Back to Journals » Breast Cancer: Targets and Therapy » Volume 12
Outcomes of Sentinel Lymph Node Biopsy Using Blue Dye Method for Early Breast Cancer – A Single-Institution Experience in the Philippines
Authors Yap RV , De La Serna FM
Received 12 December 2019
Accepted for publication 26 February 2020
Published 11 March 2020 Volume 2020:12 Pages 37—44
DOI https://doi.org/10.2147/BCTT.S242115
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Pranela Rameshwar
Ralph Victor Yap, Frances Marion De La Serna
Department of Surgery, Cebu Doctors’ University Hospital, Cebu City, Cebu, Philippines
Correspondence: Ralph Victor Yap
Department of Surgery, Cebu Doctors’ University Hospital, Osmeña Boulevard, Cebu City 6000, Cebu, Philippines
Tel +63 917 130 1923
Fax +63 32 255 5555
Email [email protected]
Purpose: This study aimed to share our experience with SLNB in the Filipino population with early breast cancer.
Patients and Methods: A retrospective review was done on all patients with confirmed invasive breast carcinoma, tumor size of 5 cm or less (T1/T2), who preoperatively had no clinical signs of axillary metastasis and subsequently underwent SLNB with blue dye method from January 01, 2008 to December 31, 2017. Clinicopathologic profiles were recorded. Outcomes of patients who had SLNB only were assessed.
Results: One hundred twenty-nine patients matched the inclusion criteria with a mean age of 54.3 years. The majority (88.4%) had a total mastectomy. Invasive ductal carcinoma (65.1%) was the most common tumor. Estrogen and progesterone receptors were positive in 69% and 61.2% respectively while only 28.7% were HER2 positive. SLNB was successfully carried out in 126 (97.7%) patients with a range of 2– 4 SLNs harvested. Thirty-four (26.4%) patients had completion ALND. With a median of 25 months follow-up, 75 out of 95 patients who underwent SLNB alone had follow-up data. Forty-six (61.3%) patients had seroma formation. One (1.3%) patient developed arm paresthesia, 2 (2.7%) local (chest wall) and 2 (2.7%) axillary recurrences after a negative SLNB. None of the patients developed lymphedema.
Conclusion: The blue dye method alone is acceptable and can be readily employed in institutions with limited resources. Even with the limited population, the morbidity and oncologic outcomes of patients who underwent SLNB alone were low and comparable to similar international published data. SLNB should be the preferred method for staging the axilla.
Keywords: breast cancer, Philippines, SLNB, outcomes, sentinel, blue dye
Introduction
In the Philippines, breast cancer is the most common type of cancer in females and the 3rd leading cause of cancer mortality.1 One of the important aspects of breast cancer management is axillary staging. The status of axillary lymph nodes is an important prognosticator that affects adjuvant treatment decisions in patients with early breast carcinoma.2 In the year 1994, Giuliano introduced the idea of sentinel lymph node (SLN) mapping for breast carcinoma.3 The SLN receives lymphatic drainage directly from the breast tumor first. Thus, axillary nodal metastases may occur in an orderly fashion by spreading first in the SLN then to the next echelon of lymph nodes. In patients with SLNs negative for metastasis, axillary lymph node dissection (ALND) is safely avoided. Sentinel lymph node biopsy (SLNB) has replaced routine ALND in women with clinically node-negative axilla. It provides an accurate assessment of axillary lymph node status and staging. As a less invasive method, it reduces ALND-associated morbidity such as lymphedema, arm paresthesia, and limitation of shoulder motion.4 Oncologic outcomes in terms of disease-free survival, overall survival, and locoregional recurrence rate were similar in the SLNB alone versus the ALND group in patients with clinically node-negative axilla.5 In doing the SLNB, a combination of radioactive technetium-99m and blue bye technique is preferred to increase the SLN identification rate than using one technique in isolation.6
As stated in the clinical practice guideline update for SLNB, patients with early breast cancer who do not have axillary lymph node metastases should not be offered ALND.7 However, in our institution, only two breast surgeons are SLNB-validated since the year 2008 thus most of the general surgeons still perform outright ALND in patients with breast cancer without clinical signs of axillary nodal metastasis. Secondly, because of the unavailability of a gamma probe, SLNBs are performed using a blue dye technique only. To the best of our knowledge, there are no published studies yet in the Philippines regarding the outcomes of SLNB in early breast cancer. Thus, this study aims to share our experience with SLNB using a blue dye method only in the Filipino population with early breast cancer.
Patients and Methods
Retrospectively, we reviewed all SLNB performed from January 01, 2008, through December 31, 2017, at Cebu Doctors’ University Hospital, Cebu City, Philippines. All patients diagnosed with invasive breast carcinoma, tumor size of 5 cm or less (T1/T2), and imaging-confirmed clinically node-negative axilla were included. Patients with ductal carcinoma in-situ and inflammatory breast cancers were excluded. The study was approved by the Cebu Doctors’ University Hospital Ethics Committee (Protocol Code 2017–032). Written informed consent was provided by all patients.
Either total mastectomy or breast conservation surgery (BCS) for the primary tumor was chosen based on the patients’ preferences and tumor characteristics. All procedures were performed by 2 SLNB-validated breast surgeons. All patients were evaluated preoperatively with a core needle biopsy, sonomammography and/or mammography. The SLNB was performed in the operating room under general anesthesia. Three to 5 mL of blue dye (either methylene blue or patent blue V) was injected in the periareolar/intradermal location. The choice of blue dye depends on availability but patent blue V (PBV) was preferably used for BCS. The site of injection is manually compressed and gently massaged for 5 mins prior to skin incision. Dissection through the subcutaneous tissue and clavipectoral fascia into the axilla was performed through the most lateral incision of planned mastectomy or a separate incision just below the axillary hairline for BCS. All blue-stained lymph nodes were harvested and sent for frozen section (FS) intraoperatively. Indications for completion ALND in one setting were (1) SLN(s) positive for tumor cells on FS and (2) failure to identify SLNs. All SLN(s) were serially sectioned at 1mm interval, embedded in paraffin, and stained with Hematoxylin and Eosin (H&E) per laboratory protocol. Macrometastasis, micrometastasis, and isolated tumor cells (ITC) were defined as more than 2 mm, more than 0.2 mm but not more than 2 mm, and 0.2 mm or less in sizes respectively. Cytokeratin immunohistochemical (IHC) staining was left at the pathologist’s discretion (selective) in case further evaluation of the SLN(s) is needed. The breast tumor specimens were routinely sent for IHC testing to determine the estrogen, progesterone, and Her2 receptors status.
All patients were advised to follow-up in the clinic 1 week from the date of surgery for wound and seroma assessment then at 2nd and 3rd-week postoperatively if needed. History and clinical examination were done every 6 months for 3 to 5 years then annually thereafter. Mammography and or chest wall/axillary ultrasound (if clinically indicated) were performed annually as well. Patients were advised adjuvant therapy post-operatively when clinically indicated. Clinical signs of tumor recurrence were subjected to a core or fine needle biopsy.
Medical records were reviewed for patients’ clinicopathologic characteristics and outcomes. The variables investigated were: age, treatment of primary tumor, number of SLNs identified, number of SLNs positive for tumor cells on FS, number of ALND, final biopsy of the specimen, biomarker status, tumor size, presence of lymphovascular invasion (LVI) and tumor grade. The primary endpoint of this study is the SLNB-related outcomes which were assessed by reviewing the outpatient records of patients who underwent SLNB alone for axillary staging. These variables included seroma and hematoma formation, infection, lymphedema, limitation of shoulder motion, paresthesia, local (breast/chest wall) and regional (axillary) recurrence. Data was encoded using Microsoft Excel (Microsoft Corp., Redmond, USA). Descriptive statistics were used and values were expressed as mean and frequency (percentage).
Results
Clinicopathologic Characteristics
Between January 01, 2008, to December 31, 2017, a total of 129 female breast cancer patients with clinically negative axillary disease preoperatively underwent SLNB. The mean age was 54.3 ± 12.4 years. The majority of the patients (88.4%) underwent a total mastectomy to address the primary tumor, while 11.6% had breast conservation surgery. The final histopathology result of the breast tumor was invasive ductal in 65.1% of the patients, 27.1% for lobular, 4.7% for mammary, and 2.3% and 0.8% for mucinous and cribriform types respectively. More than half of the patients were estrogen (69%) and progesterone (61.2%) receptor-positive, while 28.7% were HER2 positive. The tumor size was T1 (< 2cm) in 47.3% of patients and T2 (> 2cm but ≤ 5 cm) in 52.7% of patients. Lymphovascular invasion was positive in 10.9% of patients. Almost half of the patients (49.6%) had grade 1 tumors while 41.1% and 9.3% of patients were grades 2 and 3 tumors, respectively (Table 1).
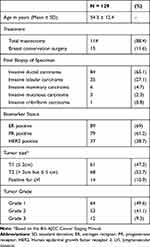 |
Table 1 Patients’ Clinical Characteristics |
Intraoperative SLN Identification
Intra-operatively, sentinel lymph node(s) were identified successfully in 126 out of 129 patients. The identification rate with the blue dye method in our series is 97.7%. Three patients with unsuccessful SLNB underwent completion ALND. The total number of SLNs harvested was one in 13 patients (10.3%), 2 in 32 patients (25.4%), 3 in 44 patients (35%), 4 in 26 patients (20.6%), and > 5 in 11 patients (8.7%). A total of 26 patients (20.1%) had metastasis in their SLN(s) upon FS analysis and underwent completion ALND. In these patients, 5 had micrometastases while ITC was found in one case. It may be worth noting the other 5 patients who had completion ALND at the time of surgery. Two patients were part of the SLNB-validation study during the year 2008, and three were done based on the surgeon’s clinical judgment intra-operatively for a high likelihood of metastasis despite a negative FS analysis (Table 2).
 |
Table 2 Details of Intraoperative SLNB Findings |
SLNB-Related Outcomes
With a median of 25 months follow-up (range 11 to 86), 75 out of 95 patients who underwent SLNB alone had follow-up data. No patient developed a hematoma, lymphedema, shoulder dysfunction, nor wound infection. However, 46 (61.3%) patients developed seroma formation (1 had BCS and 45 had a total mastectomy) and one (1.3%) arm numbness. There were two ipsilateral axillary recurrences and two ipsilateral local recurrences (chest wall) after a negative SLNB (Table 3).
 |
Table 3 SLNB-Related Outcomes in 75 (Out of 95) Patients Who Underwent SLNB Only |
Discussion
Currently, the standard method of axillary staging in breast cancer patients with clinically negative axillary nodal involvement is SLNB. However, there is a paucity of available data in the Philippines with regards to the practice of SLNB for early breast cancer among general surgeons. Even in the grey literature, most of the primary endpoint of the available local studies on SLNB is focused on its diagnostic accuracy.8,9 Our local society of general surgeons have yet to come up with its guidelines for SLNB and its validation process since its group recommendation in 2013.10 The SLNB program for patients with breast cancer in our institution started in 2008 in a multidisciplinary setting (Surgeon and Pathologist) with strict inclusion criteria. The indications for SLNB in our setting were T1 or T2 tumors and clinically negative axilla which is in concordance to internationally published guidelines. The indication of SLNB has been extended to patients with large and/or high-grade ductal carcinoma-in-situ, especially when total mastectomy is required.11
The options for the primary treatment of the breast in early invasive cancer are (1) total mastectomy and (2) BCS (with radiation therapy), with the latter being the preferred method.10 Breast conservation surgery has shown improved 10-year overall survival estimates, quality of life, and superior aesthetic outcomes.12 However, the BCS rate in our institution is still low (11.6%) and the actual numbers may even be lower if we were to include all patients with the same clinicopathologic stage who underwent total mastectomy performed by other general surgeons. Compared to developed countries, the BCS rates range from 58% to 66.8%.13,14 In Soweto, South Africa, only 91 (20%) out of 445 patients with early-stage breast cancer underwent BCS.15 Another study in Malaysia involving 730 patients with breast cancer showed a low BCS rate of 32.9%. Factors such as co-morbidities, education level, socio-economic status, marital status, and mode of diagnosis may influence the wide variation of BCS rates among countries.16
The commonly used blue dyes for SLNB include PBV, isosulfan blue, and methylene blue dye (MBD). In our setting, both PBV and MBD were routinely used because it is readily available in our country with MBD being more affordable. The use of blue dye is not without risks. Although rare and not life-threatening, the reported complications of MBD for SLNB include allergic reactions and skin lesions/necrosis.17–20 In contrast, PBV should be used with more caution because of its risk for anaphylaxis.21,22 In our study, temporary skin tattooing was observed in some patients but none developed skin necrosis nor anaphylactic reaction. The use of MBD in pregnant breast cancer patients remains controversial due to the lack of data in the literature. A cohort study involving 7 pregnant breast cancer patients with a median gestational age of 17 weeks who had SLNB with MBD method alone has shown that its use appears to be safe in this population.23 However, as of this writing, the NCCN guidelines contraindicate the use of blue dye for axillary staging in pregnant patients.24
The identification rate with the blue dye method in our institution is 97.7%. Our results were comparable to similar published studies using the blue dye method only.25,26 However, the sensitivity of the blue dye method alone is lower (81–82% vs 95–100%) compared to using a dual method (radioisotope and blue dye).27,28 A meta-analysis involving 1559 patients has shown that the MBD method alone for SLNB is acceptable but should be used with caution since it can have an unacceptable false-negative rate.29 Another meta-analysis has shown statistically significant false-negative rates between using blue dye only and the combination of radioactive tracer and blue dye (9.4% vs 5.4%).30 An even higher false-negative rate of 21.4% was reported when only the blue dye method is used.6 To optimize the SLN identification rate and minimize the false-negative rate, the use of a dual method for mapping is recommended.30,31 Sentinel lymph node should be identified in > 95% of patients undergoing SLNB with a false negative rate of 5–10% using standard protocols.32 However, in low resource settings, using blue dye for SLNB remains a feasible option.26 As a low-middle income country, there is a lack of data with regards to the availability and distribution of a nuclear medicine facility among institutions. There has been a growing interest in the use of the novel techniques in SLNB for breast cancer such as indocyanine green (ICG) fluorescence, superparamagnetic iron oxide (SPIO) nanoparticles and contrast-enhanced ultrasound (CEUS) using microbubbles. Their reported identification rates (ICG 97 – 99%, SPIO 94.8–98.8%, CEUS 60 – 100%) make them potential alternatives to the conventional method in SLN mapping.33–37 However, large-scale studies are still warranted for these newer techniques.
In our study, 70.7% of patients with successful SLNB had 3 or fewer SLNs harvested. In previous literature, most cases will have a median number of harvested SLNs of 2 but limiting the number of SLNs removed to 3 can be associated with a high false-negative rate.38 In contrast, removal of up to 5 SLNs was sufficient to identify metastatic nodes in more than 99% of patients.39 All 26 patients with positive SLNs in our study underwent completion ALND in one setting. The ACOSOG Z0011 trial has shown that patients with 1 to 2 positive SLNs do not benefit from further ALND and the 10-year overall survival was comparable to SLNB alone.40 Of these 26 patients with positive SLNs, 19.2% were a micrometastatic disease. The 10-year follow-up of the IBCSG 23–01 trial has shown that it is safe to omit axillary dissection when only micrometastasis is found in the SLN(s).41 However, there is no data yet to support the applicability of these trials in the Filipino population.
The most frequent sequela following breast cancer surgery is seroma formation and the risk is 2.5 times higher in patients who underwent a modified radical mastectomy.42 Total seroma production is significantly higher after total mastectomy than after BCS.43 The method of axillary sampling may also influence seroma production. Compared with ALND, SLNB is associated with fewer seroma formation, wound infection, and arm paresthesia.44 In our study, 61.3% of patients who had SLNB-only developed seroma post-operatively. This confirms that the extent of surgery for the primary tumor is the most important factor for seroma formation. The long-term morbidity after SLNB alone is significantly lower compared with ALND. In a previous study involving 431 patients who underwent SLNB alone resulted in a lower post-operative occurrence of arm numbness or pain, decreased shoulder range of motion, and lymphedema when compared with 210 patients who underwent SLNB with completion ALND.45 None of the patients in our study developed lymphedema after SLNB only after a median follow-up of 25 months. The reported rates of lymphedema as described in previous literature ranged from 0% to 7% with 6 to 36 months of follow-up. However, a median of 5 years of follow-up may be required to accurately determine its true incidence.46
The incidence of local recurrence after total mastectomy was previously reported to range 3% - 8.8%.47,48 In contrast, in-breast tumor recurrence following BCS range 0.3% to 5.8% (up to 10-year follow-up).49,50 However, there were no significant differences in disease-free and overall survival between mastectomy and BCS group after a 20-year follow-up.48,51 Newer data have shown that those who had BCS have significantly better outcomes in terms of local and distant control, and overall survival compared to those who had a total mastectomy.12 The risk of axillary recurrence after a negative SLNB is reported low. The Swedish Multicentre Cohort Study involving 2216 SLN-negative patients reported a 1.6% risk of regional recurrence at a median follow-up of 126 months.52 A much lower risk of 0.6% was reported in a study involving 464 patients at a median follow-up of 38 months. In our series, 2 out of 75 patients (2.7%) developed regional recurrence.53
The limitations of our study are its retrospective design, small population, and short follow-up which could have possibly contributed to the low rate of postoperative morbidity including the oncologic outcomes. Our primary reliance on only two SLNB-validated surgeons limits the generalizability of our results to all centers within the region. Also, we did not systematically collect data on the adjuvant therapy of our patients which could have affected locoregional failure rates.
Conclusion
Our study describes for the first time the clinicopathologic characteristics and outcomes of patients who underwent SLNB for early breast cancer in the Philippines. In low and/or middle-income countries such as the Philippines, the use of the blue dye method alone is acceptable and can be readily employed in institutions with limited resources such as the availability of a nuclear medicine facility. Even with the limited number of SLNB in our institution, the morbidity and oncologic outcomes of SLNB were low and comparable to similar international published data. SLNB should be the preferred method for staging the axilla in clinically node-negative breast cancer patients. However, more data on the factors affecting BCS and SLNB outcomes are needed in the Philippines.
Acknowledgments
This paper was presented as an abstract poster during the 16th St. Gallen International Breast Cancer Conference held last March 20 – 23, 2019 in Vienna, Austria.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Global Cancer Observatory. Population fact sheet: Philippines. 2018. Available from: http://gco.iarc.fr/today/data/factsheets/populations/608-philippines-fact-sheets.pdf.
2. Kim H, Cho J, Kwon SY, Kang SH. Biologic subtype is a more important prognostic factor than nodal involvement in patients with stages I and II breast carcinoma. Ann Surg Treat Res. 2016;90(1):1–9. doi:10.4174/astr.2016.90.1.1
3. Giuliano AE, Kirgan DM, Guenther JM, Morton DL. Lymphatic mapping and sentinel lymphadenectomy for breast cancer. Ann Surg. 1994;220(3):391–398. doi:10.1097/00000658-199409000-00015
4. Manca G, Rubello D, Tardelli E, et al. Sentinel lymph node biopsy in breast cancer: indications, contraindications, and controversies. Clin Nucl Med. 2016;41(2):126–133. doi:10.1097/RLU.0000000000000985
5. Krag DN, Anderson SJ, Julian TB, et al. Sentinel-lymph-node resection compared with conventional axillary-lymph-node dissection in clinically node-negative patients with breast cancer: overall survival findings from the NSABP B-32 randomised Phase 3 trial. Lancet Oncol. 2010;11(10):927–933. doi:10.1016/S1470-2045(10)70207-2
6. Peek MC, Kovacs T, Baker R, Hamed H, Kothari A, Douek M. Is blue dye still required during sentinel lymph node biopsy for breast cancer? Ecancermedicalscience. 2016;10:674. doi:10.3332/ecancer.2016.674
7. Lyman GH, Somerfield MR, Bosserman LD, Perkins CL, Weaver DL, Giuliano AE. Sentinel lymph node biopsy for patients with early-stage breast cancer: American Society of Clinical Oncology clinical practice guideline update. J Clin Oncol. 2017;35(5):561–564. doi:10.1200/JCO.2016.71.0947
8. Siguan S, Bordeos L, Baking-Fernandez S. A review of the accuracy of sentinel lymph node biopsy by comparing frozen section with final paraffin block H&E staining, and correlation with the final axillary lymph node dissection results. PJSS. 2016;71(2):41–45.
9. Tolentino-Molina M, Datay-Lim S, Alcazaren E. Intraoperative frozen section assessment of sentinel lymph nodes in breast cancer: six-year experience in a Tertiary Hospital. PJP. 2016;1(1). doi:10.21141/PJP.2016.006
10. Simbulan MH
11. Cardoso F, Kyriakides S, Ohno S, et al. Early breast cancer: ESMO clinical practice guidelines for diagnosis, treatment and follow-up. Ann Oncol. 2019;30(8):1194–1220. doi:10.1093/annonc/mdz173
12. Corradini S, Reitz D, Pazos M, et al. Mastectomy or breast-conserving therapy for early breast cancer in real-life clinical practice: outcome comparison of 7565 cases. Cancers (Basel). 2019;11(2):160. doi:10.3390/cancers11020160
13. Churilla TM, Donnelly PE, Leatherman ER, Adonizio CS, Peters CA. Total mastectomy or breast conservation therapy? How radiation oncologist accessibility determines treatment choice and quality: a SEER data-base analysis. Breast J. 2015;21(5):473–480. doi:10.1111/tbj.12449
14. Van Maaren MC, De Munck L, De Bock GH, et al. 10 year survival after breast-conserving surgery plus radiotherapy compared with mastectomy in early breast cancer in the Netherlands: a population-based study. Lancet Oncol. 2016;17(8):1158–1170. doi:10.1016/S1470-2045(16)30067-5
15. Cubasch H, Joffe M, Ruff P, et al. Breast conservation surgery versus total mastectomy among women with localized breast cancer in Soweto, South Africa. PLoS One. 2017;12(8):e0182125. doi:10.1371/journal.pone.0182125
16. Wong WJ, Mosiun JA, Hidayati Z, et al. Low Breast Conserving Surgery (BCS) rates in public hospitals in Malaysia: the effect of stage and ethnicity. Breast. 2019;46:136–143. doi:10.1016/j.breast.2019.05.016
17. Kaklamanos IG, Birbas K, Syrigos K, Bonatsos VG, Bonatsos G. Prospective comparison of peritumoral and subareolar injection of blue dye alone, for identification of sentinel lymph nodes in patients with early stage breast cancer. J Surg Oncol. 2011;104(1):37–40. doi:10.1002/jso.21897
18. Reyes F, Noelck M, Valentino C, Grasso-lebeau L, Lang J. Complications of methylene blue dye in breast surgery: case reports and review of the literature. J Cancer. 2010;2:20–25. doi:10.7150/jca.2.20
19. Salhab M, Al Sarakbi W, Mokbel K. Skin and fat necrosis of the breast following methylene blue dye injection for sentinel node biopsy in a patient with breast cancer. Int Semin Surg Oncol. 2005;2(1):26. doi:10.1186/1477-7800-2-26
20. Stradling B, Aranha G, Gabram S. Adverse skin lesions after methylene blue injections for sentinel lymph node localization. Am J Surg. 2002;184(4):350–352. doi:10.1016/S0002-9610(02)00945-5
21. Brenet O, Lalourcey L, Queinnec M, et al. Hypersensitivity reactions to patent blue V in breast cancer surgery: a prospective multicentre study. Acta Anaesthesiol Scand. 2013;57(1):106–111. doi:10.1111/aas.12003
22. Barthelmes L, Goyal A, Newcombe RG, Mcneill F, Mansel RE. Adverse reactions to patent blue V dye - The NEW START and ALMANAC experience. Eur J Surg Oncol. 2010;36(4):399–403. doi:10.1016/j.ejso.2009.10.007
23. Gropper AB, Calvillo KZ, Dominici L, et al. Sentinel lymph node biopsy in pregnant women with breast cancer. Ann Surg Oncol. 2014;21(8):2506–2511. doi:10.1245/s10434-014-3718-2
24. National Comprehensive Cancer Network. Breast cancer (Version 2.2020). Available from: https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf.
25. Alhussini MA, Awad AT, Ashour MH, Abdelateef A, Fayed H. Breast cancer sentinel node detection: an alternative solution for centers lacking nuclear technology. Breast Care (Basel). 2016;11(4):265–268. doi:10.1159/000448409
26. Seenu V, Suhani S, Srivastava A, Parshad R, Mathur S, Kumar R. Optimization of sentinel lymph node identification techniques in the Indian setting: a randomized clinical trial. Indian J Cancer. 2019;56(2):114–118. doi:10.4103/ijc.IJC_163_18
27. Motomura K, Inaji H, Komoike Y, et al. Combination technique is superior to dye alone in identification of the sentinel node in breast cancer patients. J Surg Oncol. 2001;76(2):95–99. doi:10.1002/(ISSN)1096-9098
28. Radovanovic Z, Golubovic A, Plzak A, Stojiljkovic B, Radovanovic D. Blue dye versus combined blue dye-radioactive tracer technique in detection of sentinel lymph node in breast cancer. Eur J Surg Oncol. 2004;30(9):913–917. doi:10.1016/j.ejso.2004.08.003
29. Li J, Chen X, Qi M, Li Y, Metze K. Sentinel lymph node biopsy mapped with methylene blue dye alone in patients with breast cancer: a systematic review and meta-analysis. PLoS One. 2018;13(9):e0204364. doi:10.1371/journal.pone.0204364
30. Pesek S, Ashikaga T, Krag LE, Krag D. The false-negative rate of sentinel node biopsy in patients with breast cancer: a meta-analysis. World J Surg. 2012;36(9):2239–2251. doi:10.1007/s00268-012-1623-z
31. Goyal A, Newcombe RG, Chhabra A, Mansel RE. Factors affecting failed localisation and false-negative rates of sentinel node biopsy in breast cancer–results of the ALMANAC validation phase. Breast Cancer Res Treat. 2006;99(2):203–208. doi:10.1007/s10549-006-9192-1
32. The American Society of Breast of Surgeons. Performance and practice guidelines for sentinel lymph node biopsy in breast cancer patients. 2014. Available from: https://www.breastsurgeons.org/docs/statements/Performance-and-Practice-Guidelines-for-Sentinel-Lymph-Node-Biopsy-in-Breast-Cancer-Patients.pdf.
33. He K, Chi C, Kou D, et al. Comparison between the indocyanine green fluorescence and blue dye methods for sentinel lymph node biopsy using novel fluorescence image-guided resection equipment in different types of hospitals. Transl Res. 2016;178:74–80. doi:10.1016/j.trsl.2016.07.010
34. Guo J, Yang H, Wang S, et al. Comparison of sentinel lymph node biopsy guided by indocyanine green, blue dye, and their combination in breast cancer patients: a prospective cohort study. World J Surg Oncol. 2017;15(1):196. doi:10.1186/s12957-017-1264-7
35. Taruno K, Kurita T, Kuwahata A, et al. Multicenter clinical trial on sentinel lymph node biopsy using superparamagnetic iron oxide nanoparticles and a novel handheld magnetic probe. J Surg Oncol. 2019;120(8):1391–1396. doi:10.1002/jso.v120.8
36. Man V, Wong TT, Co M, Suen D, Kwong A. Sentinel lymph node biopsy in early breast cancer: magnetic tracer as the only localizing agent. World J Surg. 2019;43(8):1991–1996. doi:10.1007/s00268-019-04977-1
37. Nielsen Moody A, Bull J, Culpan AM, et al. Preoperative sentinel lymph node identification, biopsy and localisation using contrast enhanced ultrasound (CEUS) in patients with breast cancer: a systematic review and meta-analysis. Clin Radiol. 2017;72(11):959–971. doi:10.1016/j.crad.2017.06.121
38. Chagpar AB, Scoggins CR, Martin RC, et al. Are 3 sentinel nodes sufficient? Arch Surg. 2007;142(5):456–459. doi:10.1001/archsurg.142.5.456
39. Yi M, Meric-bernstam F, Ross MI, et al. How many sentinel lymph nodes are enough during sentinel lymph node dissection for breast cancer? Cancer. 2008;113(1):30–37. doi:10.1002/cncr.23514
40. Giuliano AE, Ballman KV, Mccall L, et al. Effect of axillary dissection vs no axillary dissection on 10-year overall survival among women with invasive breast cancer and sentinel node metastasis: the ACOSOG Z0011 (Alliance) randomized clinical trial. JAMA. 2017;318(10):918–926. doi:10.1001/jama.2017.11470
41. Galimberti V, Cole BF, Viale G, et al. Axillary dissection versus no axillary dissection in patients with breast cancer and sentinel-node micrometastases (IBCSG 23-01): 10-year follow-up of a randomised, controlled phase 3 trial. Lancet Oncol. 2018;19(10):1385–1393. doi:10.1016/S1470-2045(18)30380-2
42. Hashemi E, Kaviani A, Najafi M, Ebrahimi M, Hooshmand H, Montazeri A. Seroma formation after surgery for breast cancer. World J Surg Oncol. 2004;2(1):44. doi:10.1186/1477-7819-2-44
43. Ebner F, Friedl TWP, De Gregorio A, et al. Seroma in breast surgery: all the surgeons fault? Arch Gynecol Obstet. 2018;298(5):951–959. doi:10.1007/s00404-018-4880-8
44. Giuliano AE, Hunt KK, Ballman KV, et al. Axillary dissection vs no axillary dissection in women with invasive breast cancer and sentinel node metastasis: a randomized clinical trial. JAMA. 2011;305(6):569–575. doi:10.1001/jama.2011.90
45. Langer I, Guller U, Berclaz G, et al. Morbidity of sentinel lymph node biopsy (SLN) alone versus SLN and completion axillary lymph node dissection after breast cancer surgery: a prospective Swiss multicenter study on 659 patients. Ann Surg. 2007;245(3):452–461. doi:10.1097/01.sla.0000245472.47748.ec
46. Mclaughlin SA, Wright MJ, Morris KT, et al. Prevalence of lymphedema in women with breast cancer 5 years after sentinel lymph node biopsy or axillary dissection: objective measurements. J Clin Oncol. 2008;26(32):5213–5219. doi:10.1200/JCO.2008.16.3725
47. Morrow M, Golshan M. Chapter 33: mastectomy. In: Harris JR, Lippman ME, Morrow M, Osborne CK, editors. Diseases of the Breast.
48. Veronesi U, Cascinelli N, Mariani L, et al. Twenty-year follow-up of a randomized study comparing breast-conserving surgery with radical mastectomy for early breast cancer. N Engl J Med. 2002;347(16):1227–1232. doi:10.1056/NEJMoa020989
49. Veronesi U, Luini A, Del Vecchio M, et al. Radiotherapy after breast-preserving surgery in women with localized cancer of the breast. N Engl J Med. 1993;328(22):1587–1591. doi:10.1056/NEJM199306033282202
50. Veronesi U, Marubini E, Mariani L, et al. Radiotherapy after breast-conserving surgery in small breast carcinoma: long-term results of a randomized trial. Ann Oncol. 2001;12(7):997–1003. doi:10.1023/A:1011136326943
51. Fisher B, Anderson S, Bryant J, et al. Twenty-year follow-up of a randomized trial comparing total mastectomy, lumpectomy, and lumpectomy plus irradiation for the treatment of invasive breast cancer. N Engl J Med. 2002;347(16):1233–1241. doi:10.1056/NEJMoa022152
52. De Boniface J, Frisell J, Bergkvist L, Andersson Y. Ten-year report on axillary recurrence after negative sentinel node biopsy for breast cancer from the Swedish Multicentre Cohort Study. Br J Surg. 2017;104(3):238–247. doi:10.1002/bjs.10411
53. Inoue T, Nishi T, Nakano Y, et al. Axillary lymph node recurrence after sentinel lymph node biopsy performed using a combination of indocyanine green fluorescence and the blue dye method in early breast cancer. Breast Cancer. 2016;23(2):295–300. doi:10.1007/s12282-014-0573-8
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
