Back to Journals » Biologics: Targets and Therapy » Volume 11
Paradoxical SAPHO syndrome observed during anti-TNF-α therapy for Crohn's disease
Authors Amano H, Matsuda R, Shibata T, Takahashi D, Suzuki S
Received 11 February 2017
Accepted for publication 30 March 2017
Published 22 May 2017 Volume 2017:11 Pages 65—69
DOI https://doi.org/10.2147/BTT.S134508
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Doris Benbrook
Hitoshi Amano,1 Reikei Matsuda,1 Tomohiko Shibata,2 Daisuke Takahashi,1 Shinichiro Suzuki3
1Department of Gastroenterology, Fujisawa Shonandai Hospital, Fujisawa, 2Division of Rheumatology, Department of Internal Medicine, St Marianna University School of Medicine, Yokohama City Seibu Hospital, Yokohama, 3Department of Surgery, Fujisawa Shonandai Hospital, Fujisawa, Japan
Abstract: Currently, anti-TNFα antibodies are used to treat Crohn’s disease. We report on a 45-year-old Japanese female with Crohn’s disease developing SAPHO (synovitis, acne, pustulosis, hyperostosis, and osteitis) syndrome following exposure to the anti-TNFα antibody adalimumab. Initially, adalimumab induced remission, but the patient showed SAPHO syndrome 11 weeks following the start of adalimumab therapy for the first time. Cutaneous and articular involvement were exacerbating the condition, so adalimumab was discontinued and the patient was put on low-dose methotrexate to control her symptoms. To our knowledge, this is the first report of SAPHO syndrome occurring during anti-TNF therapy, which is thought to be a paradoxical response to adalimumab.
Keywords: anti-TNFα, adalimumab, paradoxical reaction, SAPHO syndrome, Crohn’s disease
Introduction
SAPHO (synovitis, acne, pustulosis, hyperostosis, and osteitis) syndrome is a rare inflammatory multiorgan syndrome involving the skin, bones, and joints. The annual prevalence of SAPHO syndrome is reported to be around one to 40 per 100,000.1,2 The etiology of SAPHO syndrome is not fully understood, but nonsteroidal anti-inflammatory drugs (NSAIDs), antibiotics, bisphosphonates, colchicines, corticosteroids, disease-modifying antirheumatic drugs, TNFα inhibitors, and even IL1-receptor antagonists have been used to treat SAPHO syndrome.3,4
Currently, anti-TNFα biologics, including infliximab and adalimumab, are widely used to treat inflammatory bowel diseases (IBDs), which include Crohn’s disease (CD), and paradoxical events like psoriasis are not uncommon observations during anti-TNF therapy.5–7 The paradox is that anti-TNF biologics have shown efficacy in patients with psoriasis.8 Here, we report the first case of SAPHO syndrome occurring just after remission induction by the anti-TNFα antibody adalimumab. Paradoxical reaction to the biologic rather than a coincidental event was assumed.
Case report
A 45-year-old Japanese female was hospitalized due to severe abdominal discomfort, bloody diarrhea, arthritis in the limbs, nodular erythema, and high fever. She had no particular history or family history of inflammatory episodes. Despite upper endoscopy and small-bowel follow-through showing no obvious abnormalities, ileocolonoscopy revealed deep discrete longitudinal ulcers accompanied by cobblestone appearance in the colon, together with severe anal fissures. Noncaseating granuloma was detected in the biopsies from the colonic mucosa. The patient was diagnosed with CD, with colonic involvement and extraintestinal joint manifestation. Because she had a complicating anal lesion and had left her disabled child behind, we decided to treat her with an anti-TNFα antibody, hoping to shorten her hospital stay. She received subcutaneous adalimumab: 160 mg at week 0, 80 mg at week 2, and thereafter 40 mg every 2 weeks. Her symptoms improved, and the patient was discharged. Sulfasalazine was stopped after the discharge, due to signs of pancreatitis associated with sulfasalazine. After the fifth adalimumab shot, she visited our outpatient clinic with complaints of a tender shoulder and left clavicle and acne spreading over her trunk, limbs, and face (Figure 1A). Two weeks later, both submandibular saliva glands were swollen and tender. She had low-grade fever and could not raise her arms, due to unbearable pain in the bilateral acromioclavicular joints. Her anterior chest pain was painful in the sternoclavicular, and sternocostal joints.
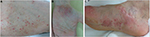  | Figure 1 (A) Acne in right lower limb, which spread to patient’s face, limbs, and trunk; (B, C) palmoplantar pustulosis. |
Laboratory tests showed elevated CRP of 0.73 mg/dL, serum amylase of 248 IU/L, and erythrocyte-sedimentation rate of 40 mm/hour without elevated white blood-cell count. Serum antinuclear antibody, anticyclic citrullinated peptide antibody, rheumatoid factor, and anti-Sjögren’s syndrome A and B antibodies were negative and IgG4 was within the normal range. Similarly, human leukocyte antigen B27 blood culture, procalcitonin, 1,3-β-D-glucan, and IFNγ-release assays (QuantiFeron®-TB Gold; Quest Diagnostics, Madison, NJ, US) were negative. Further, because NSAIDs showed inadequate efficacy, we added 20 mg/day prednisolone orally, but the syndrome reappeared when the dose of prednisolone was reduced to 15 mg/day. Additionally, the pain in her low back was diagnosed to be bilateral sacroiliitis. Oral minocycline and corticosteroid ointment seemed to be effective on acne, but ineffective on other symptoms.
Because we had assumed that her cutaneous, bone, and joint manifestations were adverse effects of adalimumab, the anti-TNF was discontinued after the fifth shot, but her cutaneous and articular symptoms continued to exacerbate. Ileocolonoscopy was undertaken again, and showed mucosal healing in the colon and at the anal lesion. Fourteen weeks after the cessation of adalimumab, pustulosis appeared on her palms and soles. The patient was diagnosed to have developed cutaneous lesions like acne and palmoplantar pustulosis, together with articular features like anterior chest pain and sacroiliitis, which appeared after the administration of adalimumab and were consistent with SAPHO syndrome (Figure 1B and C). She was referred to a rheumatologist in a tertiary hospital and received radiological examination, which supported the diagnosis of SAPHO syndrome. Computerized tomography showed bone erosions with edema in the bilateral sternoclavicular joints (Figure 2). Additionally, bone-scintigraphy findings showed extensive uptake of radiopharmaceutical 99mTc at the sternoclavicular joints and sternum, which is called a “bull’s head” sign (Figure 3). Intensive uptake was also observed in the bilateral sacroiliac joints. She started receiving low-dose methotrexate (6 mg per week), which did not induce adequate efficacy; it was increased to 12 mg/week 3 months later to induce and maintain clinical remission.
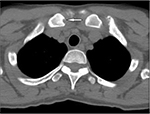  | Figure 2 Computed tomography scan of clavicles. Erosion in right clavicle (white arrow). |
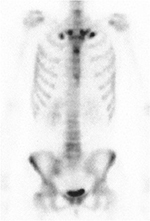  | Figure 3 Bone-scintigraphy findings showed intensive uptake of 99mTc at the sternoclavicular joints and sternum, called a “bull’s head” sign. |
Ethical considerations
Written informed consent was provided by the patient after explaining the purpose, the nature of the treatments, and the procedures involved. Further, she agreed to the use of the information, including all images to appear in this case report, stating that she fully understood this journal to be a publication that is freely accessible through the Internet. The patient’s anonymity should be protected in this report.
Discussion
Synovitis, acne, pustulosis, hyperostosis, and osteitis form the acronym “SAPHO”.9 Aseptic osteitis, mainly in the anterior chest wall, accompanied by cutaneous manifestations like acne and psoriatic lesions are the known morbidities.3 The diagnostic criteria described by Benhamou et al10 are frequently used by physicians in clinical practice settings. However, SAPHO syndrome has been thought to be a multifactorial phenomenon comprising genetic susceptibility, infection, and immune malfunction.11–13
More recently, SAPHO syndrome was categorized to be seronegative spondyloarthropathy and occasionally overlapped with diseases like psoriasis and IBD.3,14,15 Naves et al16 reported three patients in an IBD cohort of 1,309 (0.2%) had SAPHO syndrome from their hospital database. In their study, they undertook a systematic literature review of SAPHO to see its association with IBD. Their literature review showed that ~60% of patients who had both SAPHO syndrome and IBD were females, and the average interval for the occurrence of the two diseases was 8.5 years (range: 3–20 years) from the time when IBD was first diagnosed. CD accounted for 69% of cases, in which 79% had colonic involvement.16 Although the clinical features described by Naves et al16 are in line with our observations, in this patient, SAPHO syndrome occurred just after the patient’s CD appeared to have responded to adalimumab, which has been used to treat SAPHO syndrome as well.
It is also appropriate to mention here that psoriasis, systemic lupus erythematosus, and sarcoidosis are viewed as anti-TNFα-induced morbidities or paradoxical phenomena.7,14,17 Further, most cases of psoriasis in IBD patients under anti-TNF therapy are considered paradoxical reactions for the fact that anti-TNF biologics have shown efficacy in non-IBD patients with psoriasis.8 In line with this assertion, Guerra et al7 found 125 psoriasis cases from a total of 7,415 patients treated with TNFα inhibitors for IBD. In fact, all of the TNFα inhibitors available on the market are reported to have the potential to cause psoriasis-like adverse cutaneous side effects.5,8 Therefore, topical treatment without discontinuing the biologic is given priority over withdrawing the biologic in IBD cases, which become complicated by psoriasis, unless the cutaneous lesions are severe enough to impair patient’s quality of life seriously. According to a systematic literature review14 and a cohort study7 of psoriasis cases associated with anti-TNFα therapy in IBD patients, recurrence or aggravation of psoriasis were seen in 60%–72% of patients when switched from one anti-TNFα inhibitor causing psoriasis to another anti-TNFα. Therefore, there was no evidence in favor of switching to another anti-TNFα when SAPHO syndrome occurred in this case.
Our patient’s SAPHO manifestation was exacerbated even after withdrawal of adalimumab. The rheumatologist had to start methotrexate, which was added to NSAIDs, but the dose of methotrexate had to be increased, due to unremitting joint and bone manifestations. Further, in situations when a TNFα inhibitor has been continued after occurrence of psoriasis, it was because the underlying IBD was seen to be responding well. In line with this assertion, we reviewed 12 case reports, and found that all cases (18 of 18 patients) with IBD were in remission or responding favorably to anti-TNF biologics when the psoriasis was first observed.18–29
Apart from anti-TNF biologic-induced psoriatic skin lesions, in a small fraction of IBD patients, the underlying IBD is complicated by psoriasis.14,30 Denadai et al14 found the incidence of psoriasis among IBD patients to be up to 11%, although it is only 1.5% in the general population.14 The same genes (IL23R, IL12B, TYK2) are thought to predispose to both IBD and psoriasis.14 One may assume that IBD patients bear another gene or genes that predispose them to anti-TNF induced psoriasis, which is a common paradoxical reaction after biologics.31 However, we believe that an understanding of the association of psoriasis with IBD, and anti-TNF biologic-induced psoriasis as a paradoxical reaction could at least in part explain the paradoxical SAPHO syndrome during anti-TNF treatment in the present CD case. Bilateral inflammation of the submandibular glands seen with concomitant acne and articular involvement might also have been caused by adalimumab. Therefore, we worked with reputable rheumatologists to understand the SAPHO syndrome in our CD patient better, related to IBD or to the biologic.
To determine whether or not there had been case reports of SAPHO syndrome associated with an anti-TNFα inhibitor, we searched PubMed with Medical Subject Headings, with the terms “Crohn’s disease, anti-TNF-α, adalimumab, infliximab, etanercept, certolizumab, and golimumab combined with SAPHO syndrome”, limited to articles in English. We found no report of paradoxical reaction caused by a TNFα inhibitor. We also undertook a search for “SAPHO syndrome and Crohn’s disease” and found 14 relevant articles, but none reporting a diagnosis of SAPHO syndrome following anti-TNFα therapy. Therefore, this appears to be the first case of a paradoxical reaction to adalimumab. Similarly, the precise mechanism(s) for paradoxical reactions to anti-TNFα biologics are currently not well understood. Nonetheless, our extensive review of the relevant published work suggests that an imbalance in cytokine interactions triggers a cascade of events leading to a paradoxical phenomenon as an adverse reaction to a drug, which triggered the events. More specifically, for paradoxical psoriasis, a shift toward a TH1 cytokine profile or an uncontrolled release of IFNα has been hypothesized. Indeed, it has been reported that during anti-TNFα therapy, there is an excess IFNα release by plasmacytoid dendritic cells. IFNα promotes expression of CXCR3 on T cells, favoring T-cell homing in the skin.32
Conclusion
Here, we report the first case of SAPHO syndrome occurring during adalimumab therapy in a patient with colonic CD. We undertook multiple laboratory tests to exclude other or similar morbidities seen in CD patients. Accordingly, the SAPHO syndrome in our CD case was unlikely to be a coincidental event, but was associated with the anti-TNF biologic we used to treat the patient’s CD. Cessation of adalimumab administration was not followed by disappearance of the SAPHO features, and thus switching to another anti-TNF biologic was unlikely to benefit the patient’s CD.
Disclosure
The authors report no conflicts of interest in this work.
References
McPhillips A, Wolford LM, Rodrigues DB. SAPHO syndrome with TMJ involvement: review of the literature and case presentation. Int J Oral Maxillofac Surg. 2010;39(12):1160–1167. | ||
Takigawa T, Tanaka M, Nakanishi K, et al. SAPHO syndrome associated spondylitis. Eur Spine J. 2008;17(10):1391–1397. | ||
Rukavina I. SAPHO syndrome: a review. J Child Orthop. 2015;9(1):19–27. | ||
Garcovich S, Amelia R, Magarelli N, Valenza V, Amerio P. Long-term treatment of severe SAPHO syndrome with adalimumab: case report and a review of the literature. Am J Clin Dermatol. 2012;13(1):55–59. | ||
Wollina U, Hansel G, Koch A, Schönlebe J, Köstler E, Haroske G. Tumor necrosis factor-alpha inhibitor-induced psoriasis or psoriasiform exanthemata: first 120 cases from the literature including a series of six new patients. Am J Clin Dermatol. 2008;9(1):1–14. | ||
Iborra M, Beltrán B, Bastida G, Aquas M, Nos P. Infliximab and adalimumab-induced psoriasis in Crohn’s disease: a paradoxical side effect. J Crohns Colitis. 2011;5(2):157–161. | ||
Guerra I, Pérez-Jeldres T, Iborra M, et al. Incidence, clinical characteristics, and management of psoriasis induced by anti-TNF therapy in patients with inflammatory bowel disease: a nationwide cohort study. Inflamm Bowel Dis. 2016;22(4):894–901. | ||
Zweegers J, Roosenboom B, van de Kerkhof PC, et al. Frequency and predictors of a high clinical response in psoriasis patients on biologic therapy in daily practice. Br J Dermatol. 2017;176(3):786–793. | ||
Chamot AM, Benhamou CL, Kahn MF, Beraneck L, Kaplan G, Prost A. Le syndrome acné pustulose hyperostose ostéïte (SAPHO). Réultats d’une enquête nationale: 85 observations. [Acne-pustulosis-hyperostosis-osteitis syndrome: results of a national survey – 85 cases]. Rev Rhum Mal Osreoartic. 1987;54(3):187–196. French. | ||
Benhamou CL Chamot ML, Kahn MF. Synovitis-acne-pustulosis hyperostosis-osteomyelitis syndrome (SAPHO): a new syndrome among the spondyloarthropathies? Clin Exp Rheumatol. 1988;6(2):109–112. | ||
Hurtado-Nedelec M, Chollet-Martin S, Nicaise-Roland P, et al. Characterization of the immune response in the synovitis, acne, pustulosis, hyperostosis, osteitis (SAPHO) syndrome. Rheumatology (Oxford). 2008;47(8):1160–1167. | ||
Assmann G, Simon P. The SAPHO syndrome: are microbes involved? Best Pract Res Clin Rheumatol. 2011;25(3):423–434. | ||
Burgemeister LT, Baeten DL, Tas SW. Biologics for rare inflammatory diseases: TNF blockade in the SAPHO syndrome. Neth J Med. 2012;70(10):444–449. | ||
Denadai R, Teixeira FV, Steinwurz F, Romiti R, Saad-Hossbe R. Induction or exacerbation of psoriatic lesions during anti-TNF-α therapy for inflammatory bowel disease: a systematic literature review based on 222 cases. J Crohns Colitis. 2013;7(7):517–524. | ||
Nguyen MT, Borchers A, Selmi C, Naguwa SM, Cheema G, Gershwin ME. The SAPHO syndrome. Semin Arthritis Rheum. 2012;42(3):254–265. | ||
Naves JE, Cabré E, Maňosa M, Grados D, Olivé A, Domènech E. A systematic review of SAPHO syndrome and inflammatory bowel disease association. Dig Dis Sci. 2013;58(8):2138–2147. | ||
Fiorino G, Allez M, Malesci A, Danese S. Review article: anti TNF-α induced psoriasis in patients with inflammatory bowel disease. Aliment Pharmacol Ther. 2009;29(9):921–927. | ||
Severs GA, Lawlor TH, Purcell SM, Adler JA, Thompson R. Cutaneous adverse reaction to infliximab: report of psoriasis developing 3 patients. Cutis. 2007;80(3):231–237. | ||
Peramiquel L, Puig L, Dalmau J, Ricart E, Roe E, Alomar A. Onset of flexural psoriasis during infliximab treatment for Crohn’s disease. Clin Exp Dermatol. 2005;30:713–714. | ||
Pirard D, Arco D, Debrouckere V, Heenen M. Anti-tumor necrosis factor α-induced psoriasiform eruptions: three further cases and current overview. Dermatology. 2006;213(3):182–186. | ||
Takahashi H, Hashimoto Y, Ishida-Yamamoto A, Ashida T, Kohgo Y, Iizuka H. Psoriasiform and pustular eruption induced by infliximab. J Dermatol. 2007;34(7):468–472. | ||
Umeno J, Matsumoto T, Jo Y, Ichikawa M, Urabe K, Iida M. Psoriasis during anti-tumor necrosis factor-α therapy for Crohn’s disease. Inflamm Bowel Dis. 2007;13(9):1188–1189. | ||
Sladden MJ, Clarke PJ, Wettenhall J. Infliximab-induced palmoplantar pustulosis in a patient with Crohn disease. Arch Dermatol. 2007;143(11):1449. | ||
English PL, Vender R. Occurrence of plantar pustular psoriasis during treatment with infliximab. J Cutan Med Surg. 2009;13(1):40–42. | ||
Manni E, Barachini P. Psoriasis induced by infliximab in a patient suffering from Crohn’s disease. Int J Immunopathol Pharmacol. 2009;22(3):841–844. | ||
Medkour F. Babai S, Chanteloup E, Buffard V, Delchier JC, Le-Louet H. Development of diffuse psoriasis with alopecia during treatment of Crohn’s disease with infliximab. Gastroenterol Clin Biol. 2010;34(2):140–141. | ||
El Shabrawi-Caelen L, La Placa M, Vincenzi C, Haidn T, Muellegger R, Tosti A. Adalimumab-induced psoriasis of the scalp with diffuse alopecia: a severe potentially irreversible cutaneous side effect of TNF-α blockers. Inflamm Bowel Dis. 2010;16(2):182–183. | ||
Cohen JD, Bournerias I, Buffard V, et al. Psoriasis induced by tumor necrosis factor-α antagonist therapy: a case series. J Rheumatol. 2007;34(2):380–385. | ||
Thurber M, Feasel A, Stroehlein J, Hymes SR. Pustular psoriasis induced by infliximab. J Drugs Dermatol. 2004;3(4):439–440. | ||
Allison MC, Dhillon AP, Lewis WG, Pounder RE. Inflammatory Bowel Disease. London: Mosby; 1998. | ||
Cho JH, Brant SR. Recent insights into the genetics of inflammatory bowel disease. Gastroenterology. 2011;140(6):1704–1712. | ||
Toussirot E, Aubin F. Paradoxical reactions under TNF-α blocking agents and other biological agents given for chronic immune-mediated diseases: an analytical and comprehensive overview. RMD Open. 2016;2(2):e000239. |
 © 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2017 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
