received more respiratory medicine and had higher rate of hospitalization for lung diseases, more contacts to general practice and lower income than individuals with FEV1/FVC >75%. This study suggests that preventative measures in young adults with early signs of obstructive airways may reduce respiratory morbidity later in life.44
COPD Severity and Other Health Conditions
Low peak lung function in early adulthood increases the risk of COPD later in life and is associated with a higher prevalence of respiratory, cardiovascular, and metabolic comorbidities8,9 Therefore, we hypothesized that parental COPD is a prognostic factor for a worse course of COPD and poor general health outcomes in adult offspring with COPD. However, the review revealed a vast knowledge gap on this topic. Only three studies evaluated COPD-related outcomes in offspring and no studies reported on other health-related outcomes. Hersh et al was the only study with the aim of assessing the impact of parental COPD on the severity of COPD in offspring. That study found parental COPD to be associated with lower lung function, greater dyspnea, higher share of severe exacerbations and lower quality of life at baseline. Moll et al evaluated the predictive performance of a prognostic model and likewise found parental COPD to be associated with several COPD-related outcomes. However, cross-sectional studies cannot assess how disease burden and other health-care outcomes (eg, comorbidities) vary from the time of diagnosis and onwards. Thus, it is still unclear whether assessing parental COPD history can be used to identify individuals in risk of worse course of COPD and generally worse health outcomes.
Strengths and Limitations
This scoping review has several strengths. The search strategy was assisted by an academic librarian, the reviewing process was conducted independently by two authors, and the reference review included extra studies that were not captured by the search. There are some limitations: the search was restricted to English and Nordic languages, and some articles in other languages may have been missed. This was a scoping review and no quality assessment of the studies was performed.16,17 Therefore, the results on prevalence and OR drawn from this scoping review must be interpreted carefully. We excluded studies that combined sibling and parental COPD or solely evaluated sibling COPD as the exposure; thus, the results do not provide information on the share of siblings with COPD, which would be valuable information when assessing the impact of parental COPD on offspring.
Conclusion
The literature on the association between parental COPD and COPD in offspring is sparse. The studies vary vastly in design and population characteristics, which makes comparison of results difficult. We do not currently find grounds for a systematic review and meta-analysis based on this scoping review. Only eight studies would be eligible (ie, studies using an antecedents approach), and three of these studies use COPDGene data with overlapping cases, which makes a synthesis of the data problematic.
We identified a lack of follow-up studies, and in particular follow-up studies that assess whether parental COPD is a risk factor for worse course of COPD and other health-related outcomes.
More recent publications with spirometry confirmed diagnosis, more uniform definitions of COPD and elaboration on environmental exposures are lacking. Population-based studies designed to evaluate parental COPD as a risk factor for COPD in adulthood are called for, and, importantly, follow-up studies evaluating the health-related consequences of parental COPD.
Nevertheless, the studies included in this scoping review indicate that parental COPD is associated with a higher risk of COPD in offspring. Thus, based on the current literature, evaluating parental COPD history may aid in early detection of individuals in high risk of COPD.
Abbreviations
A1AT deficiency, alpha-1 antitrypsin deficiency; AO, airway obstruction; BODE index, body mass index, obstruction, dyspnoea and exercise capacity; CB, chronic bronchitis; CI, confidence intervals; COPD, chronic obstructive pulmonary disease; FEV1, forced expiratory volume in 1 second; FVC, forced vital capacity; GOLD, global initiative for chronic obstructive lung disease; Kco, carbon monoxide transfer coefficient; LF, lung function; LLN, lower limit of normal; mMRC, modified Medical Research Council; NA, not available; OR, odds ratio; PB-FEV1, post-bronchodilator FEV1; PY, pack-years SGRQ, total St. George’s Respiratory Questionnaire score; SIR, standardized incidence rate; 6MWD, 6-minute walking test.
Ethical Approval
The study did not involve human participants, and ethical approval was not required.
Acknowledgment
The authors thank research librarian Sebrina Maj-Britt Hansen from the University Library of Southern Denmark, for her assistance with the search strategy.
Author Contributions
MGS is the guarantor of the work. MGS, AL, AK and OH contributed with the conception of the study. MGS extracted data, summarized the articles, prepared the first draft of the manuscript, and created the tables and figure. MGS and AK conducted the systematic search and literature review. AK, AL and OH critically revised the manuscript. All authors revised the manuscript and accepted the final version and agreed to publish the article and have agreed on the journal to which the article has been submitted. All authors take responsibility and are accountable for the integrity of the work as a whole, from inception to published article.
Funding
The study was supported by an unrestricted grant from the Danish Lung Association’s Fund. The funder was not involved in any way during the conduct of the research project.
Disclosure
Dr Melina Gade Sikjær reports grants from Danish Lung Association’s Fund, during the conduct of the study. The authors report no other conflicts of interest in this work.
References
1. Rabe KF, Watz H. Chronic obstructive pulmonary disease. Lancet. 2017;389(10082):1931–1940. doi:10.1016/s0140-6736(17)31222-9
2. Roth G, Abate D, Abate K. Global, regional, and national age-sex-specific mortality for 282 causes of death in 195 countries and territories, 1980–2017: a systematic analysis for the Global Burden of Disease Study 2017. Lancet. 2018;392(10159):1736–1788. doi:10.1016/s0140-6736(18)32203-7
3. Celli BR, Wedzicha JA, Drazen JM. Update on clinical aspects of chronic obstructive pulmonary disease. N Engl J Med. 2019;381(13):1257–1266. doi:10.1056/NEJMra1900500
4. Agusti A, Hogg JC. Update on the pathogenesis of chronic obstructive pulmonary disease. N Engl J Med. 2019;381(13):1248–1256. doi:10.1056/NEJMra1900475
5. Lokke A, Lange P, Scharling H, Fabricius P, Vestbo J. Developing COPD: a 25 year follow up study of the general population. Thorax. 2006;61(11):935–939. doi:10.1136/thx.2006.062802
6. Lundback B, Lindberg A, Lindstrom M, et al. Not 15 but 50% of smokers develop COPD?–Report from the obstructive lung disease in Northern Sweden Studies. Respir Med. 2003;97(2):115–122. doi:10.1053/rmed.2003.1446
7. Kohansal R, Martinez-Camblor P, Agustí A, Buist AS, Mannino DM, Soriano JB. The natural history of chronic airflow obstruction revisited: an analysis of the Framingham offspring cohort. Am J Respir Crit Care Med. 2009;180(1):3–10. doi:10.1164/rccm.200901-0047OC
8. Lange P, Celli B, Agustí A, et al. Lung-function trajectories leading to chronic obstructive pulmonary disease. N Engl J Med. 2015;373(2):111–122. doi:10.1056/NEJMoa1411532
9. Agustí A, Noell G, Brugada J, Faner R. Lung function in early adulthood and health in later life: a transgenerational cohort analysis. Lancet Respir Med. 2017;5(12):935–945. doi:10.1016/s2213-2600(17)30434-4
10. Duan P, Wang Y, Lin R, et al. Impact of early life exposures on COPD in adulthood: a systematic review and meta-analysis. Respirology. 2021;26(12):1131–1151. doi:10.1111/resp.14144
11. Hooper R, Burney P, Vollmer WM, et al. Risk factors for COPD spirometrically defined from the lower limit of normal in the BOLD project. Eur Respir J. 2012;39(6):1343–1353. doi:10.1183/09031936.00002711
12. Nihlen U, Nyberg P, Montnemery P, Lofdahl CG. Influence of family history and smoking habits on the incidence of self-reported physician’s diagnosis of COPD. Respir Med. 2004;98(3):263–270. doi:10.1016/j.rmed.2003.10.006
13. Montnemery P, Lanke J, Lindholm LH, et al. Familial related risk-factors in the development of chronic bronchitis/emphysema as compared to asthma assessed in a postal survey. Eur J Epidemiol. 2000;16(11):1003–1007. doi:10.1023/a:1011004420173
14. Li LS, Paquet C, Johnston K, Williams MT. “What are my chances of developing COPD if one of my parents has the disease?” A systematic review and meta-analysis of prevalence of co-occurrence of COPD diagnosis in parents and offspring. Int J Chron Obstruct Pulmon Dis. 2017;12:403–415. doi:10.2147/copd.S123933
15. Postma DS, Bush A, van den Berge M. Risk factors and early origins of chronic obstructive pulmonary disease. Lancet. 2015;385(9971):899–909. doi:10.1016/s0140-6736(14)60446-3
16. Munn Z, Peters MDJ, Stern C, Tufanaru C, McArthur A, Aromataris E. Systematic review or scoping review? Guidance for authors when choosing between a systematic or scoping review approach. BMC Med Res Methodol. 2018;18(1):143. doi:10.1186/s12874-018-0611-x
17. Peters MD, McInerney P, Munn Z, Tricco AC, Khalil H. Chapter 11: scoping Reviews (2020 version). Secondary Chapter 11: scoping Reviews (2020 version); 2020. Available from: https://synthesismanual.jbi.global.
18. Tricco AC, Lillie E, Zarin W, et al. PRISMA extension for scoping reviews (PRISMA-ScR): checklist and explanation. Ann Intern Med. 2018;169(7):467–473. doi:10.7326/m18-0850
19. Veritas Health Innovation M, Australia. Covidence systematic review software. Available from www.covidence.org.
20. Moll M, Lutz SM, Ghosh AJ, et al. Relative contributions of family history and a polygenic risk score on COPD and related outcomes: cOPDGene and ECLIPSE studies. BMJ Open Respir Res. 2020;7(1):e000755. doi:10.1136/bmjresp-2020-000755
21. Kueppers F, Miller RD, Gordon H, Hepper NG, Offord K. Familial prevalence of chronic obstructive pulmonary disease in a matched pair study. Am J Med. 1977;63(3):336–342. doi:10.1016/0002-9343(77)90270-4
22. Foreman MG, Zhang L, Murphy J, et al. Early-onset chronic obstructive pulmonary disease is associated with female sex, maternal factors, and African American race in the COPDGene Study. Am J Respir Crit Care Med. 2011;184(4):414–420. doi:10.1164/rccm.201011-1928OC
23. Silverman EK, Chapman HA, Drazen JM, et al. Genetic epidemiology of severe, early-onset chronic obstructive pulmonary disease. Risk to relatives for airflow obstruction and chronic bronchitis. Am J Respir Crit Care Med. 1998;157(6 Pt 1):1770–1778. doi:10.1164/ajrccm.157.6.9706014
24. Khoury MJ, Beaty TH, Tockman MS, Self SG, Cohen BH. Familial aggregation in chronic obstructive pulmonary disease: use of the log linear model to analyze intermediate environmental and genetic risk factors. Genet Epidemiol. 1985;2(2):155–166. doi:10.1002/gepi.1370020206
25. Higgins M, Keller J. Familial occurrence of chronic respiratory disease and familial resemblance in ventilatory capacity. J Chronic Dis. 1975;28(4):239–251. doi:10.1016/0021-9681(75)90053-3
26. Hersh CP, Hokanson JE, Lynch DA, et al. Family history is a risk factor for COPD. Chest. 2011;140(2):343–350. doi:10.1378/chest.10-2761
27. Zoller B, Li X, Sundquist J, Sundquist K. Familial transmission of chronic obstructive pulmonary disease in adoptees: a Swedish nationwide family study. BMJ open. 2015;5(4):e007310. doi:10.1136/bmjopen-2014-007310
28. Cosío BG, Pascual-Guardia S, Borras-Santos A, et al. Phenotypic characterisation of early COPD: a prospective case-control study. ERJ Open Res. 2020;6(4):00047–2020. doi:10.1183/23120541.00047-2020
29. McCloskey SC, Patel BD, Hinchliffe SJ, Reid ED, Wareham NJ, Lomas DA. Siblings of patients with severe chronic obstructive pulmonary disease have a significant risk of airflow obstruction. Am J Respir Crit Care Med. 2001;164(8 Pt 1):1419–1424. doi:10.1164/ajrccm.164.8.2105002
30. Lu B, He Q. Correlation of pulmonary functions of COPD patients to those of their first-degree children. Chin Med J. 2003;116(7):991–995.
31. Amra B, Borougeni VB, Golshan M, Soltaninejad F. Pulmonary function tests and impulse oscillometry in severe chronic obstructive pulmonary disease patients’ offspring. J Res Med Sci. 2015;20(7):697–700. doi:10.4103/1735-1995.166229
32. Mostovoĭ IuM. Kliniko-geneticheskie issledovaniia pri khronicheskom bronkhite [Clinico-genetic research in chronic bronchitis]. Ter Arkh. 1988;60(3):52–55. Russian.
33. Goldman RE, Mennillo L, Stebbins P, Parker DR. How do patients conceptualize chronic obstructive pulmonary disease? Chron Respir Dis. 2017;14(3):245–255. doi:10.1177/1479972316680845
34. Martinez CH, Mannino DM, Jaimes FA, et al. Undiagnosed obstructive lung disease in the United States. Associated factors and long-term mortality. Ann Am Thorac Soc. 2015;12(12):1788–1795. doi:10.1513/AnnalsATS.201506-388OC
35. Lindberg A, Jonsson AC, Rönmark E, Lundgren R, Larsson LG, Lundbäck B. Prevalence of chronic obstructive pulmonary disease according to BTS, ERS, GOLD and ATS criteria in relation to doctor’s diagnosis, symptoms, age, gender, and smoking habits. Respiration. 2005;72(5):471–479. doi:10.1159/000087670
36. Lamprecht B, Soriano JB, Studnicka M, et al. Determinants of underdiagnosis of COPD in national and international surveys. Chest. 2015;148(4):971–985. doi:10.1378/chest.14-2535
37. Çolak Y, Afzal S, Nordestgaard BG, Vestbo J, Lange P. Prognosis of asymptomatic and symptomatic, undiagnosed COPD in the general population in Denmark: a prospective cohort study. Lancet Respir Med. 2017;5(5):426–434. doi:10.1016/s2213-2600(17)30119-4
38. Wilson BJ, Qureshi N, Santaguida P, et al. Systematic review: family history in risk assessment for common diseases. Ann Intern Med. 2009;151(12):878–885. doi:10.7326/0003-4819-151-12-200912150-00177
39. Berg AO, Baird MA, Botkin JR, et al. National institutes of health state-of-the-science conference statement: family history and improving health. Ann Intern Med. 2009;151(12):872–877. doi:10.7326/0003-4819-151-12-200912150-00165
40. Li LS, Williams MT, Johnston KN, Frith P, Hyppönen E, Paquet C. Parental and life-course influences on symptomatic airflow obstruction. ERJ Open Res. 2020;6(1). doi:10.1183/23120541.00343-2019
41. Meigs JB, Cupples LA, Wilson PW. Parental transmission of type 2 diabetes: the Framingham offspring study. Diabetes. 2000;49(12):2201–2207. doi:10.2337/diabetes.49.12.2201
42. Williams RR, Hunt SC, Heiss G, et al. Usefulness of cardiovascular family history data for population-based preventive medicine and medical research (the Health Family Tree Study and the NHLBI Family Heart Study). Am J Cardiol. 2001;87(2):129–135. doi:10.1016/s0002-9149(00)01303-5
43. Lloyd-Jones DM, Nam BH, D’Agostino RB
44. Ørts LM, Bech BH, Lauritzen T, Carlsen AH, Sandbæk A, Løkke A. Lung function in adults and future burden of obstructive lung diseases in a long-term follow-up. NPJ Prim Care Respir Med. 2020;30(1):10. doi:10.1038/s41533-020-0169-z
45. Page MJ, McKenzie JE, Bossuyt PM, et al. The PRISMA 2020 statement: an updated guideline for reporting systematic reviews. BMJ. 2021;372:n71.
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
