Back to Journals » Journal of Pain Research » Volume 12
Pain reduction and improved vascular health associated with daily consumption of an anti-inflammatory dietary supplement blend
Authors Hamilton DE , Jensen GS
Received 28 September 2018
Accepted for publication 24 April 2019
Published 15 May 2019 Volume 2019:12 Pages 1497—1508
DOI https://doi.org/10.2147/JPR.S189064
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Katherine Hanlon
Debby E Hamilton,1 Gitte S Jensen2
1Researched Nutritionals, Los Olivos, CA, USA; 2NIS Labs, Klamath Falls 1437, OR, USA
Purpose: The objective for this clinical pilot study was to evaluate changes to chronic pain, vascular health, and inflammatory markers when consuming a dietary supplement blend (DSB, CytoQuel®), containing curcumin, resveratrol, tocotrienols, N-Acetylcysteine, and epigallocatechin gallate.
Materials and methods: An open-label study design was used where 21 study participants were evaluated at baseline and at 2 and 8 weeks after consuming DSB. Participants were randomized to consume 3 capsules once daily versus 2 capsules twice daily. Pain and activities of daily living questionnaires were used to gather subjective data on pain levels and interference with daily living. Blood pressure was measured in both arms and ankles, and the ankle-brachial index (ABI) calculated. Blood samples were used to evaluate markers associated with inflammation and cardiovascular health.
Results: Highly significant reduction of chronic pain was seen after 8 weeks (p<0.01), both at rest and when physically active. Faster improvement was seen when consuming 3 capsules once daily, compared to 2 capsules twice daily. The pain reduction resulted in improved sleep quality (p<0.1), and improved social functioning (p<0.01), and less need for support from others (p<0.05), Normalization of mildly elevated ABI at study start was seen after 2 weeks. Plasma fibrinogen and von Willebrand Factor and serum matrix metalloproteinase-9 (MMP-9) showed reduction after 2 weeks (not significant), whereas a reduction in serum interleukin-1 receptor antagonist-a (IL-1ra) was statistically significant after 2 weeks (p<0.05). Correlation between pain reduction and changes to MMP-9 after 8 weeks was highly significant (P<0.01), whereas correlation between pain reduction and changes to IL-1ra reached significance at 2 weeks for the group consuming 3 caps once daily (p<0.04).
Conclusion: Consuming DSB helped manage pain, increased comfort during daily activities, and improved vascular function. This was associated with selective effects on specific blood biomarkers associated with inflammation and vascular health.
Keywords: ankle-brachial index, cardiovascular disease, fibrinogen, interleukin-1 receptor antagonist, matrix metalloproteinase-9, von Willebrand factor
Introduction
Chronic pain is defined as persistent pain for greater than three months. In the United States alone, based on a 2012 survey, an estimated 23.4 million adults (10.3%) had chronic pain on a daily basis for three months.1 An even greater percentage (55.7%) or 126 million adults had intermittent pain over the past 12 weeks before the survey. For those people suffering with the most severe pain, they were more likely to report overall worse quality of life.2 With the aging population expanding, these numbers are predicted to increase.
The symptoms of pain may arise from a multitude of factors. Inflammation is one mechanism leading to chronic pain through the activation of NFKB. Activation of the NFKB pathway has been implicated in contributing to pain in chronic conditions ranging from migraines to nerve injury to autoimmune diseases such as Rheumatoid Arthritis.3,4 The NFKB pathway, once initiated causes an increase in pro-inflammatory cytokines such as IL-1B, IL-6, IL-8, and TNF-alpha. Elevated pro-inflammatory cytokines can then activate NFKB further causing chronic inflammation.5
Pain can also originate from inflammation in the cardiovascular system.6 This inflammation causes a decrease in circulation and stiffening of the arteries diminishing vascular function. Inflammation along with oxidative stress depleting nitric oxide bioavailability leads to this vascular endothelial dysfunction resulting in pain.7
Treatment for chronic pain has traditionally been focused on pharmaceuticals including non-steroidal anti-inflammatories (NSAIDs) and opioid medications. Each of these classes of medications have potentially significant side effects. NSAIDs can cause gastrointestinal bleeding, have adverse cardiovascular and renal effects, and may delay healing and may be ineffective for certain people in severe pain.8 Opioids have a significant risk for addiction and misuse.9 With the significant side effects associated with standard pain medicines, safe and effective alternatives are needed.
Herbal and nutritional supplements are being investigated for pain control based on their anti-inflammatory and anti-oxidative stress properties.10 Curcumin, the active ingredient in turmeric can decrease inflammation in multiple chronic diseases.11 One mechanism of action appears to be NFKB inhibition.12–15 Because of the poor absorption of natural curcumin, advanced formulations of curcumin have been developed for increased absorption with higher and longer lasting blood levels.16
Resveratrol is another polyphenolic compound with anti-inflammatory and anti-oxidant properties including blocking NFKB.17 In addition, the compound appears to be beneficial for the inflammation and oxidative stress involved in the development of cardiovascular disease. Resveratrol improves cardiovascular function by stimulating endothelial production of nitric oxide, decreasing oxidative stress, inhibiting vascular inflammation, and decreasing clotting.18
Epigallocatechin gallate (EGCG) is another phenolic herbal component derived from tea that has been studied for inflammation and pain control. Research has shown EGCG to decrease inflammatory cytokines such as Interleukin-1β (IL-1β) and TNF-αalpha through inhibition of NFKB.19 Similarly, to Resveratrol, EGCG seems to have a beneficial impact on inflammation and vascular function in cardiovascular disease.20–22
Nutritional components in addition to herbs have also been found to decrease pain and inflammation. Vitamin E is a known anti-oxidant but research on use in chronic disease has shown mixed results possibly due to various sources and quality of materials studied, and lack of systematic comparison between studies on tocopherols versus tocotrienols.23 In most research studies, the form of Vitamin E used is alpha-tocopherol. Vitamin E is formed from a combination of four tocopherols (alpha, beta, gamma, and delta) and four tocotrienols (alpha, beta, gamma, and delta). Tocotrienols may display 40 to 60 times more antioxidant potential than tocopherols.24,25 In addition, tocotrienols have been found to have anti-inflammatory properties impacting NFKB, signal transducers such as STAT3, and COX-2 pathways.26–28 Tocotrienols with their anti-inflammatory and anti-oxidant properties have shown to be beneficial in cardiovascular disease including lowering lipids, CRP, nitric oxide, and atherosclerotic plaques.29–32
N-acetyl-cysteine (NAC) is a unique nutritional supplement that is a precursor of glutathione and the amino acid cysteine. Traditionally in medicine, NAC has been used as a mucolytic medicine and as an antidote for liver toxicity associated with acetaminophen overdose. NAC is now researched for its anti-inflammatory mechanisms since it can inhibit NFKB and decrease proinflammatory cytokines.33,34 Since NAC is a precursor of glutathione, its use has been shown to improve markers of oxidative stress.35,36
Traditional medicinal herbs are often used in combination for synergistic and strengthening effects. Research has elaborated on the specific mechanisms involved in their synergy. Individual herbs may have different abilities to regulate enzymes and transporters in digestive and detoxification pathways, but when combined may support the pathway through separate mechanisms.37,38 Results from research studies addressing pain with herbs and nutritional supplements in single ingredient formulas have been inconsistent.
The dietary supplement blend (DSB) was developed for more efficacious management of pain and inflammation by combining targeted herbs and nutritional supplements and is a blend of curcumin with enhanced absorption, resveratrol, tocotrienols, NAC, and EGCG. Based on the research supporting the ingredients in DSB individually for inflammation and oxidative stress, the range of ingredients in DSB should engage multiple mechanisms of action in inflammatory pathways to improve consistent treatment of pain. The primary purpose of this research study was to show efficacy for treating pain and to find an optimal dosing regimen since neither have been established previously.
Materials and methods
Study design
An open-label study design was used to evaluate the effects of consumption of a nutraceutical product. The study was of 8 weeks’ duration, with evaluation at baseline, 2, and 8 weeks of product consumption. People were recruited for the study if they met the inclusion criteria of being 30–75 years of age, having a body mass index between 20.0 and 34.9 kg/m2, and experiencing chronic pain in at least one specific anatomical area for more than 6 months. People were excluded from the study if they had active uncontrolled auto-immune illness, known active cardiovascular health issues, cancer and/or chemotherapy in the past 12 months, were prescribed prescription medications for pain, hypertension, blood thinning, hyperlipidemia, and if they had surgery or trauma during the past six months. This study was conducted in accordance with the Declaration of Helsinki. The study was conducted over a period of 4 months, from September to December 2017. The location was Klamath Falls, Oregon, USA, which has a high-desert climate. During the season of the study, the weather was changing from hot and very dry to colder and more humid weather conditions. Twenty-one study participants were recruited after providing written informed consent (as approved by the registered Institutional Review Board Sky Lakes Medical Center Institutional Review Board FWA 2603) and passing a screening process. Study participants were instructed to maintain a constant diet and lifestyle during the study. Study participants were instructed to consume similar breakfasts on the morning of each clinic visit and to avoid vitamins, nutritional supplements, and exercise the morning of a clinic visit. They were instructed to avoid coffee, tea, nicotine, and energy drinks for at least an hour before arrival. Twenty people completed the study.
Consumable
The consumable test product for the study was a nutraceutical formulation, namely the dietary supplement blend CytoQuel®, designed to reduce inflammation, and provided by the manufacturer, Researched Nutritionals, Los Olivos, California USA. Study participants were assigned to either consume three capsules once daily or consume two capsules twice daily. One daily dose, as recommended on the product label, consisted of 3 veggie capsules. This daily dose provides 1850 mg of a proprietary blend of black tea extract (50% of the antioxidant EGCG), NAC, the enhanced bioavailable curcumin product CurcuWin™, tocotrienols (a form of Vitamin E), and resveratrol. Some medical practitioners prefer to give a smaller dose (two capsules), but to give it twice daily (2467 mg of the blend). Therefore, study participants were randomized to one of two groups, taking either three capsules once daily, or two capsules twice daily. At the baseline, 2-week, and 8-week visits, study participants were given a supply of the test product to last until the subsequent visit. They were instructed to consume the capsules with food. Study participants were instructed to return the bottles with any unused capsules. Capsule counts were used to document compliance with respect to consumption of test product. The average compliance was 91%.
Blood pressure
Blood pressure was monitored at all study visits, using an Omron BP 742 monitor. To calculate ankle-brachial indexes (ABIs), blood pressures were taken on both right and left arms, and on both legs above the ankle such that the bottom edge of the blood pressure cuff was two inches above the lateral malleolus bone.39,40
Questionnaire-based data collection
At each study visit, four questionnaires were administered where the study participant would answer the questionnaires by use of a tablet computer in the presence of clinic staff available to answer any questions or provide clarifications. The Pain scoring questionnaire assessed pain levels both at rest and while active and included pain in different anatomical regions; the Activities of Daily Living questionnaire monitored many aspects of daily functioning; a Wellness questionnaire contained questions regarding general health and wellness, and a Health questionnaire recorded relatively minor but noticeable health issues such as changes in allergies or frequency of headaches.
Testing of inflammatory biomarkers
At each visit, a blood draw was performed and used for evaluation of a panel of biomarkers for inflammation and vascular health. The measurement of high sensitivity C-reactive protein was performed at a clinical diagnostic laboratory. Testing of all other biomarkers was performed at NIS Labs using utilizing xMAP technology (Luminex, Austin, TX, USA).
Serum levels of the following cytokines were tested: IL-1β, IL-1ra, IL-6, IL-8 (CXCL8), IL-10, IL-13, IL-17, interferon gamma (IFNγ), MIP-1α, MIP-1β, MCP-1, and tumor necrosis factor alpha (TNFα), using Bio-Plex Pro™ multiplex Luminex immunoassays (Bio-Rad Laboratories, Hercules, CA). Serum levels of 5 matrix metalloproteinases (MMP-1, MMP-2, MMP-7, MMP-9, and MMP-10) and plasma levels of fibrinogen and von Willebrand Factor (vWF) were tested using MilliPlex Luminex panels (Millipore, Burlington, MA USA).
Statistical analysis
Group averages and standard error of the means (SEM) for each data set were calculated using Microsoft Excel. Statistical significance of changes from baseline to later assessments was evaluated by between-treatment analysis using ’within-subject’ analysis using the two-tailed, paired t-test. Analysis was performed for people consuming three capsules daily and for people consuming two capsules twice daily. Analysis was also performed on all study participants, since they all consumed a minimum of three capsules daily. Additional ‘between-groups’ analysis examined whether there were significant differences between the two dosing regimens, using the two-tailed, unpaired t-test.
The outcome variables: Primary pain at rest, IL-1ra, vWF, and MMP-9 were analyzed using a linear mixed-effects model. Unstructured covariance structure was used to model the dependence between observations for each subject, and the type 3 F-test was used to test if the effect of a predictor (ie, the fixed effect) was statistically significant. For factors with more than two levels (for example, time), if the effect was significant, pairwise comparison was performed to evaluate at which two levels the statistically significance occurred. To control for the family wise error rate, the multiple comparison procedure, Tukey–Kramer test was implemented.41
The quantile-quantile (QQ) plots of the scaled residuals (obtained after multiplying the raw residuals by Cholesky decomposition) were used to assess the multivariate normality assumption of the linear mixed-effects model.42–44 Wilcoxon rank-sum tests were conducted to determine if there was a statistically significant difference in the outcome variables between the two groups (A vs B). Normality of the data was assessed via the QQ plots. Analyses were conducted using SAS version 9.4 (SAS Institute, Cary, NC).
In order to determine the correlations between the following outcome measures, including ‘Pain at rest‘ and ‘change in pain at rest‘, and the biomarkers vWF, MMP-9, and IL-1ra, Spearman’s correlations were computed for the overall sample, and by stratifying the sample by time and group.
For any tests, a p-value less than 0.05 indicated significance, and a p-value less than 0.01 indicated a high level of significance.
Results
Study population
Seventy-three people were pre-screened, 23 people were screened, and 21 people recruited based on the inclusion/exclusion criteria listed above. Please see the Consort flow chart in Figure 1. Twenty participants completed the study. Table 1 shows the number of participants, average age, and BMI for each gender.
 | Table 1 Demographics of study participants |
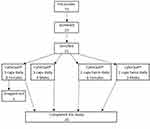 | Figure 1 Consort flow chart. The chart shows screening and randomization, as well as the number of people completing the 8-week study. |
Pain levels and physical functioning
Significant pain reduction was observed during the study and positively affected activities of daily living. The reduction in primary pain when being physically active was statistically significant already after 2 weeks and reached a high level of statistical significance at 8 weeks (p<0.01) (Table 2). Pain reduction was also seen for both primary and secondary pain areas at rest, and the reduction was highly significant after 8 weeks (p<0.01). The improvements resulted in significant reduced need for support from others (p<0.05), a statistical trend towards improved sleep quality (P<0.1), and highly significant reduction in pain interfering in social life (p<0.01). In addition, reduction in both stiffness and pain interfering with daily activities was highly significant at 8 weeks (Table 3).
 | Table 2 Pain scores |
 | Table 3 Activities of daily living |
When the data were analyzed separately for people consuming 3 capsules once daily versus people consuming 2 capsules twice daily, it showed that people consuming 3 capsules once daily showed a faster rate of improvement for both pain (Figure 2) and stiffness (Figure 3). This was especially clear for the primary pain when being physically active, where the two groups started at similar levels of pain, and the group consuming 3 capsules once daily showed significantly greater pain reduction already at 2 weeks than the other group (p<0.05). Furthermore, despite the higher average initial pain and stiffness in the groups consuming 3 capsules once daily, this group showed a faster improvement than the other group.
Blood pressure and ABI
Blood pressure measurements in both right and left arms remained constant over the course of the study, showing no significant differences over time or between groups. In contrast, blood pressure readings in the ankles were on average higher at study start; suggestive of wall stiffness as a result of inflammatory factors and altered vascular muscle tone. Ankle blood pressure results showed significant reduction over the course of the study. The reduction in systolic blood pressure for both right and left ankle, as well as the ABI for both right and left side, reached a high level of statistical significance after 8 weeks (Table 4). When the ABI was analyzed separately for people consuming 3 capsules once daily versus people consuming 2 capsules twice daily, it showed that people consuming 3 capsules once daily showed a faster rate of improvement, with the decrease for the right systolic index reaching statistical significance at 2 weeks (Figure 4).
 | Table 4 Blood pressure and ankle-brachial index |
Blood biomarkers
Blood samples were tested for biomarkers pertaining to inflammation and cardiovascular risk factors. Plasma fibrinogen and von Willebrand Factor (vWF) showed decreases but did not reach statistical significance (Figure 5A and B). Matrix metalloproteinase-9 (MMP-9) showed a reduction over time and reached a statistical trend already after 2 weeks (p<0.1) (Figure 5C). The Interleukin-1 receptor antagonist (IL-1ra) showed a statistically significant reduction already at 2 weeks of consumption and remained at the reduced level for the remainder of the study (Figure 5D).
There was a mild reduction in Tumor Necrosis Factor, which reached a statistical trend at 2 weeks for the group consuming 2 capsules twice daily, and at 8 weeks for the group consuming 3 capsules daily (p<0.09, data not shown). No significant changes were seen for C-reactive protein, IL-8, MMP-1, MMP-2, MMP-7, or MMP-10.
Discussion
With the rising incidence of chronic pain and chronic disease, finding safe treatments in support of clinical improvement is important. The study reported here showed that consumption of DSB for 8 weeks was associated with a rapid reduction in pain scores during physical activity that reached statistical significance after 2 weeks. The results were highly significant after 8 weeks, when compared to baseline scores. Pain at rest and improvement in sleep quality, which often reflects a decrease in pain, were also improved with the 8-week supplement regimen. The study was conducted during seasonal change going from warm and dry to colder and more humid, so it would be expected that the weather changes should have increased pain and stiffness; it is therefore unlikely that weather changes had a major effect on the pain reduction we observed. That said, a placebo effect may have had some contribution to the pain relief. The causes of decreased pain may arise from a combination of direct anti-inflammatory effects that helped reduce the inflammatory conditions causing the pain and may also be associated with a change in pain perception. Both peripheral and central sensitization mechanisms are involved in amplifying pain perception and are affected by inflammation.45 Due to the recognized side effects of steroid and non-steroid anti-inflammatory drugs, research into natural products is seeking new modalities to reduce local inflammation as well as neuroinflammation associated with pain perception.46 Given the reported effects on nociception by both curcumin47 and resveratrol,48,49 this suggests that part of the pain-relieving effects reported in our study may be due to reduced neuroinflammation associated with altered pain perception.
Cytokines are viewed as the primary biological markers in inflammation. They can be categorized as pro and anti-inflammatory. TNF-alpha and IL-1 have been classified as pro-inflammatory cytokines causing activation of the NFKB pathway.50,51 Both of these cytokines are rapidly released upon tissue injury or infection. Once activated NFKB causes an increase in macrophages which triggers both the innate and adaptive immune responses.52 In order to resolve acute inflammation, the NFKB system needs to be inhibited. Unfortunately, with many chronic diseases such as rheumatoid arthritis, multiple sclerosis, inflammatory bowel disease, and atherosclerosis, the NFKB pathway is continually activated leading to chronic inflammation.52 One of the primary issues with monitoring inflammation by measurement of cytokines is the short amount of time they exist in the blood leading to difficulties in finding consistent patterns. In our study, multiple cytokines were measured although several of them were below detection limits at baseline, so no significant changes were seen. However, specific pro- and anti-inflammatory cytokines and chemokines including TNF-alpha, MIP-1B, IL-1ra, and MMP-9 showed varying decreases over the course of the study. IL-1ra is a potent anti-inflammatory cytokine; however, it can be increased in inflammatory conditions, likely as a mechanism to attempt to control and reduce inflammation. Reduced IL-1ra levels were seen in previous clinical studies linked to inflammation,53 and the results showed a significant correlation between pain reduction and changes to IL-1ra already at 2 weeks of study participation. Decreases in these cytokines would be expected to correlate with decreases in pain, suggesting that multiple mechanisms appear to be involved in inflammation and the development and perception of pain.
Recently, research has found coagulation factors such as fibrinogen and vWF, markers involved in hemostasis, to be involved in the development of inflammation. In the pilot study described here, fibrinogen and vWF levels decreased throughout the study as pain symptoms decreased. Although these factors have traditionally been involved in the acute clotting cascade, elevated levels of these components have been found in inflammatory diseases such as stroke, cardiac disease, brain trauma, multiple sclerosis, and Alzheimer’s Dementia.54 Blood coagulation and inflammatory processes are both part of the innate immune system, and there is a bidirectional interaction between the two, where blood vessel injury initiates both the coagulation cascade, and simultaneously, the inflammatory response with an increase in vascular permeability, allowing an influx of leukocytes into the tissue and release of cytokines and chemokines.55 Fibrinogen and its peptides are also able to induce inflammatory changes by binding to specific receptors on multiple types of immune cells.56 One of these receptors is Toll-like Receptor-4 which is involved in macrophage activation and release of cytokines and chemokines including MCP-1, MIP-l, IL-6, Il-8, TNF-alpha, and MMP-9.57 Multiple hemostatic factors including von Willebrand Factor (vWF) and fibrinogen are involved in the development of atherosclerosis and vascular disease.58 Elevated levels of fibrinogen in the blood is a marker of increased risk for developing cardiovascular diseases such as hypertension and atherosclerosis.50 Specifically for peripheral artery disease, elevated fibrinogen is one of the best-established risk factors along with smoking, hypertension, hyperlipidemia, and diabetes.59 Increased levels of vWF have been associated with many cardiovascular diseases including ischemic heart disease, peripheral vascular disease, aneurysms, atrial fibrillation, deep vein thrombosis, and cerebrovascular disease.60 Since there is a known association between vWF, formation of thrombosis and atherosclerosis, elevated levels of vWF can be used to follow the health of a patient with cardiovascular disease.60
An elevated ABI has also been used as a predictive value for cardiovascular disease and peripheral artery disease.61 The primary symptom of peripheral artery disease is intermittent claudication or pain in the legs with walking. Finding an elevated ABI is more common than a history of claudication in people with peripheral artery disease which makes this measurement a good screening tool for peripheral artery disease.62 Peripheral artery disease is correlated with an increased risk of cardiovascular and cerebrovascular disease disability and mortality.62,63 The use of the ABI in our study was used as a measurement of health and found to decrease toward a normal value. People were screened and excluded for diagnosed cardiovascular disease, yet people had mild-to-moderate elevation of ABI levels at baseline, that decreased towards normal levels with intake of DSB. This suggests that consumption of DSB may decrease risks of developing future cardiovascular disease. The clinical study reported herein was focused on recruiting study participants with chronic pain, and the observations pertaining to vascular health were therefore not in a study population with known cardiovascular risks.
Both chronic pain and cardiovascular disease are associated with inflammation, and pain-mediated activation of the stress response can increase blood pressure and cardiovascular stress. Therefore, it is not surprising that reducing chronic pain may also reduce risks for cardiovascular disease. Cardiovascular disease is a leading cause of death and disability in the United States so identifying patients with cardiovascular disease is critically important.63 While many people have identifiable risk factors for cardiovascular disease such as smoking and diabetes, approximately 15% of the men and 10% of the women do not have any of the standard risk factors.64 Even with people having classic risk factors for cardiovascular disease, research has shown that health care practitioners can be poor estimators of disease risk.65 Therefore, further research with DSB should include placebo-controlled trials and address cardiovascular health in people with symptomatic and high-risk profiles including elevated ABI levels, elevated inflammatory cytokines, and hemostatic factors.
The study presented here took a novel approach to low-risk approach to pain management and also evaluated whether this could have secondary beneficial effects on cardiovascular health and reduction of inflammation. The study combined pain scores with functional changes. Importantly, the total lower daily dose was more effective than the higher daily dose; the data suggest that the once-daily dose provided more robust support of reducing pain and inflammation than the higher dose split into two smaller doses. That said, the limitations of the study were its small size and lack of a placebo control. A placebo effect may have had some contribution to the observed pain reduction, and a larger placebo-controlled study size, using a one-dose regimen, which appears to ideally be 3 capsules daily, is warranted. Such further clinical evaluation should examine the effects of DSB, specifically in study populations with the presence of cardiovascular risk factors.
Conclusion
Inflammation is a primary pathological factor seen in many chronic diseases, and a primary manifestation involves pain. Finding safe treatments for pain is therefore a goal for many health practitioners. DSB offers a safe alternative for decreasing pain based on clinical pain scores at rest and with physical activity. Intake of the nutraceutical blend for as little as 2 weeks’ duration helped decrease chronic pain during rest and with physical activity, along with improving sleep. The lessening of pain correlated with a decrease in pro-inflammatory cytokines and chemokines including TNF-alpha, IL-1ra, and MMP-9, along with the hemostatic factors fibrinogen and vWF. Significant improvement of ankle-brachial blood pressure index also illustrated a clinically relevant decrease in inflammation related to an improvement in cardiovascular function.
Acknowledgments
The clinical trial was conducted at NIS Labs, an independent contract research organization that specializes in natural products research and testing. The study was sponsored by Researched Nutritionals LLC, the manufacturer of the product tested in the clinical trial.
Author contributions
DEH and GSJ designed the study. GSJ oversaw the trial and the biomarker testing. GSJ performed the data analysis. DEH and GSJ wrote the manuscript. Both authors contributed to data analysis, drafting and revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
DEH is employed as the Director of Physician Education and Clinical Trials for the study sponsor, Research Nutritionals, LLC. The authors report no other conflicts of interest in this work.
References
1. Nahin RL. Estimates of pain prevalence and severity in adults: United States, 2012. J Pain. 2015;16(8):769–780. doi:10.1016/j.jpain.2015.05.002
2. Leadley RM, Armstrong N, Reid KJ, Allen A, Misso KV, Kleijnen J. Healthy aging relation to chronic pain and quality of life in Europe. Pain Pract. 2014;14(6):547–558. doi:10.1111/papr.12125
3. Niderberger E, Geisslinger G. The IKK-NF-kappaB pathway: a source for novel molecular drug targets in pain therapy? FASEB J. 2008;22(10):3432–3442. doi:10.1096/fj.08-109355
4. Hartung JE, Eskew O, Wong T, et al. Nuclear factor-kappa B regulates pain and COMT expression in a rodent model of inflammation. Brain Behav Immun. 2015;50:196–202. doi:10.1016/j.bbi.2015.07.014
5. Liu T, Zhang L, Joo D, Sun SC. NF-kB signaling in inflammation. Signal Transduct Target Ther. 2017;2:1–24. doi:10.1038/sigtrans.2017.23
6. Li J, Li JJ, Li Q, Li Z, Qian HY. A rational connection of inflammation with peripheral arterial disease. Med Hypothesis. 2007;69(6):1190–1195. doi:10.1016/j.mehy.2007.02.043
7. Taddei S, Virdis A, Ghiadoni L, et al. Age‐related reduction of NO availability and oxidative stress in humans. Hypertension. 2001;38:274–279.
8. Harirforoosh S, Asghar W, Jamali F. Adverse effects of nonsteroidal antiinflammatory drugs: an update of gastrointestinal, cardiovascular and renal complications. J Pharm Sci. 2013;16(5):821–847.
9. Seth P, Scholl L, Rudd RA, Bacon S. Overdose deaths involving opioids, cocaine, and psychostimulants-United States, 2015–2016. MMWR Morb Mortal Wkly Rep. 2018;67(12):349–358. doi:10.15585/mmwr.mm6712a1
10. Shen CL, Smith BJ, Lo DF, et al. Dietary polyphenols and mechanisms of osteoarthritis. J Nutr Biochem. 2012;23(11):1367–1377. doi:10.1016/j.jnutbio.2012.04.001
11. Ghosh S, Banerjee S, Sil PC. The beneficial role of curcumin on inflammation, diabetes, and neurodegenerative disease: A recent update. Food Chem Toxicol. 2015;83:111–124. doi:10.1016/j.fct.2015.05.022
12. Bengmark S. Curcumin, an atoxic anti-oxidant and natural NF-KB, cyclooxygenase-2, lipoxygenase, and inducible nitric oxide synthase inhibitor: A shield against acute and chronic disease. J Parenter Enteral Nutr. 2006;30(1):45–51. doi:10.1177/014860710603000145
13. Chainini-Wu N. Safety and anti-inflammatory activity of curcumin: a component of turmeric (Curcuma Longa). J Altern Complement Med. 2003;9(1):161–168. doi:10.1089/107555303321223035
14. Epstein J, Sanderson IR, Macdonald TT. Curcumin as a therapeutic agent: the evidence from in vitro, animal and human studies. Br J Nutr. 2010;103(11):1545–1557. doi:10.1017/S0007114509993667
15. Sahin K, Pala R, Tuzcu M, et al. Curcumin prevents muscle damage by regulating NF-kB and Nrf2 pathways and improves performance: an in vivo model. J Inflamm Res. 2016;9:147–154. doi:10.2147/JIR.S110873
16. Jäger R, Lowery RP, Calvanese AV, Joy JM, Purpura M, Wilson JM. Comparative Absorption of Curcumin Formulations. Nutr J. 2014;13(1):11. doi:10.1186/1475-2891-13-11
17. Ma C, Wang Y, Dong L, Li M, Cai W. Anti-inflammatory effect of resveratrol through the suppression of NF-kB and JAK/STAT signaling pathways. Acta Biochim Biophys Sin (Shanghai). 2015;47(3):207–213. doi:10.1093/abbs/gmu135
18. Xia N, Daiber A, Förstermann U, Li H. Antioxidant effects of resveratrol in the cardiovascular system. Br J Pharmacol. 2017;174(12):1633–1646. doi:10.1111/bph.13492
19. Jiang J, Mo ZC, Yin K, et al. Epigallocatechin-3-gallate prevents TNF-alpha-induced NF-kB activations thereby upregulating ABCA1 via the Nrf2/Keap 1 pathway in macrophage foam cells. Int J Mol Med. 2012;29(5):946–956. doi:10.3892/ijmm.2012.924
20. Bahoran T, Luximon-Ramma A, Gunness TK, et al. Aruoma OI Black tea reduces uric acid and C-reactive protein levels in humans susceptible to cardiovascular diseases. Toxicology. 2010;278(1):68–74. doi:10.1016/j.tox.2009.11.024
21. DeBacquer D, Clays E, Delanghe J, DeBacker G. Epidemiological evidence for an association between habitual tea consumption ad markers of chronic inflammation. Atherosclerosis. 2006;189(2):428–435. doi:10.1016/j.atherosclerosis.2005.12.028
22. Steptoe A, Gibson EL, Vuononvirta R, et al. The effects of chronic tea intake on platelet activation and inflammation: a double-blind placebo-controlled trial. Atherosclerosis. 2007;193(2):277–282. doi:10.1016/j.atherosclerosis.2006.08.054
23.
24. Serbinova E, Kagan V, Han D, Packer L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic Biol Med. 1991;10:263–275. doi:10.1016/0891-5849(91)90033-Y
25. Suzuki YJ, Tsuchiya M, Wassall SR, et al. Structural and dynamic membrane properties of alpha-tocopherol and alpha-tocotrienol: implication to the molecular mechanism of their antioxidant potency. Biochemistry. 1993;32:10692–10699. doi:10.1021/bi00091a020
26. Wong W-Y, Ward LC, Fong CW, Yap WN, Brown L. Anti-inflammatory γ- and δ-tocotrienols improve cardiovascular, liver and metabolic function in diet induced obese rats. Eur J Nutr. 2015;56(1):133–150. doi:10.1007/s00394-015-1064-1
27. Nesaretnam K, Meganathan P. Tocotrienols: inflammation and cancer. Ann N Y Acad Sci. 2011;1229:18–22. doi:10.1111/j.1749-6632.2011.06088.x
28. Wu SJ, Liu PL, Ng LT. Tocotrienol-rich fraction of palm oil exhibits anti-inflammatory property by suppressing the expression of inflammatory mediators in human monocytic cells. Mol Nutr Food Res. 2008;52(8):921–929. doi:10.1002/mnfr.200700418
29. Shibata A, Kobayashi T, Asai A, et al. High purity tocotrienols attenuate atherosclerotic lesion formation in apoE-KO mice. J Nutr Biochem. 2017;48:44–50. doi:10.1016/j.jnutbio.2017.06.009
30. Qureshi AA, Khan DA, Mahjabeen W, Trias AM, SilswalN QN. Impact of delta-tocotrienol on inflammatory biomarkers and oxidative stress in hypercholesterolemic subjects. Clin Exp Cardol. 2015;6(4):1000367.
31. Qureshi AA, Khan D, Mahjabeen W, Qureshi N. Dose-dependent modulation of lipid parameters, cytokines, and RNA by delta-tocotrienol in hypercholesterolemic subjects restricted to AHA Step-1 diet. Br J Med Res. 2015;6(4):351–366. doi:10.9734/BJMMR
32. Ramanathan N, Tan E, Loh LJ, Soh BS, Yap WN. Tocotrienol is a cardioprotective agent against ageing-associated cardiovascular disease and its associated morbidities. Nutr Metab (Lond). 2018;15:6. doi:10.1186/s12986-018-0244-4
33. Pei Y, Liu H, Yang Y, et al. Biological Activities and Potential Oral Applications of N-Acetylcysteine: progress and Prospects. Oxid Med Cell Longev. 2018;2018:2835787. doi:10.1155/2018/2835787
34. Radomska-Lesniewska DM, Skopińska-Rózewska E, Jankowska-Steifer E, et al. N-Acetyl Cysteine IL-8 and MMP-9 release and ICAM-1 expression by bronchoalveolar cells from interstitial lung disease patients. Pharmacol Rep. 2010;62(1):131–138.
35. Csontos C, Rezman B, Foldi V, et al. Effect of N-Acetyl-Cysteine treatment on oxidative stress and inflammation after severe burn. Burns. 2012;38(3):428–437. doi:10.1016/j.burns.2011.09.011
36. Nascimento MM, Suliman ME, Silva M, et al. Effect of oral N-Acetyl Cysteine treatment on plasma inflammatory and oxidative stress markers in peritoneal dialysis patients: a placebo-controlled study. Perit Dial Int. 2010;30(3):336–342. doi:10.3747/pdi.2009.00073
37. Yang Y, Zhang Z, Li S, Ye X, Li X, He K. Synergy effects of herbs extracts: pharmacokinetics and pharmacodynamic basis. Fitoterapia. 2014;92:133–147. doi:10.1016/j.fitote.2013.10.010
38. Zhou X, Seto SW, Chang D, et al. Synergistic effects of chinese herbal medicine: a comprehensive review of methodology and current research. Front Pharmacol. 2016;7:201. doi:10.3389/fphar.2016.00323
39. Chongthawonsatid S, Dutsadeevettakul S. Validity and reliability of the ankle-brachial index by oscillometric blood pressure and automated ankle-brachial index. J Res Med Sci. 2017;22:44. doi:10.4103/jrms.JRMS_976_16
40. Pillois DX, Benchimol A, Houitte A, Sagardiluz P, Tortelier L, Bonnet J. Accuracy of ankle-brachial index using an automatic blood pressure device to detect peripheral artery disease in preventive medicine. Arch Cardiovasc Dis. 2009;102(6–7):519–524. doi:10.1016/j.acvd.2009.03.011
41. Neter J, Wasserman W, Kutner M. Applied Linear Statistical Models. Boston, MA: Richard D. Irwin, Inc; 1990.
42. Verbeke G, Molenberghs G. Linear Mixed Models for Longitudinal Data. New York: Springer-Verlag; 2000.
43. Fitzmaurice G, Laird N, Ware J. Applied Longitudinal Analysis. Hobokon, New Jersey: John Wiley & Sons, inc; 2004.
44. Houseman E, Ryan L, Coull B. Cholesky residuals for assessing normal errors in a linear model with correlated outcomes. J Am Stat Assoc. 2004;99:383–394. doi:10.1198/016214504000000403
45. Ronchetti S, Migliorati G, Delfino DV. Association of inflammatory mediators with pain perception. Biomed Pharmacother. 2017;96:1445–1452. doi:10.1016/j.biopha.2017.12.001
46. Ni GL, Cui R, Shao AM, Wu ZM. Salidroside ameliorates diabetic neuropathic pain in rats by inhibiting neuroinflammation. J Mol Neurosci. 2017;63(1):9–16. doi:10.1007/s12031-017-0951-8
47. Xiao L, Ding M, Fernandez A, Zhao P, Jin L, Li X. Curcumin alleviates lumbar radiculopathy by reducing neuroinflammation, oxidative stress and nociceptive factors. Eur Cell Mater. 2017;33:279–293. doi:10.22203/eCM.v033a21
48. Bazzo KO, Souto AA, Lopes TG, et al. Evidence for the analgesic activity of resveratrol in acute models of nociception in mice. J Nat Prod. 2013;76(1):13–21. doi:10.1021/np300529x
49. Takehana S, Sekiguchi K, Inoue M, et al. Systemic administration of resveratrol supress the nociceptive neuronal activity of spinal trigeminal nucleus caudalis in rats. Brain Res Bull. 2016;120:117–122. doi:10.1016/j.brainresbull.2015.11.011
50. Hayden MS, Ghosh S. Regulation of NF-kB by TNF family cytokines. Semin Immunol. 2014;26(3):253–266. doi:10.1016/j.smim.2014.05.004
51. Tak PP, Firestein GS. NF-kB: A key role in inflammatory diseases. J Clin Invest. 2001;107:7–11. doi:10.1172/JCI11830
52. Lawrence T. The nuclear factor NF-kB pathway in inflammation. Cold Spring Harb Perspect Biol. 2009;1:
53. Herder C, Brunner EJ, Rathmann W, et al. elevated levels of the anti-inflammatory interleukin-1 receptor antagonist precede the onset of type 2 diabetes: the whitehall II study. Diabetes Care. 2009;32(3):421–423. doi:10.2337/dc08-1161
54. Davalos D, Akassoglou K. Fibrinogen as a key regulator of inflammation in disease. Semin Immunopath. 2012;34(1):43–62. doi:10.1007/s00281-011-0290-8
55. Schoenmaker S, Reitsma PH, Spek A. Blood coagulation factors as inflammatory mediators. Blood Cells Mol Dis. 2005;34:30–37. doi:10.1016/j.bcmd.2004.09.001
56. Adams RA, Passino M, Sachs BD, Nuriel T, Akassoglou K. Fibrin mechanisms and functions in nervous system pathology. Mol Interv. 2004;4(3):163–176. doi:10.1124/mi.4.3.6
57. Smiley ST, King JA, Hancock WW. Fibrinogen stimulates macrophage chemokine secretion through toll-like receptor 4. J Immunol. 2001;167(5):2887–2894.
58. Kannel WB. Overview of hemostasis factors involved in atherosclerotic cardiovascular disease. Lipids. 2005;40(12):1215–1220.
59. Paraskevas KI, Baker DM, Vrentzos GE, Mikhailidis DP. The role of fibrinogen and fibrinolysis in peripheral artery disease. Thromb Res. 2008;122(1):1–12. doi:10.1016/j.thromres.2007.06.003
60. Lip GYH, Blann AD. von Willebrand Factor and its relevance to cardiovascular disorders. Br Heart J. 1995;74:580–583.
61. Li Q, Zeng H, Liu F, et al. High ankle-brachial index indicates cardiovascular and peripheral arterial disease in patients with type 2 diabetes. Angiology. 2015;66(10):918–924. doi:10.1177/0003319715573657
62. Criqui MH, Aboyans V. Epidemiology of peripheral artery disease. Circ Res. 2015;116(9):1509–1526. doi:10.1161/CIRCRESAHA.116.303849
63. Wallace ML, Ricco JA, Barrett B. Screening strategies for cardiovascular disease in asymptomatic adults. Prim Care. 2014;41(2):371–397. doi:10.1016/j.pop.2014.02.010
64. Khot UN, Khot MB, Bajzer CT, et al. Prevalence of conventional risk factors in patients with coronary heart disease. JAMA. 2003;290(7):898–904. doi:10.1001/jama.290.7.898
65. Matheny M, McPheeters ML, Glasser A, et al. systematic review of cardiovascular disease risk assessment tools. Agency for healthcare research and quality (US); 2011 May. Report No.: 11-05155-EF-1. U.S. Preventive Services Task Force Evidence Syntheses, formerly Systematic Evidence Reviews.
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




