Back to Journals » Drug Design, Development and Therapy » Volume 14
Paeoniflorin Ameliorates Chronic Hypoxia/SU5416-Induced Pulmonary Arterial Hypertension by Inhibiting Endothelial-to-Mesenchymal Transition
Authors Yu M , Peng L , Liu P , Yang M , Zhou H , Ding Y , Wang J, Huang W, Tan Q , Wang Y, Xie W, Kong H , Wang H
Received 18 October 2019
Accepted for publication 25 February 2020
Published 19 March 2020 Volume 2020:14 Pages 1191—1202
DOI https://doi.org/10.2147/DDDT.S235207
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Yan Zhu
Min Yu,1,* Liyao Peng,1,* Ping Liu,1 Mingxia Yang,2 Hong Zhou,1 Yirui Ding,1 Jingjing Wang,3 Wen Huang,1 Qi Tan,1 Yanli Wang,1 Weiping Xie,1 Hui Kong,1 Hong Wang1
1Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Nanjing Medical University, Nanjing, Jiangsu 210029, People’s Republic of China; 2Department of Respiratory and Critical Care Medicine, The Affiliated Changzhou No.2 People’s Hospital of Nanjing Medical University, Changzhou 213003, People’s Republic of China; 3Department of Respiratory Medicine, Shanghai Pulmonary Hospital, Tongji University School of Medicine, Shanghai 200433, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Hui Kong; Hong Wang
Department of Respiratory and Critical Care Medicine, The First Affiliated Hospital of Nanjing Medical University, 300 Guangzhou Road, Nanjing, Jiangsu 210029, People’s Republic of China
Tel +86-25-68136426
Fax +86-25-68136269
Email [email protected]; [email protected]
Background: Endothelial cells dysfunction is one of the hallmark pathogenic features of pulmonary arterial hypertension (PAH). Paeoniflorin (PF) is a monoterpene glycoside with endothelial protection, vasodilation, antifibrotic, anti–inflammatory and antioxidative properties. However, the effects of PF on PAH remain unknown.
Methods: Here, we investigated the efficacy of PF in the SU5416/hypoxia (SuHx) rat model of PAH. Human pulmonary arterial endothelial cells (HPAECs) were exposed to 1% O2 with or without PF treatment.
Results: Hemodynamics analysis showed that prophylactic treatment with PF (300 mg/kg i.g. daily for 21 days) significantly inhibited chronic hypoxia/SU5416-induced elevations of right ventricular systolic pressure (RVSP) and right ventricular hypertrophy index in rats. Meanwhile, PF significantly reduced pulmonary vascular remodeling, as well as alleviated collagen deposition in lungs and right ventricles in SuHx rats. Additionally, PF inhibited SuHx–induced down-regulation of endothelial marker (vascular endothelial cadherin) and up-regulation of mesenchymal markers (fibronectin and vimentin) in lung, suggesting that PF could inhibit SuHx–induced endothelial-to-mesenchymal transition (EndMT) in lung. Further in vitro studies confirmed that PF treatment suppressed hypoxia-induced EndMT in HPAECs, which was abolished by the knockdown of bone morphogenetic protein receptor type 2 (BMPR2) in HPAECs.
Conclusion: Taken together, our findings suggest that PF ameliorates BMPR2 down-regulation-mediated EndMT and thereafter alleviates SuHx–induced PAH in rats.
Keywords: paeoniflorin, pulmonary arterial hypertension, endothelial-to-mesenchymal transition, BMPR2, hypoxia
Introduction
Pulmonary arterial hypertension (PAH) is a lethal disorder characterized by pulmonary arterial obstruction and sustained elevation of pulmonary arterial pressure, eventually resulting in right ventricle hypertrophy and failure.1 The key pathological feature of PAH is pulmonary vascular remodeling, comprising the dysfunction of pulmonary arterial endothelial cells, excessive proliferation and apoptosis resistance of smooth muscle cells and abnormal activation of adventitial fibroblasts.2 Despite major advances achieved in the management of PAH in the past decade, current approaches mainly target pulmonary vasoconstriction rather than proliferative vascular remodeling, and the long-term clinical outcome improvement for this devastating disease is limited.3 Thus, novel therapeutic approaches are urgently required for better treatment of PAH.
Endothelial cell dysfunction plays a key role in the development of PAH through altered production of endothelial vasoactive mediators, impaired vasoconstriction, unbalanced endothelial cell proliferation and apoptosis, and aberrant endothelial-to-mesenchymal transition (EndMT).2 Generally, EndMT is a complex biological process in which endothelial cells (ECs) lose their specific phenotype and gradually transdifferentiate into cells with a mesenchymal phenotype characterized by loss of cell-cell junctions and polarity, and the acquisition of cellular motility and invasive properties. During this process, ECs lose specific endothelial markers such as CD31, vascular endothelial cadherin (VE-cadherin) and progressively express mesenchymal markers such as fibronectin, vimentin and α-smooth muscle actin (α-SMA). EndMT has been shown to play a vital role not only in embryonic developmental processes but also in the pathogenesis of fibrotic lung disease.4 Recently, EndMT has emerged as a critical player in the pulmonary vascular remodeling in human PAH and experimental models of PAH.5,6
Paeoniflorin (PF), a monoterpene glucoside extracted from the root of Paeonia lactiflora, possesses many pharmacological activities, such as anti–inflammatory, antioxidative, analgesic, anticancer and immunoregulatory effects.7–10 PF is widely used in clinical practice for the treatment of rheumatoid arthritis and systemic lupus erythematosus.11 A recent study demonstrated that PF exerts a direct vasculoprotective effect through antioxidative and anti–inflammatory effects in fluctuant hyperglycemia-induced vascular endothelial injuries.8 Moreover, it has been documented that PF could protect against lipopolysaccharide, oxidized low-density lipoprotein or radiation-induced endothelial cell dysfunction.12–14 Recently, PF was reported to prevent hypoxia-induced epithelial-mesenchymal transition (EMT) in human breast cancer cells.15 However, the effects of PF on pulmonary vascular remodeling and EndMT process in PAH are still unknown. Therefore, we explored the effects of PF on pulmonary hemodynamics, pulmonary artery thickness, and right ventricular remodeling in the SU5416/hypoxia (SuHx) rat model of PAH, and investigated its possible molecular mechanism.
Materials and Methods
Animals
All animal procedures were conducted following the guidelines published by the National Institutes of Health Guide for the Care and Use of Laboratory Animals (NIH publication No. 85–23, revised 1996). All study protocols were approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (NJMU/IACUC-1809008). Male Sprague Dawley rats (Nanjing Medical University Experimental Animal Center, Nanjing, China) weighing 200–220 g were randomly assigned into 4 groups: Control, Control+PF (300 mg/kg), SuHx, SuHx+PF (300 mg/kg) (n=6 in each). In order to investigate the preventive efficacy of PF in PAH, the SuHx rat model of PAH was induced by a single subcutaneous injection of SU5416 at 20 mg/kg (MedChemexpress, USA, a vascular endothelial growth factor receptor inhibitor) in a solution (composed of 0.5% (w/v) carboxymethylcellulose sodium, 0.9% (w/v) sodium chloride, 0.4% (v/v) polysorbate 80, 0.9% (v/v) benzyl alcohol in deionized water) followed by 3 weeks chronic hypoxia (10% O2) using a ventilated chamber as published previously.16,17 Control rats were injected only the vehicle. The experimental rats received saline as the vehicle control or paeoniflorin (Nantong Jingwei biological science and Technology Co, Ltd, Nantong, China) at the dose of 300 mg/kg by intragastric administration once daily for 3 weeks from the first day (Figure 1). At the end of the hypoxia exposure period, rats were sacrificed for the following experimental measurements.
Hemodynamics
At the end of the 21-days exposure period, the rats were anesthetized with 45mg/kg of pentobarbital sodium (Sigma-Aldrich, St. Louis, MO, USA) by intraperitoneal injection. A polyethylene catheter was inserted through the right jugular vein into the right ventricle (RV) to measure the right ventricular systolic pressure (RVSP). The signals were continuously recorded using the Power Lab data acquisition system (ADI Instruments). Then, the thorax was opened and the lung tissues were flushed with cold saline through the pulmonary artery. The left lung was immediately removed and frozen for protein isolation. The right lung and RV heart tissue were excised for histological assessment. The RV hypertrophy was presented as the weight ratio of the RV to the left ventricle and interventricular septum (RV/LV+S).
Morphological Measurements
The isolated lung and RV heart tissue were fixed in 4% paraformaldehyde, embedded in paraffin and sectioned at 5-μm thickness. Then, lung sections were stained with hematoxylin-eosin (H&E) and examined by a light microscope (DM2500, Leica, Wetzlar, Germany) using a standard protocol. Morphometric analysis was performed in the pulmonary arteries (external diameter: 25–100 μm) at a magnification of 400×. Pulmonary arterial wall thickness was calculated as medial wall thickness index (%) = (external diameter – internal diameter)/external diameter × 100. For quantitative analysis, 20 randomly selected vessels from each rat were analyzed, and the average was calculated. Right ventricular hypertrophy was measured by the cardiomyocyte cross-sectional area (CSA) in HE-stained sections. To evaluate the collagen deposition in lung and RV heart tissue, Masson’s trichrome and Picrosirius red staining were performed. The degree of fibrosis in the lung and RV heart tissue was calculated by measuring the percentage of the collagen-positive area using Image-Pro Plus software.
Culture of Human Pulmonary Artery Endothelial Cells (HPAECs)
HPAECs were obtained from ScienCell Research Laboratories (ScienCell, Carlsbad, CA), maintained in endothelial cell medium (ScienCell) with 5% fetal bovine serum, 1% endothelial cell growth supplement, and 1% penicillin/streptomycin solution at 5% CO2 and 37 °C. Cells between passages 3 and 8 were used for further experiments.
Endothelial-Mesenchymal Transition of HPAECs in vitro
Cells were cultured at approximately 80% confluency. To determine the effects of paeoniflorin on EndMT, the cells were cultured under normoxia or hypoxia (1% O2) with or without paeoniflorin (10 μM, Sigma-Aldrich, St. Louis MO, USA) treatment for 0 h, 6 h, 12 h, 24 h, 48 h, and 72 h. EndMT was assessed by loss of the endothelial marker (VE-cadherin) and gain of the mesenchymal markers (α-SMA, fibronectin and vimentin) and EndMT-related transcription factor (snail and twist) using immunoblot analysis and immunofluorescence.
RNA Interference
For small interfering RNA (siRNA) transfection, HPAECs were grown in 24-well plates until the confluence of cells reached approximately 50%. siRNA against human bone morphogenetic protein receptor type II (BMPR2) (forward, 5ʹ-GCAGCAAGCACAAAUCAAATT-3ʹ; reverse, 5ʹ-UUUGAUUUGUGCUUGCUGCTT-3ʹ) and scrambled control (NC) siRNA were purchased from GenePharma (Shanghai, China). Cells were transfected with BMPR2 siRNA (50 nM) or scrambled siRNA (50 nM) using the siRNA transfection reagent siRNA-mate (GenePharma), following the manufacturer’s protocol. The knockdown efficiency of siRNAs was performed 24 h after transfection by Western blotting. After 24 h of transfection, the cells were cultured under normoxia or hypoxia with or without paeoniflorin for another 48 h, followed by lysis buffer for Western blot analysis.
Immunofluorescence Staining
HPAECs were fixed in 4% paraformaldehyde for 30 min, permeabilized with PBS containing 0.1% Triton-X100, then blocked with 2% BSA (Beyotime, Jiangsu, China) at room temperature. To access the EndMT process, samples were further incubated with anti–VE-cadherin antibody (Abcam, Cambridge, MA) and anti–vimentin (Abcam, Cambridge, MA) antibody overnight at 4°C. Subsequently, cells were incubated with secondary antibody (donkey anti-mouse IgG, Alexa Fluor 555; and donkey anti-rat IgG, Alexa Fluor 488) for 1 h at room temperature. Nuclei were visualized by 4, 6-diamidino-2-phenylindole (DAPI, Sigma-Aldrich). Fluorescence was observed with the Leica 2500 microscope. All samples were examined, and at least five images from each were acquired.
Western Blotting Analysis
Total proteins were extracted from the lung tissues and HPAECs with RIPA Lysis and Extraction buffer (Thermo Scientific, Rockford) containing protease inhibitors, phosphatase inhibitors, and PMSF. The protein concentrations were measured using BCA protein assay kit (Beyotime, Jiangsu, China). An equal amount of protein from each sample was subjected to electrophoresis on 10% SDS-PAGE gels and transferred to nitrocellulose/polyvinylidene difluoride membranes (Millipore, Bedford, MA) in a Bio-Rad Trans-Blot system. The membranes were blocked in TBST containing 5% powdered non-fat dry milk for 1 h at room temperature and then probed with primary antibodies, followed by incubation with the corresponding secondary antibodies. Antibodies against VE-cadherin, α-SMA, vimentin, fibronectin, p-SMAD1/5/SMAD1/5 and BMPR2 were obtained from Abcam (Cambridge, MA). β-actin, snail, twist antibody was obtained from Proteintech (Proteintech Group, CHI, USA). Protein signal was visualized by a WesternBright ECL (Advansta, CA). The band intensity was measured by Quantity One software (Bio-Rad, USA).
Statistical Analysis
All data were presented as the mean ± standard error of the mean (SEM). One-way or two-way ANOVA followed by Fisher’s LSD post-hoc test was used to compare the data of multiple groups. P < 0.05 was considered statistically significant.
Results
Effects of PF on RVSP and Pulmonary Vascular Remodeling in the SuHx Rat Model of PAH
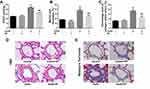
Hemodynamic data showed that rats in the SuHx group developed pulmonary hypertension as indicated by a significant increase in right ventricular systolic pressure (RVSP) on the 21th day, which was prevented by PF (Figure 2A). Pulmonary vascular remodeling is a key pathological feature of pulmonary hypertension, which is characterized by the thickening of all three layers of the blood vessel wall and exaggerated collagen deposition and perivascular infiltration of inflammatory cells. As is shown in Figure 2B–E, treated with PF (300 mg/kg) significantly decreased the thickness of pulmonary artery medial wall and the degree of collagen deposition in the distal pulmonary arteries induced by SU5416/hypoxia. Together, these results demonstrate that PF could prevent the development of experimental PAH and inhibited pulmonary vascular remodeling.
Effect of PF on Right Ventricular Hypertrophy and Fibrosis in the SuHx Rat Model of PAH
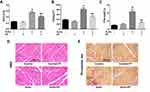
We next evaluate the efficacy of PF on right ventricular changes in experimental PAH in vivo. As shown in Figure 3A, RV/LV+S in the SuHx group showed a significant elevation compared to the control groups, which was alleviated by PF treatment. CSA of RV cardiomyocytes was calculated to evaluate the effect of PF on RV remodeling. Statistical analyses indicated that PF effectively attenuated SU5416/hypoxia-induced increment in CSA (Figure 3B). Representative images of H&E staining are shown in Figure 3D. Similar changes were observed in the interstitial RV fibrosis. As revealed by picrosirius red staining (Figure 3C and E), a significant elevation of interstitial fibrosis was observed in the right ventricle of SuHx rats, which was markedly decreased by the treatment of PF. Thus, the above observations suggest that PF attenuated right ventricular hypertrophy and fibrosis in the SuHx model in rats.
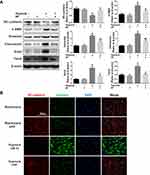
Paeoniflorin Decreased EndMT in Lung in the SuHx Rat Model of PAH
EndMT is a critical process in the progression of pulmonary hypertension and pulmonary vascular remodeling. The expression of EndMT-related markers was measured to evaluate the effects of PF on EndMT in vivo (Figure 4A). Our data indicated that SU5416/hypoxia treatment significantly decreased the expression of the endothelial marker VE-cadherin and increased the expression of mesenchymal markers including fibronectin and vimentin. Pretreated with PF restored the down-regulation of VE-cadherin and partially inhibited up-regulation of the mesenchymal markers induced by SU5416/hypoxia (Figure 4B–D). These results suggest that PF could inhibit EndMT in the SuHx rat model of PAH.
Hypoxia Induced EndMT in HPAECs
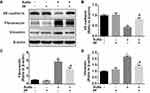
To induce the EndMT of HPAECs in vitro, cells were exposed to hypoxia for 0 h, 6 h, 12 h, 24 h, 48 h, and 72 h, respectively. As shown in Figure 5A, hypoxia induced a gradually upregulation of mesenchymal markers (α-SMA, fibronectin and vimentin) and EndMT-related transcription factor (snail and twist), but a downregulation of the endothelial marker (VE-cadherin), indicating hypoxia induced EndMT in HPAECs in a time-dependent manner. To validate this result, the expressions of EndMT-related markers were determined by immunofluorescence staining. After hypoxia for 48 h, the expression of vimentin was raised in the cytoplasm and the expression of VE-cadherin was decreased on cellular membrane compared to normoxia (Figure 5B). Taken together, these data demonstrated that hypoxia induced EndMT in HPAECs.
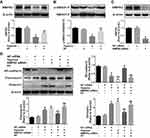
PF Prevented Hypoxia-Induced EndMT in HPAECs
Western blotting analysis showed that 10 μΜ PF reversed hypoxia-induced down-regulation of endothelial marker (VE-cadherin), and partially inhibited the up-regulation of mesenchymal markers (vimentin and fibronectin) (Figure 6A). Further immunofluorescence staining confirmed that PF inhibited hypoxia-induced loss of VE-cadherin on the cellular membrane and over-expression of vimentin in the cytoplasm in HPAECs (Figure 6B).
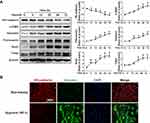
PF Inhibited Hypoxia-Induced EndMT in HPAECs by BMPR2 Up-Regulation
BMPR2 signaling dysfunction is known as one of the key contributors to EndMT in PAH. Our results showed that hypoxia significantly reduced the expression of BMPR2 in HPAECs, which could be partially restored by PF treatment (Figure 7A). In order to investigate the downstream effectors of the BMPR2 signaling pathways to support the hypothesis that the effects of paeoniflorin are partially mediated by BMPR2, we evaluated the phosphorylation levels of SMAD1/5, a downstream signal of BMPR2. Western blotting results showed that hypoxia significantly inhibited the phosphorylation level of SMAD1/5 in HPAECs, while PF treatment partially reversed the decreased phosphorylation levels of SMAD1/5 (Figure 7B). To further explore whether PF could attenuate hypoxia-induced EndMT via BMPR2 upregulation, HPAECs were transfected with BMPR2-siRNA or NC siRNA. The expression of the BMPR2 was significantly decreased after HPAECs transfected with BMPR2 siRNA for 24 h (Figure 7C). As shown in Figure 7D, BMPR2 knockdown by siRNA reduced the inhibitory effect of PF on hypoxia-induced EndMT. Collectively, these data suggest that BMPR2 is involved in the inhibitory effects of PF in hypoxia-induced EndMT in HPAECs.
Discussion
In the current study, we investigated the effects of PF on the development of pulmonary hypertension induced by SU5416 and hypoxia in rats. PF effectively attenuates SU5416/hypoxia-induced PAH, pulmonary vascular remodeling, right ventricular hypertrophy and fibrosis in rats. Besides, we found that PF attenuates EndMT in lung in the SuHx rat model of PAH. Furthermore, our data suggest that BMPR2 is involved in the inhibitory effects of PF in hypoxia-induced EndMT of cultured HPAECs.
Generally, three animal models have been popular for preclinical testing of new PAH pharmacotherapies: the monocrotaline lung injury model, the chronic hypoxia-induced pulmonary hypertension model and the SuHx model, which leads to severe PAH and closely mimics the vascular changes seen in patients with severe PAH and is now being extensively used.18 Our data corroborated with previous findings that we observed a significant increase in RVSP, accompanied by pulmonary vascular remodeling and right ventricular hypertrophy in the SuHx rat model. Paeoniflorin, a monoterpene glycoside, has beneficial effects on diverse diseases such as rheumatoid arthritis, liver fibrosis, chronic heart failure, and asthma.19–22 However, its efficacy against the development and progression of PAH has never been tested. In the present study, we found that paeoniflorin significantly ameliorated SU5416/hypoxia-induced PAH and reduced pulmonary vascular remodeling and right ventricular hypertrophy. A growing body of data from patients with idiopathic pulmonary arterial hypertension suggests that RV fibrosis and pulmonary interstitial fibrosis impair cardiac function, and plays a crucial role in the development of pulmonary hypertension and RV failure.23 PF have also been reported to attenuate fluctuant hyperglycemia-induced vascular endothelial injuries, and to improve bleomycin-induced pulmonary fibrosis in rats by suppressing collagen synthesis.8,24 Besides, the anti-remodeling effect of PF on cardiovascular diseases, including acute myocardial infarction and pressure overload-induced cardiac remodeling, might also contribute to the beneficial effect of PF on PH.21,25 In the present study, we also found a marked attenuation of the collagen deposition in the pulmonary arterial and RV heart tissue in the PF-treated SuHx rats compared to vehicle-treated SuHx rats. Thus, PF significantly inhibited cardiopulmonary fibrosis, which may represent a novel therapeutic agent for the treatment of PAH.
Vascular cell phenotype switching such as EndMT plays an important role in abnormal pulmonary vascular remodeling and tissue fibrosis in PAH and it has been reported that 5% of ECs express both endothelial and mesenchymal phenotypes in SU5416/hypoxia-induced PAH.26,27 Generally, EndMT is characterized by the acquisition of mesenchymal phenotype while the loss of endothelial cell markers in endothelium. EndMT is one source of matrix-generating fibroblasts in pulmonary hypertension, leading to extracellular matrix production and collagen deposition, which contributes to pulmonary vascular remodeling. Previous studies have indicated that PF protected against TGFβ1-induced EMT in pulmonary fibrosis.24 In our study, we found that endothelial marker was down-regulated and mesenchymal markers were significantly up-regulated in SuHx rat lungs. However, these changes were partially reversed by the treatment of PF. Thus, the present results suggest that PF inhibits EndMT in the SuHx rat model of PAH. This result was further confirmed in cultured HPAECs in vitro. Notably, hypoxia induced upregulation of mesenchymal markers (α-SMA, fibronectin and vimentin) and EndMT-related transcription factor (Snail and Twist), but downregulation of the endothelial marker (VE-cadherin) in HPAECs in a time-dependent manner, which was inhibited by PF. Thus, our data from either in vivo or in vitro studies suggest that PF could suppress EndMT in lung, which subsequently inhibited the development of PAH.
BMPR2 and its related signaling pathway have been recognized as a key regulator of pulmonary vascular homeostasis, as an estimated 80% of familial and 20% of idiopathic PAH patients carrying a heterozygous BMPR2 mutation.28,29 BMPR2 gene mutations have also been found in patients with pulmonary hypertension caused by other factors (congenital heart disease, appetite suppressant drugs, pulmonary vein occlusion, etc.). Moreover, BMPR2 hypofunction have been shown to contribute to PAH pathogenesis, even though without BMPR2 mutations.30 A growing number of studies have linked dysfunctional BMPR2 signaling, including BMPR2, p-SMADs and Id1 to the development of EndMT process in PAH, and targeted gene delivery of BMPR2 has a therapeutic effect in the animal models of PAH.31–33 Moreover, it was reported that TGF-β1 induced EndMT in human pulmonary microvascular ECs is associated with reduced BMPR2 expression, and stimulating BMPR2 signaling partially ameliorated TGF-β1 induced EndMT in ECs.33 There are multiple causes for the downregulation of BMPR2 signaling, such as RAGE, 17-β estradiol.34,35 Under hypoxic conditions, HIF-1α induces miR-322, which targets BMPR1a and SMAD5, thereby downregulating BMPR2 signaling.36,37 There is strong evidence showing that PF can effectively prevent renal interstitial fibrosis through blocking EMT via BMP-7 recovery.38 Previous studies also reported that PF attenuated cobalt chloride-induced apoptosis of endothelial cells via HIF-1α pathway.39 Our study is consistent with previous reports that hypoxia significantly reduced the expression of BMPR2 and downstream phosphorylation levels of SMAD1/5 in HPAECs, which was partially reversed by PF. Moreover, down-regulating the expression of BMPR2 in HPAECs by siRNA reduced the inhibitory role effect of PF on hypoxia-induced EndMT in HPAECs. Therefore, our results showed that BMPR2 is involved in the inhibitory effects of PF in hypoxia-induced EndMT.
Conclusion
In summary, our study suggests that PF is a potential therapeutic agent for PAH. Our research results demonstrate that PF alleviates pulmonary vascular remodeling and right ventricular hypertrophy in the SuHx rat model of PAH. The underlying mechanism of PF may involve the inhibition of BMPR2 down-regulation-mediated EndMT. Thus, paeoniflorin might be a promising drug for the treatment of PAH.
Ethic Approval
The study was approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (NJMU/IACUC-1809008).
Acknowledgments
This work was supported by the National Natural Science Foundation of China (81870054, 81800011, 81800090, 81472199), the Priority Academic Program Development of Jiangsu Higher Education Institutions (JX10231802), the National Natural Science Foundation of Jiangsu Province of China (M.Y.) (grant numbers BK20141162), the Fundamental Research Funds for the Central Universities (grant numbers 22120180610), and the Key Project of National Science & Technology for Infectious Diseases of China (grant numbers 2015ZX10003001, 2018ZX10722301).
Author Contributions
All authors contributed to data analysis, drafting or revising the article, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest in this work.
References
1. Simonneau G, Montani D, Celermajer DS, et al. Haemodynamic definitions and updated clinical classification of pulmonary hypertension. Eur Respir J. 2019;53(1):Jan. doi:10.1183/13993003.01913-2018
2. Humbert M, Guignabert C, Bonnet S, et al. Pathology and pathobiology of pulmonary hypertension: state of the art and research perspectives. Eur Respir J. 2019;53(1):1801887. doi:10.1183/13993003.01887-2018
3. Sitbon O, Gomberg-maitland M, Granton J, et al. Clinical trial design and new therapies for pulmonary arterial hypertension. Eur Respir J. 2019;53(1):Jan. doi:10.1183/13993003.01908-2018
4. Zhang H, Lui KO, Zhou B. Endocardial cell plasticity in cardiac development, diseases and regeneration. Circ Res. 2018;122(5):774–789. doi:10.1161/CIRCRESAHA.117.312136
5. Ranchoux B, Antigny F, Rucker-martin C, et al. Endothelial-to-mesenchymal transition in pulmonary hypertension. Circulation. 2015;131(11):1006–1018. doi:10.1161/CIRCULATIONAHA.114.008750
6. Ranchoux B, Harvey LD, Ayon RJ, et al. Endothelial dysfunction in pulmonary arterial hypertension: an evolving landscape (2017 Grover Conference Series). Pulm Circ. 2018;8(1):2045893217752912. doi:10.1177/2045893217752912
7. Wen J, Xu B, Sun Y, et al. Paeoniflorin protects against intestinal ischemia/reperfusion by activating LKB1/AMPK and promoting autophagy. Pharmacol Res. 2019;146:104308. doi:10.1016/j.phrs.2019.104308
8. Wang JS, Huang Y, Zhang S, et al. A protective role of paeoniflorin in fluctuant hyperglycemia-induced vascular endothelial injuries through antioxidative and anti-inflammatory effects and reduction of PKCbeta1. Oxid Med Cell Longev. 2019;2019:5647219.
9. Kong M, Liu HH, Wu J, et al. Effects of sulfur-fumigation on the pharmacokinetics, metabolites and analgesic activity of Radix Paeoniae Alba. J Ethnopharmacol. 2018;212:95–105. doi:10.1016/j.jep.2017.10.023
10. Yu G, Wang Z, Zeng S, et al. Paeoniflorin inhibits hepatocyte growth factor- (HGF-) induced migration and invasion and actin rearrangement via suppression of c-Met-mediated RhoA/ROCK signaling in glioblastoma. Biomed Res Int. 2019;2019:9053295. doi:10.1155/2019/9053295
11. Tu J, Guo Y, Hong W, et al. The regulatory effects of paeoniflorin and its derivative paeoniflorin-6ʹ-O-benzene sulfonate CP-25 on inflammation and immune diseases. Front Pharmacol. 2019;10:57. doi:10.3389/fphar.2019.00057
12. Wang Y, Che J, Zhao H, Tang J, Shi G. Paeoniflorin attenuates oxidized low-density lipoprotein-induced apoptosis and adhesion molecule expression by autophagy enhancement in human umbilical vein endothelial cells. J Cell Biochem. 2019;120(6):9291–9299. doi:10.1002/jcb.v120.6
13. Chen J, Zhang M, Zhu M, et al. Paeoniflorin prevents endoplasmic reticulum stress-associated inflammation in lipopolysaccharide-stimulated human umbilical vein endothelial cells via the IRE1alpha/NF-kappaB signaling pathway. Food Funct. 2018;9(4):2386–2397. doi:10.1039/C7FO01406F
14. Yu J, Zhu X, Qi X, Che J, Cao B. Paeoniflorin protects human EA.hy926 endothelial cells against gamma-radiation induced oxidative injury by activating the NF-E2-related factor 2/heme oxygenase-1 pathway. Toxicol Lett. 2013;218(3):224–234. doi:10.1016/j.toxlet.2013.01.028
15. Zhou Z, Wang S, Song C, Hu Z. Paeoniflorin prevents hypoxia-induced epithelial-mesenchymal transition in human breast cancer cells. Onco Targets Ther. 2016;9:2511–2518. doi:10.2147/OTT.S102422
16. Farkas D, Thompson AAR, Bhagwani AR, et al. Toll-like receptor 3 is a therapeutic target for pulmonary hypertension. Am J Respir Crit Care Med. 2019;199(2):199–210. doi:10.1164/rccm.201707-1370OC
17. Yung LM, Nikolic I, Paskin-flerlage SD, Pearsall RS, Kumar R, Yu PB. A selective transforming growth factor-beta ligand trap attenuates pulmonary hypertension. Am J Respir Crit Care Med. 2016;194(9):1140–1151. doi:10.1164/rccm.201510-1955OC
18. de Raaf MA, Schalij I, Gomez-arroyo J, et al. SuHx rat model: partly reversible pulmonary hypertension and progressive intima obstruction. Eur Respir J. 2014;44(1):160–168. doi:10.1183/09031936.00204813
19. Xu H, Cai L, Zhang L, et al. Paeoniflorin ameliorates collagen-induced arthritis via suppressing nuclear factor-kappaB signalling pathway in osteoclast differentiation. Immunology. 2018. doi:10.1111/imm.12907
20. Hu Z, Qin F, Gao S, Zhen Y, Huang D, Dong L. Paeoniflorin exerts protective effect on radiation-induced hepatic fibrosis in rats via TGF-beta1/Smads signaling pathway. Am J Transl Res. 2018;10(3):1012–1021.
21. Chen H, Dong Y, He X, Li J, Wang J. Paeoniflorin improves cardiac function and decreases adverse postinfarction left ventricular remodeling in a rat model of acute myocardial infarction. Drug Des Devel Ther. 2018;12:823–836. doi:10.2147/DDDT
22. Shou Q, Jin L, Lang J, et al. Integration of metabolomics and transcriptomics reveals the therapeutic mechanism underlying paeoniflorin for the treatment of allergic asthma. Front Pharmacol. 2018;9:1531. doi:10.3389/fphar.2018.01531
23. Andersen S, Nielsen-kudsk JE, Vonk Noordegraaf A, de Man FS. Right ventricular fibrosis. Circulation. 2019;139(2):269–285. doi:10.1161/CIRCULATIONAHA.118.035326
24. Ji Y, Dou YN, Zhao QW, et al. Paeoniflorin suppresses TGF-beta mediated epithelial-mesenchymal transition in pulmonary fibrosis through a Smad-dependent pathway. Acta Pharmacol Sin. 2016;37(6):794–804. doi:10.1038/aps.2016.36
25. Liu X, Chen K, Zhuang Y, et al. Paeoniflorin improves pressure overload-induced cardiac remodeling by modulating the MAPK signaling pathway in spontaneously hypertensive rats. Biomed Pharmacother. 2019;111:695–704. doi:10.1016/j.biopha.2018.12.090
26. Suzuki T, Carrier EJ, Talati MH, et al. Isolation and characterization of endothelial-to-mesenchymal transition cells in pulmonary arterial hypertension. Am J Physiol Lung Cell Mol Physiol. 2018;314(1):L118–L126. doi:10.1152/ajplung.00296.2017
27. Good RB, Gilbane AJ, Trinder SL, et al. Endothelial to mesenchymal transition contributes to endothelial dysfunction in pulmonary arterial hypertension. Am J Pathol. 2015;185(7):1850–1858. doi:10.1016/j.ajpath.2015.03.019
28. Cogan JD, Pauciulo MW, Batchman AP, et al. High frequency of BMPR2 exonic deletions/duplications in familial pulmonary arterial hypertension. Am J Respir Crit Care Med. 2006;174(5):590–598. doi:10.1164/rccm.200602-165OC
29. Evans JD, Girerd B, Montani D, et al. BMPR2 mutations and survival in pulmonary arterial hypertension: an individual participant data meta-analysis. Lancet Respir Med. 2016;4(2):129–137. doi:10.1016/S2213-2600(15)00544-5
30. Tielemans B, Delcroix M, Belge C, Quarck R. TGFbeta and BMPRII signalling pathways in the pathogenesis of pulmonary arterial hypertension. Drug Discov Today. 2019;24(3):703–716. doi:10.1016/j.drudis.2018.12.001
31. Orriols M, Gomez-puerto MC, Ten Dijke P. BMP type II receptor as a therapeutic target in pulmonary arterial hypertension. Cell Mol Life Sci. 2017;74(16):2979–2995. doi:10.1007/s00018-017-2510-4
32. Hopper RK, Moonen JR, Diebold I, et al. In pulmonary arterial hypertension, reduced BMPR2 promotes endothelial-to-mesenchymal transition via HMGA1 and its target slug. Circulation. 2016;133(18):1783–1794. doi:10.1161/CIRCULATIONAHA.115.020617
33. Reynolds AM, Holmes MD, Danilov SM, Reynolds PN. Targeted gene delivery of BMPR2 attenuates pulmonary hypertension. Eur Respir J. 2012;39(2):329–343. doi:10.1183/09031936.00187310
34. Meloche J, Courchesne A, Barrier M, et al. Critical role for the advanced glycation end-products receptor in pulmonary arterial hypertension etiology. J Am Heart Assoc. 2013;2(1):e005157.
35. Austin ED, Hamid R, Hemnes AR, et al. BMPR2 expression is suppressed by signaling through the estrogen receptor. Biol Sex Differ. 2012;3(1):6. doi:10.1186/2042-6410-3-6
36. Zeng Y, Liu H, Kang K, et al. Hypoxia inducible factor-1 mediates expression of miR-322: potential role in proliferation and migration of pulmonary arterial smooth muscle cells. Sci Rep. 2015;5:12098. doi:10.1038/srep12098
37. Yu H, Alruwaili N, Hu B, et al. Potential role of cartilage oligomeric matrix protein in the modulation of pulmonary arterial smooth muscle superoxide by hypoxia. Am J Physiol Lung Cell Mol Physiol. 2019;317:L569–L577. doi:10.1152/ajplung.00080.2018
38. Zeng J, Dou Y, Guo J, Wu X, Dai Y. Paeoniflorin of Paeonia lactiflora prevents renal interstitial fibrosis induced by unilateral ureteral obstruction in mice. Phytomedicine. 2013;20(8–9):753–759. doi:10.1016/j.phymed.2013.02.010
39. Ji Q, Yang L, Zhou J, et al. Protective effects of paeoniflorin against cobalt chloride-induced apoptosis of endothelial cells via HIF-1alpha pathway. Toxicol in Vitro. 2012;26(3):455–461. doi:10.1016/j.tiv.2012.01.016
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.