Back to Journals » Cancer Management and Research » Volume 12
PACE4 Expression is a Novel Independent Prognostic Factor in Nasopharyngeal Carcinoma
Authors Lin Y, Long H, Tan X, Zhang D, Jiang L
Received 28 May 2020
Accepted for publication 2 August 2020
Published 18 September 2020 Volume 2020:12 Pages 8623—8629
DOI https://doi.org/10.2147/CMAR.S264143
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 5
Editor who approved publication: Dr Ahmet Emre Eşkazan
Yunen Lin,1,* Huidong Long,2,* Xiaojun Tan,1,* Donghui Zhang,1 Liwen Jiang1
1Department of Pathology, Affiliated Cancer Hospital & Institute of Guangzhou Medical University, Guangzhou, Guangdong, 510120, People’s Republic of China; 2Department of Medical Oncology, Affiliated Cancer Hospital & Institute of Guangzhou Medical University, Guangzhou, Guangdong, 510095, People’s Republic of China
*These authors contributed equally to this work
Correspondence: Huidong Long
Affiliated Cancer Hospital & Institute of Guangzhou Medical University, No. 78 Hengzhi Gang, Guangzhou 510095, People’s Republic of China
Tel +86-13631319747
Email [email protected]
Background: Paired basic amino acid-cleaving enzyme 4 (PACE4) belongs to the family of proprotein convertase and is essential for tumor progression, whereas its role in cancer remains controversial and little is known about its role in nasopharyngeal carcinoma (NPC). The aim of this study was to examine if the expression of PACE4 is a prognostic biomarker for patients with NPC.
Methods: Immunofluorescence (IF) and immunohistochemistry (IHC) were used to analyze PACE4 expression in NPC cell line CNE1 and 172 clinicopathologically characterized NPC tissues. The data were analyzed by Chi-square test, Kaplan–Meier plots, and Cox proportional hazards regression model.
Results: IF and IHC staining results showed that PACE4 was mainly located in the cytoplasm of NPC cell line (CNE1) and NPC tissues. Expression of PACE4 was observed in 46/172 (26.7%) of NPC tissues. Further analysis showed that expression of PACE4 was positively associated with late N stage, distant metastasis, and late clinical stage (P< 0.05). High expression of PACE4 predicted shorter 5-year overall survival of patients with NPC, especially for the patients in advanced stage (32.7% vs 77.3%, P< 0.001). Furthermore, multivariate analysis showed that PACE4 expression may serve as a potential prognostic factor for NPC.
Conclusion: Our results suggest that PACE4 may play a crucial role in tumor progression and may serve as a valuable prognostic biomarker for patients with NPC.
Keywords: paired basic amino acid-cleaving enzyme 4, immunohistochemistry, nasopharyngeal carcinoma, overall survival, prognostic biomarker
Introduction
Nasopharyngeal carcinoma (NPC) is a prevalent malignant tumor in South China, especially in the Guangdong area.1 Genetic susceptibility and environmental factors including Epstein-Barr virus infection have been suggested to be associated with the pathogenesis of NPC.1–3 NPC is characterized by a multi-sequential process, which appears to be dominated by multiple abnormal genetic changes, and this process is related to the progression of NPC.1 However, the precise genetic alterations underlying NPC progression are largely unclear. Thus, the identification of novel genetic markers for the early detection of this malignant tumor is essential, which may provide more effective targeted therapies and ultimately improve the clinical outcome of patients with NPC.
Paired basic amino acid-cleaving enzyme 4 (PACE4), also known as proprotein convertase subtilisin/kexin type 6 (PCSK6), is a member of the proprotein convertase family, which plays regulatory roles in the proteolytic activity of various precursor proteins and in the regulation of protein maturation.4 In addition, PACE4 is involved in the regulation of various cellular processes such as proliferation, differentiation, and apoptosis.5–8 Previous studies showed that PACE4 was clearly down-regulated in ovarian cancer cell lines, such as Hey and Hey-C cells, and a series of primary ovarian tumors.9,10 Nevertheless, accumulating studies have highlighted PACE4 for its potential role in certain human malignancies such as oral tongue carcinoma,11 hepatocellular carcinoma,12 glioma,13 skin cancer,6,14 prostate cancer,15 and lung cancer.16 These studies suggested that PACE4 is a positive regulator of cancer progression and is associated with the tumorigenesis of cancers. PACE4 overexpression can lead to the enhanced susceptibility to carcinogenesis and tumor progression, indicating a novel target for suppressing tumor cell invasion.6
To date, the dynamic expression of PACE4 in NPCs and its clinicopathologic/prognostic implication have not been studied. In the present study, immunofluorescence (IF) and immunohistochemistry (IHC) were utilized to analyze the distribution of PACE4 protein expression in the NPC cohort and nasopharyngeal mucosal tissues. Moreover, the relationships between PACE4 expression and clinicopathologic and prognostic implication on the NPC patients were assessed to determine whether the expression of PACE4 is a prognostic biomarker for patients with NPC.
Materials and Methods
Patients and Tissue Specimens
In this study, paraffin-embedded pathologic specimens from 172 patients with NPC and 12 normal nasopharyngeal epithelial tissues from healthy volunteers (fiberoptic nasopharyngeal biopsy) were obtained from the Department of Pathology, Cancer Centre of Guangzhou Medical College and The First Affiliated Hospital of Guangzhou Medical University between July 2005 and June 2008. The 12 healthy volunteers were recruited during routine physical examination in our hospital. These NPC cases included 113 (65.7%) males and 59 (34.3%) females, with a median age of 45 years old (range from 18 to 72 years old). The inclusion criteria for the patients were as follows: 1) All subjects were newly diagnosed without radiotherapy, chemotherapy, or synchronous cancer. 2) All subjects had no inflammatory diseases, diabetes, hypertension, or other complications. 3) Diagnosis of the NPC was confirmed by histological examination. The average follow-up time was 64.62 months (range from 8.0 to 102 months). The clinicopathologic characteristics of these patients, including age, sex, histological type, T stage, N stage, distant metastasis, and clinical stage, are described in Table 1. After enrolment, the NPC patients received radiotherapy via the intensity-modulated radiation therapy technique, and concurrent chemotherapy. Ethical approval for the study was approved by the Ethics Committee of the Affiliated Cancer Hospital & Institute of Guangzhou Medical University and all the patients signed written informed consent forms. The disease stages of all of the patients were classified or reclassified according to the 1992 nasopharyngeal carcinoma staging system in China.17
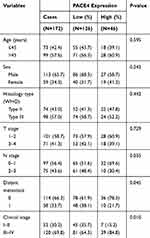 |
Table 1 Relationships Between PACE4 Expression and Clinicopathological Parameters |
Cell Line and Cell Culture
The nasopharyngeal carcinoma cell line (CNE1) was provided by the Affiliated Cancer Hospital & Institute of Guangzhou Medical University. The CNE1 cells were cultured with RPMI1640 culture medium (Sigma-Aldrich, St. Louis, MO, USA) supplied with 10% fetal bovine serum (FBS; Thermo Fisher Scientific, Waltham, MA, USA) in an incubator supplied with 5% CO2 at 37°C. The use of the cell line was approved by the Ethics Committee of Cancer Centre of Guangzhou Medical University, and the cells were authenticated by STR profile.
Immunofluorescence
The CNE1 cells were harvested and washed three times with phosphate-buffered saline (PBS), suspended in PBS, and seeded on slides. The slides were fixed with 95% alcohol for 30 minutes and permeabilized. After 1 hour of blocking in PBS+0.1% Tween+1% bovine serum albumin, the cells were incubated with the PACE4 primary antibody (Rabbit mAb; Abcam, Cambridge, UK) at 37°C for 1 hour and then with a secondary antibody for 30 minutes at 37°C. After counterstaining with 4ʹ,6-diamidino-2-phenylindole (DAPI; 1 μg/mL) for 10 minutes, the slides were observed by fluorescence microscopy and photographed.
Immunohistochemistry (IHC) of the Clinical Tissues
IHC was performed to investigate the expression of PACE4 in 172 human NPC tissues and 12 normal nasopharyngeal epithelial tissues. The sections were incubated with a PACE4 primary antibody (1:100 dilution; Abcam, Cambridge, UK) for 1 hour at room temperature. Mayer’s hematoxylin was used for nuclear counterstaining. The sections were mounted using a synthetic medium. The slides were reviewed by two or three pathologists blinded to the study. To evaluate the PACE4 expression levels, immune-stained slides were evaluated using a method described previously.16 The percentage of tumor cells with positive staining of PACE4 was determined at high magnification (×200). For PACE4, cytoplasmic immunostaining in tumor cells was considered to be positive. The staining intensity was judged as no staining, −; light brown, +; brown, ++; and strong brown, +++. The percentage of positive cells <10% was considered as negative and the detailed information is as follows: <10%, −; 11–25%, +; 26–50%, ++; >50%, +++. These two scores were added together to form a total score, score <2 was considered to be low expression, and score ≥2 was considered to be high expression.
Statistical Analysis
All the data analyses were performed using SPSS 16.0 (SPSS, Chicago, IL, USA). Chi-square test (two-sided) was used to analyze the significant difference for categorical data. Kaplan–Meier’s method with log rank test or Cox regression method for univariate or multivariate overall survival (OS) analysis were performed to assess the correlation between expression of PACE4 and clinicopathological indices by P-values less than 0.05 were considered to indicate statistical significance.
Results
Expression of PACE4 in NPCs Cell Line and Tissues
IF staining showed that PACE4 is mainly located in the cytoplasm of CNE1 cells (Figure 1). PACE4 staining was not detected in the normal nasopharyngeal epithelial tissues (Figure 2A), whereas IHC staining consistently showed that PACE4 is located in the cytoplasm of NPC tissues (Figure 2B and C). Furthermore, PACE4 protein expression was detected in 26.7% (46/172) of the NPC samples.
 |
Figure 1 IF staining for PACE4 in CNE1 cell line. PACE4 is mainly located in the cytoplasm of CNE1 cells (green for PACE4 and blue for DAPI). |
Clinicopathological Significance of PACE4 Expression in Patients with NPCs
The positive rates of PACE4 expression in NPCs with respect to several standard clinicopathologic features are presented in Table 1. The positive rate of PACE4 expression was higher in patients with late N stage (P=0.035), distant metastasis (P=0.045), and late clinical stage (P=0.010). There was no statistically significant association between PACE4 expression and other clinicopathologic parameters including age, sex, histological type, and T stage (P>0.05; Table 1).
PACE4 Expression is Closely Related to the Long-Term Survival of Patients with NPCs
The 5-year OS rate of the cohort of 172 NPC patients was 58.1% (Figure 3A). The prognostic value of PACE4 was evaluated through the estimation of OS using the Kaplan–Meier method and Log rank test. The results showed that high PACE4 expression was significantly related to poor OS compared with low PACE4 expression (38.8% vs 77.9%, respectively; P<0.001; Figure 3B). Next, we stratified the clinical stages into two groups that include early stages (I+II) and advanced stages (III+IV), and found that the association between high PACE4 expression and shorter OS was significantly stronger in patients at the advanced stages than early stages. At the early stages, the 5-year OS rates were 79.2% and 71.4% among the high- and low-expression patients, respectively (P>0.05; Figure 3C). At the advanced stages, the 5-year OS rate of late-stage patients with high or low expression of PACE4 was 32.7% or 77.3%, respectively (P<0.001; Figure 3D).
Expression of PACE4 is an Independent Factor to Predict the Poor Prognosis of Patients with NPCs
Moreover, multivariate analysis was performed to determine the impact of PACE4 expression and the clinicopathologic variables (ie, distant metastasis and clinical stage) on 5-year OS in patients with NPC. Our results showed that the expression of PACE4 was an independent prognostic factor for NPC patients with shorter 5-year OS (hazard ratio=3.025; 95% confidence interval (CI)=1.949–4.695, P<0.001; Table 2).
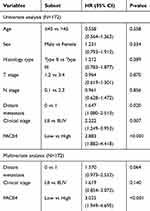 |
Table 2 Univariate and Multivariate Analysis with Respect to Over Survival in NPCs |
Discussion
The prognosis of NPC patients of uniform stage following radiotherapy is substantially different, and this underlying variation remains poorly understood. Thus, it is extremely urgent that new objective strategies that can effectively distinguish patients with favorable vs unfavorable prognoses for NPC are needed. The prognosis not only depends on the clinicopathological staging, but also on the molecular pathological classification. Despite NPC being widely investigated, the specific molecular biomarkers in NPC cells that have clinical/prognostic significance have yet to be identified.
PACE4, a candidate kexin-like mammalian proprotein-processing enzyme, was identified initially in DNA coding sequences,18 and its activation has been correlated with transcription factor basic helix-loop-helix regulation.19,20 PACE4 has the potential to participate in various cellular functions including cell proliferation, differentiation, and apoptosis.5,20 Recently, several studies have documented the involvement of PACE4 in oncogenic processes in various types of cancers including oral tongue cancer, liver cancer, skin cancer, prostate cancer, lung cancer, and glioma.6,11-15,18 However, until now, no study has explored the expression of PACE4 and its potential impact in NPC tumorigenesis.
To address this question, it is the first time that IF and IHC were used to analyze the PACE4 expression and its clinical significance in NPC. Localization of the PACE4 protein was shown in the cytoplasm of nasopharyngeal carcinoma cell line CNE1 and tissues. Statistical evaluation indicated more frequent expression of PACE4 in NPC tissues than in non-nasopharyngeal carcinoma tissues. Furthermore, the expression of PACE4 in NPCs is associated with N stage, distant metastasis, and clinical stage. On the other hand, no significant relationship was found between the PACE4 immunohistochemical data and age, sex, histological type, and T stage. Studies have demonstrated that T and N stage influenced independently on the clinical outcomes of NPC,21 which was not detected in this study, and this inconsistency may be due to limited sample size in our study. Nevertheless, the expression of PACE4 was a strong and independent predictor of poor OS as evidenced by univariate and multivariate analysis. In addition, stratified analysis of NPC in the light of clinical stage evaluated PACE4 expression to be closely related to survival of NPC patients with stage III or IV. Our observations imply that the expression of PACE4 in NPC may highlight increased malignant traits and/or poorer prognosis of this tumor subtype. Hence, its clinical value lies in that more rigorous monitoring and more aggressive regimens favored for tumors expressing PACE4. This may be a valuable way to reduce mortality and prolong the lifetime as much as possible.
Several features of PACE4 have indicated an important role of the PACE4 protein in oncogenic processes.19 PACE4 expression was shown to be a target in prostatic malignancy and associated with its invasiveness.20 Similarly, in human breast cancer, knockdown of PACE4 with MDA-MB-231 cells showed significantly reduced proliferation, migration, and invasion.22 Additionally, in lung carcinoma, our previous study exhibited that overexpression of PACE4 is indicative of poor clinical outcome.23 Mechanistically, Panet et al24 showed that PCAE4 was the key driver of ZR-75-1 estrogen receptor-positive breast proliferation and tumor progression. PACE4 can modulate apoptosis in human prostate cancer cells via endoplasmic reticulum stress and mitochondrial signaling pathways.25 In the present study, increased PACE4 expression was associated with the aggressive features of NPC and demonstrated potential as a new and independent predictor of worse cancer-specific survival. Thereby, in combination with other biomarkers of NPC, PACE4 expression status may be useful to stratify patients for novel therapeutic strategies such as adjuvant chemotherapy, radio-sensitization effect, or the establishment of appropriate treatment selection criteria for NPC patients.
To our knowledge, our study was the first to evaluate the prognostic value of PACE4 expression in the NPC clinical tissue specimens at protein level by immunohistochemical analysis. The most valuable finding of this study is that the 5-year OS of our study cohort was significantly poorer in high PACE4 expression cases than in low PACE4 expression cases. Furthermore, accentuated PACE4 expression may be a specific molecule predictor for NPC patients. Although the detailed molecular mechanism involved in this process is unclear, the present study has potential clinical benefits. Finally, the expression of PACE4, which could be detected by IHC, might be a useful molecular marker to provide evidence for molecular target therapy of NPC patients. Nevertheless, several limitations should be considered in the study. Firstly, a relatively small sample size from only a single center was included, which might cause some selective bias affecting the results, and future studies require more enrolled NPC patients to confirm the findings. Secondly, the current study lacks the NPC patients’ information including lymphovascular invasion and perineural invasion, which should be included in future studies with more NPC patients. Thirdly, the impact of PACE4 on disease-specific survival, recurrence-free survival, and metastasis survival has not been analyzed, and further studies may consider to incorporate the comprehensive analysis in order to reveal the prognostic significance of PACE4 in NPC patients. Fourthly, bioinformatics analysis may be performed in the public databases to confirm the prognostic potential of PACE4 in NPC patients.
Conclusions
Our study demonstrated that PACE4 expression plays an important role in the acquisition of an aggressive phenotype in NPC, suggesting that the expression of PACE4, as assessed by IHC, provides a promising independent biomarker for poorer OS in NPC patients. To obtain a better understanding of the mechanism of carcinogenesis and tumor progression in patients with NPC, further multifarious experimental model studies are needed. Moreover, it will be of paramount importance to analyze quantitatively the predictive power of PACE4 overexpression for NPC and other tumor subsets in further investigations.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising, or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Disclosure
The authors declare that they have no conflicts of interest for this work.
References
1. Lo KW, Huang DP. Genetic and epigenetic changes in nasopharyngeal carcinoma. Semin Cancer Biol. 2002;12(6):451–462.
2. Tao Q, Chan AT. Nasopharyngeal carcinoma: molecular pathogenesis and therapeutic developments. Expert Rev Mol Med. 2007;9(12):1–24.
3. Vasef MA, Ferlito A, Weiss LM. Nasopharyngeal carcinoma, with emphasis on its relationship to Epstein-Barr virus. Ann Otol Rhinol Laryngol. 1997;106(4):348–356.
4. Wang F, Wang L, Jiang H, Chang X, Pan J. Inhibition of PCSK6 may play a protective role in the development of rheumatoid arthritis. J Rheumatol. 2015;42(2):161–169.
5. Wang Y, Wang XH, Fan DX, Zhang Y, Li MQ, Wu HX, Jin LP. PCSK6 regulated by LH inhibits the apoptosis of human granulosa cells via activin A and TGFbeta2. J Endocrinol. 2014;222(1):151–160.
6. Bassi DE, Lopez De Cicco R, Cenna J, Litwin S, Cukierman E, Klein-Szanto AJ. PACE4 expression in mouse basal keratinocytes results in basement membrane disruption and acceleration of tumor progression. Cancer Res. 2005;65(16):7310–7319.
7. Kim H, Tabata A, Tomoyasu T, et al. Estrogen stimuli promote osteoblastic differentiation via the subtilisin-like proprotein convertase PACE4 in MC3T3-E1 cells. J Bone Miner Metab. 2015;33(1):30–39.
8. Bassi DE, Fu J, Lopez de Cicco R, Klein-Szanto AJ. Proprotein convertases: “master switches” in the regulation of tumor growth and progression. Mol Carcinog. 2005;44(3):151–161.
9. Fu Y, Campbell EJ, Shepherd TG, Nachtigal MW. Epigenetic regulation of proprotein convertase PACE4 gene expression in human ovarian cancer cells. Mol Cancer Res. 2003;1(8):569–576.
10. Matei D, Graeber TG, Baldwin RL, Karlan BY, Rao J. Gene expression in epithelial ovarian carcinoma. Oncogene. 2002;21(41):6289–6298.
11. Estilo CL, Oc P, Talbot S, et al. Oral tongue cancer gene expression profiling: identification of novel potential prognosticators by oligonucleotide microarray analysis. BMC Cancer. 2009;9:11.
12. Kurokawa Y, Matoba R, Nakamori S, et al. PCR-array gene expression profiling of hepatocellular carcinoma. J Exp Clin Cancer Res. 2004;23(1):135–141.
13. Delic S, Lottmann N, Jetschke K, Reifenberger G, Riemenschneider MJ. Identification and functional validation of CDH11, PCSK6 and SH3GL3 as novel glioma invasion-associated candidate genes. Neuropathol Appl Neurobiol. 2012;38(2):201–212.
14. Mahloogi H, Bassi DE, Klein-Szanto AJ. Malignant conversion of non-tumorigenic murine skin keratinocytes overexpressing PACE4. Carcinogenesis. 2002;23(4):565–572.
15. D’Anjou F, Routhier S, Perreault JP, et al. Molecular Validation of PACE4 as a Target in Prostate Cancer. Transl Oncol. 2011;4(3):157–172.
16. Lin YE, Wu QN, Lin XD, Li GQ. Expression of paired basic amino acid-cleaving enzyme 4 (PACE4) correlated with prognosis in non-small cell lung cancer (NSCLC) patients. J Thorac Dis. 2015;7(5):850–860.
17. Wang HY, Chang YL, To KF, et al. A new prognostic histopathologic classification of nasopharyngeal carcinoma. Chin J Cancer. 2016;35:41.
18. Van de Ven WJ, Roebroek AJ, Van Duijnhoven HL. Structure and function of eukaryotic proprotein processing enzymes of the subtilisin family of serine proteases. Crit Rev Oncog. 1993;4(2):115–136.
19. Koide S, Yoshida I, Tsuji A, Matsuda Y. The expression of proprotein convertase PACE4 is highly regulated by Hash-2 in placenta: possible role of placenta-specific basic helix-loop-helix transcription factor, human achaete-scute homologue-2. J Biochem. 2003;134(3):433–440.
20. Yoshida I, Koide S, Hasegawa SI, Nakagawara A, Tsuji A, Matsuda Y. Proprotein convertase PACE4 is down-regulated by the basic helix-loop-helix transcription factor hASH-1 and MASH-1. Biochem J. 2001;360(Pt 3):683–689.
21. Yang X-L, Wang Y, Liang S-B, et al. Comparison of the seventh and eighth editions of the UICC/AJCC staging system for nasopharyngeal carcinoma: analysis of 1317 patients treated with intensity-modulated radiotherapy at two centers. BMC Cancer. 2018;18(1):606.
22. Kang S, Zhao Y, Hu K, et al. miR-124 exhibits antiproliferative and antiaggressive effects on prostate cancer cells through PACE4 pathway. Prostate. 2014;74(11):1095–1106.
23. Wang F, Wang L, Pan J. PACE4 regulates proliferation, migration and invasion in human breast cancer MDA-MB-231 cells. Mol Med Rep. 2015;11(1):698–704.
24. Panet F, Couture F, Kwiatkowska A, Desjardins R, Guérin B, Day R. PACE4 is an important driver of ZR-75-1 estrogen receptor-positive breast cancer proliferation and tumor progression. Eur J Cell Biol. 2017;96(5):469–475.
25. Yao Z, Sun B, Hong Q, et al. Guo H: PACE4 regulates apoptosis in human prostate cancer cells via endoplasmic reticulum stress and mitochondrial signaling pathways. Drug Des Devel Ther. 2015;9:5911–5923.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.


