Back to Journals » Clinical Ophthalmology » Volume 17
Outcomes of Pars Plana Vitrectomy with Panretinal Photocoagulation for Treatment of Proliferative Diabetic Retinopathy Without Retinal Detachment: A Seven-Year Retrospective Study
Authors Patel V, Rohowetz LJ , Pakravan P, Kalavar M, Yannuzzi NA, Sridhar J
Received 15 December 2022
Accepted for publication 20 January 2023
Published 1 February 2023 Volume 2023:17 Pages 471—478
DOI https://doi.org/10.2147/OPTH.S400474
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Veshesh Patel, Landon J Rohowetz, Parastou Pakravan, Meghana Kalavar, Nicolas A Yannuzzi, Jayanth Sridhar
Department of Ophthalmology, Bascom Palmer Eye Institute University of Miami, Miami, FL, USA
Correspondence: Jayanth Sridhar, Department of Ophthalmology, Bascom Palmer Eye Institute, 900 NW 17th St, Miami, FL, 33136, USA, Tel +1 305 326-6124, Email [email protected]
Objective: To review clinical outcomes of patients with proliferative diabetic retinopathy (PDR) and vitreous hemorrhage (VH) who underwent pars plana vitrectomy (PPV) with endolaser panretinal photocoagulation (PRP) without retinal detachment (RD) repair.
Methods: Retrospective chart review of the rate of postoperative clinical findings and visual acuity in patients with PDR from May 2014 to August 2021.
Results: Pars plana vitrectomy with endolaser PRP was performed in 81 eyes of 81 patients (mean age of 62.1 ± 10.5 years). At a median follow-up of 18 months, mean Snellen best-corrected visual acuity (BCVA) significantly improved from 20/774 preoperatively to 20/53 at last follow-up (P < 0.001). Postoperative complications and clinical findings included VH (12.3%), diabetic macular edema (DME) (12.3%), ocular hypertension (8.6%), RD (4.9%), and need for additional PPV (6.2%). Eyes with PRP performed within 6 months before surgery had a lower frequency of developing postoperative VH (5.3%) compared to eyes that received PRP more than 6 months before surgery (27.3%, P = 0.04). Eyes that received preoperative anti-vascular endothelial growth factor (VEGF) treatment (2.0%) had a lower frequency of postoperative VH compared to eyes that did not receive anti-VEGF treatment (14.3%, P = 0.04). Eyes that received intraoperative sub-tenon triamcinolone acetonide developed postoperative DME (4.0%) less frequently than eyes that did not receive sub-tenon triamcinolone acetonide (26.7%, P = 0.04).
Conclusion: In patients with PDR and VH, PPV with PRP yielded significant improvements in visual acuity and resulted in overall low rates of recurrent postoperative VH. Preoperative anti-VEGF and PRP laser treatment were associated with lower rates of postoperative VH. Furthermore, intraoperative use of sub-tenon triamcinolone acetonide was associated with a lower rate of postoperative DME. Pars plana vitrectomy with endolaser PRP in conjunction with the aforementioned pre- and intraoperative therapies is an effective treatment for patients with PDR and VH.
Keywords: pars plana vitrectomy, panretinal photocoagulation, vitreous hemorrhage, vascular endothelial growth factor, sub-tenon triamcinolone acetonide
Introduction
Diabetic retinopathy is a leading cause of severe vision loss, and proliferative diabetic retinopathy (PDR) represents the most severe form of this disease.1 Treatment options for PDR include panretinal photocoagulation (PRP), intravitreal anti-vascular endothelial growth factor (anti-VEGF) therapy, and pars plana vitrectomy (PPV). Indications for PPV in patients with PDR include non-clearing vitreous hemorrhage (VH), vitreomacular traction, tractional retinal detachment, and diabetic macular edema (DME). Pars plana vitrectomy is typically accompanied with intraoperative endolaser PRP treatment to ischemic peripheral retina.2,3
Previous authors have reported outcomes of eyes with PDR undergoing clinic-based PRP or PPV.4–12 However, the preoperative factors associated with better post-procedural anatomic and functional status remain unclear. The purpose of this study was to determine the relative safety and efficacy of PPV with PRP and to identify clinical and demographic factors associated with postoperative outcomes.
Methods
The study was approved by the Institutional Review Board at the University of Miami and conformed to the requirements of the United States Health Insurance Portability and Privacy Act. The need for patient consent was waived by the Institutional Review Board due to the retrospective nature of the study. This retrospective, interventional case series included patients with PDR who underwent PPV with PRP from May 1, 2014, to August 31, 2021, with a minimum of 3 months of follow-up time. Patients with proliferative diabetic retinopathy were identified using ICD-10 codes. The current procedural terminology (CPT) code 67040 was used to identify patients who underwent PPV with endolaser PRP without RDs within the study time period. Exclusion criteria included patient age less than 18, pregnancy at time of surgery, less than 3 months of follow-up, history of prior PPV, presence of retinal detachment (RD), and neovascular glaucoma.
The following pre-operative data was collected: gender, date of birth, eye (OD/OS), race/ethnicity, duration of diabetes prior to surgery, type of diabetes, Hemoglobin A1c (HbA1c), insulin-dependence, past medical history, medications, duration of PDR prior to surgery, follow-up time, lens status, prior interventions including preoperative clinic-based PRP and anti-VEGF therapy, physical exam findings, and BCVA. Recorded intraoperative data included internal limiting membrane (ILM) peeling, intravitreal anti-VEGF therapy, sub-tenon triamcinolone acetonide (ie, given in eyes with findings of DME), tamponade agents, cutter gauge, and sclerotomy suturing. Recorded postoperative data included best corrected visual acuity (BCVA) at 3 months, 6 months, 12 months, 2 years, and 3 years (when available) as well as additional PPV procedure(s), anti-VEGF therapy, and clinic-based PRP therapy. Postoperative clinical findings recorded included retinal detachment (RD), persistent neovascularization of the disc (NVD), persistent neovascularization elsewhere (NVE), DME, VH, glaucoma, cataract, macular hole, and endophthalmitis.
Statistical analyses were performed using IBM SPSS Statistics Version 26 (IBM Corp., Armonk, NY). BCVA was converted to Logarithm of the Minimum Angle of Resolution (logMAR) for statistical analyses. Counting fingers, hand motions, light perception, and no light perception were given values of 1.9, 2.3, 2.7, and 3.0, respectively.13,14 Multivariate and univariate analyses were performed for comparing BCVA and postoperative outcomes between groups (ie, gender, race/ethnicities, type of diabetes, phakic status, etc.). Pearson Chi-square tests were used to compare categorical outcomes between groups. A p-value of less than 0.05 was considered significant.
Results
Study Population
Of the 344 total subjects initially screened for this study, 151 were excluded due to history of RD or prior PPV, 110 for insufficient follow-up, and 2 due to history of neovascular glaucoma. The study included 81 eyes (42 OD, 39 OS) of 81 patients (55.6% male). Mean age was 62.1 ± 10.5 years (SD) at initial examination with a mean follow-up time of 24.8 ± 21.4 months (median: 17.7 months). Of the eyes included, 41 (50.6%) were Hispanic, 17 (21.0%) were African American, 12 (14.8%) were White, 2 (2.5%) were American Indian/Alaska Native, 1 (1.2%) was Asian, and the race of 8 eyes (9.8%) were unknown/not reported (Table 1). The most common primary language spoken by patients was English (56.8%) followed by Spanish (34.6%).
 |
Table 1 Demographics of Patients Undergoing Pars Plana Vitrectomy with Panretinal Photocoagulation |
Medical History
Of the included patients, 73 (90.1%) had type 2 diabetes mellitus and 8 (9.9%) had type 1 diabetes mellitus. Sixty-three eyes (77.8%) were from patients who required insulin therapy. Mean duration of diabetes was 16.3 ± 15.3 years (median: 13.5 years) and mean HbA1C at time of PPV was 8.0 ± 1.8%. Systemic hypertension was recorded in 63 (77.8%) patients prior to surgery, and eighteen (22.2%) patients had a history of hemodialysis. At the time of surgical sign-up, 43 (53.1%) patients were taking an antiplatelet or anticoagulant medication.
Ocular History
The mean time from diagnosis of PDR to PPV was 18.8 ± 42.7 months (median: 6.0 months). Prior clinic-based PRP treatment had occurred in 48 eyes (59.3%), with mean time from clinic-based PRP treatment to surgery of 21.9 ± 35.2 months (median: 9.5 months). Forty-nine eyes (60.5%) had received anti-VEGF therapy before PPV. The mean number of anti-VEGF injections before surgery was 2.0 ± 2.7. Ten eyes (12.3%) received anti-VEGF therapy within 2 weeks before PPV. At the time of surgery, 45 eyes (55.6%) were phakic and 36 eyes (44.4%) were pseudophakic.
Preoperative Clinical Findings
Of the included eyes (all 81 eyes with preoperative VH), 35 eyes (43.2%) had preoperative DME (evidenced on optical coherence tomography [OCT]), 23 eyes (28.4%) had preoperative NVE, and 10 eyes (12.3%) had preoperative NVD. The mean logMAR BCVA prior to PPV was 1.58 ± 0.70 (Snellen equivalent: 20/774, Table 2).
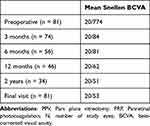 |
Table 2 Visual Acuity Outcomes After PPV with PRP |
Intraoperative Interventions
Intraoperatively, phacoemulsification with intraocular lens placement was performed in 18 eyes (22.2%) while ILM peeling was performed in 8 eyes (9.9%). Intravitreal anti-VEGF therapy was administered in 34 eyes (42.0%), and sub-tenon triamcinolone acetonide was administered in 31 eyes (38.3%). In the 30 eyes that received tamponade agents, air was the most frequent tamponade utilized (n = 25, 30.9%), followed by sulfur hexafluoride (SF6; n = 3, 3.7%) and perfluoropropane gas (C3F8; n = 2, 2.5%). The most frequent vitrector cutter gauge used in this study was 23-gauge (n = 41, 50.6%), followed by 25-gauge (n = 38, 46.9%), 20-gauge (n = 1, 1.2%), and 27-gauge (n = 1, 1.2%). Twenty eyes (24.7%) underwent suturing of the trocar sclerotomies, 13 with polyglactin 910 and 7 with gut sutures.
Visual Acuity Outcomes
Mean postoperative logMAR BCVA significantly improved to 0.60 ± 0.62 (20/84), 0.58 ± 0.61 (20/81), 0.50 ± 0.49 (20/62), and 0.41 ± 0.36 (20/51) at 3 months (n = 74, 91.4%), 6 months (n = 56, 69.1%), 12 months (n = 46, 56.8%), and 24 months (n = 34, 42.0%), respectively (P < 0.001 at all postoperative timepoints compared to preoperative BCVA, Table 2). At most recent follow-up for all eyes, the mean logMAR BCVA was 0.43 ± 0.33 (20/53) (P < 0.001). Of note, 3-month follow-up data were not available for 7 eyes that missed that follow-up visit but returned at either 6 months or 12 months.
Impact of Study Cohort Factors on Visual Acuity Outcomes
On multivariate analysis, there were no significant differences in BCVA between patient demographics, including gender, race, and age at any timepoint (P > 0.05). When analyzing medical history and its effects on postoperative BCVA, there were no significant differences in BCVA between type 1 and type 2 diabetics, insulin-dependence, hemodialysis status, systemic hypertension status, blood thinner use, and preoperative HbA1C level (P > 0.05). Longer duration of diabetes was inversely correlated with BCVA at final examination (r = 0.27, P = 0.02). Additionally, longer duration of PDR was inversely correlated with BCVA at 12 months (r = 0.32, P = 0.03) and final examination (r = 0.42, P = 0.047).
Preoperative clinic-based PRP, prior to the decision to pursue surgery, was not associated with improved postoperative mean BCVA compared to individuals that did not have prior PRP (P > 0.05). However, after stratification, individuals that had clinic-based PRP in the 6 months before surgery had a significantly better mean postoperative BCVA at 3 months (0.49 ± 0.44 [20/62]) compared to individuals that did not have any prior PRP performed (0.72 ± 0.48 [20/105], P = 0.04). Preoperative anti-VEGF use and phakic status were not associated with improved postoperative BCVA. When evaluating the effects of preoperative physical exam findings on postoperative BCVA, there were no significant difference in BCVA between eyes with preoperative NVD and NVE compared to eyes without those findings. Eyes without preoperative DME had a significantly better mean BCVA at 6 months than eyes with preoperative DME (0.33 ± 0.40 [20/43] vs 0.84 ± 0.36 [20/137], P = 0.01); however, no significance was found at the other timepoints.
Impact of Intraoperative Interventions on Visual Acuity Outcomes
There were no significant differences in mean BCVA at any time point between eyes that had phacoemulsification or ILM peel compared to those that did not receive the interventions during the surgery. There were no significant differences in mean BCVA at any time point between eyes that had intraoperative anti-VEGF or sub-tenon triamcinolone acetonide compared to those that did not receive these treatments. The type of tamponade and decision for sclerotomy suturing did not yield significant differences in mean BCVA at any time point.
Postoperative Outcomes and Complications
Ten eyes (12.3%) experienced postoperative VH and 4 eyes (4.9%) developed postoperative RD. Additionally, 10 eyes (12.3%) were noted to have DME, 3 eyes (3.7%) had NVE, and 1 eye (1.2%) had NVD. Nineteen eyes (23.5%) received postoperative anti-VEGF therapy. Seven eyes (8.6%) developed ocular hypertension, defined by use of topical aqueous suppressants, with one eye requiring incisional glaucoma surgery. Of the 24 phakic eyes that had long-term follow-up (>12 months) and did not undergo phacoemulsification at the time of surgery, 7 eyes (29.2%) developed cataracts requiring cataract extraction with intraocular lens insertion. One eye (1.2%) developed a macular hole and 1 eye (1.2%) developed endophthalmitis. Notably, two eyes (2.5%) underwent further PRP therapy, and five eyes (6.2%) underwent an additional PPV, 4 for RD and 1 for recurrent VH.
Impact of Study Cohort Factors on Postoperative Outcomes and Complications
There were no significant differences in postoperative outcomes when evaluating patient demographics, including gender, race, and age, or when considering medical history, including type of diabetics, duration of diabetes, insulin dependence, systemic hypertension status, hemodialysis status, and blood thinner use. Hemoglobin A1c was positively correlated with development of postoperative VH (r = 0.27, P = 0.02). When examining the effects of the study cohort’s ocular history, there were no relationships between postoperative outcomes and duration of PDR or lens status. However, eyes with PRP performed within 6 months of surgery had a lower frequency of postoperative VH (5.3%) compared to eyes that received PRP more than 6 months before surgery (27.3%, P = 0.04) (Table 3). Eyes that received preoperative anti-VEGF treatment had a lower frequency of postoperative VH (2.0%) compared to eyes that had not received anti-VEGF treatment (14.3%, P = 0.04). There were no significant differences in postoperative outcomes between eyes with or without preoperative NVD, NVE, and DME.
 |
Table 3 Significant Differences in Postoperative Outcomes Based on Preoperative and Intraoperative Interventions |
Impact of Intraoperative Interventions on Postoperative Outcomes and Complications
When examining intraoperative interventions, there were no significant differences in postoperative outcomes between eyes that underwent phacoemulsification or ILM peel compared to those that did not receive the interventions during surgery. Eyes that received sub-tenon triamcinolone acetonide developed postoperative DME (4.0%) less frequently than eyes that did not receive sub-tenon triamcinolone acetonide (26.7%, P = 0.04) (Table 3). The type of tamponade and decision for sclerotomy suturing were not significantly associated with increased or decreased risk of postoperative complications. There were no significant differences in postoperative outcomes between eyes that received or did not receive intraoperative anti-VEGF therapy.
Discussion
Panretinal photocoagulation and intravitreal anti-VEGF therapy are essential to the management of PDR. As part of the Diabetic Retinopathy Clinical Research Network (DRCR.net) Protocol S, Gross et al found that of 305 PDR patients (mean age of 52 years, 56% male, 52% White) that underwent either PRP or ranibizumab injection, VA in most study eyes was relatively stable and there was no difference in major adverse event over the course of 5 years.15 In the current study, in-office therapy seemed to influence ultimate surgical outcomes. Eyes receiving preoperative PRP in the 6 months before the surgery experienced better postoperative visual outcomes compared to eyes without preoperative PRP, and preoperative PRP within 6 months and use of preoperative anti-VEGF also were associated with a lower rate of postoperative VH.
Where PPV fits into the treatment algorithm for PDR remains debatable. In DRCR.net Protocol AB, Antosyzk et al found that the treatment of PDR with VH using intravitreal aflibercept compared to early PPV with PRP showed no significant difference in long-term VA outcomes (over 24 weeks).16 However, their study demonstrated faster recovery of vision when baseline vision was worse than 20/800 and quicker clearance of VH in eyes that underwent initial vitrectomy with PRP.17 The current study and Protocol AB both demonstrated the safety and efficacy of PPV with PRP for PDR with VH; an optimal approach to PDR is still likely use of traditional first-line in-office options followed by PPV if necessary and indicated.
In addition, the current study demonstrated worse visual prognosis in individuals with longstanding diabetes mellitus and PDR, which may be explained by cumulative injury to the retina, such as retinal hypoxia.18 The chronic effects of damage and deterioration can permanently change the microenvironment in the retina19 and, as a result, the visual prognosis of these patients may be worse. Moreover, in the current study, individuals with higher preoperative hemoglobin A1c were more likely to develop postoperative VH, demonstrating the effect of poor systemic glycemic control on surgical recovery and complications. In contrast, Khuthaila et al demonstrated no difference between preoperative HbA1c levels and the incidence of postoperative VH.12 Despite the conflicting data surrounding HbA1C as a prognostic factor for outcomes after PPV, optimizing systemic disease is essential for preventing progression of diabetic eye disease and surgical complications.
The impact of antiplatelet and anticoagulant use on the incidence of postoperative hemorrhage in PDR patients is controversial.20 The current study showed no significant association between blood thinner usage and postoperative complications, including VH. One prospective study of 374 eyes that underwent diabetic vitrectomy did not demonstrate an elevated risk of perioperative complications, including VH, in patients on antiplatelet or anticoagulant agents.21 In contrast, other authors have demonstrated an increased risk of postoperative vitreous hemorrhage in patients on warfarin but not aspirin.22
It is well known that sub-tenon corticosteroids reduce macular thickening by decreasing intraocular inflammation.23 Previous work has demonstrated a significant decrease in the rate of postoperative DME with postoperative sub-tenon triamcinolone acetonide.24 Supporting the current literature, the current study found preoperative intravitreal anti-VEGF and intraoperative sub-tenon triamcinolone acetonide treatment were associated with a reduced rate of postoperative VH and DME, respectively.25,26
Previous work has showed that the most common complication following PPV is VH in patients with PDR. A study by Yorston et al evaluated postoperative outcomes following PPV in patients with PDR and reported postoperative VH in 22% of patients.11 In another study by Novak et al, VH on postoperative day 1 was seen in 63% of eyes and delayed or persistent VH was seen in 23% of eyes.27 In another similar study performed by Schachat et al, immediate VH was seen in 75% of eyes and delayed VH was seen in 29% of eyes.28 In contrast, only 12% of eyes in the current study had postoperative VH following PPV with concurrent PRP, suggesting that postoperative VH may be relatively infrequent in patients undergoing PPV with endolaser PRP.
Previous work has compared vitreous substitution with sulfur hexafluoride (SF6) gas and balanced salt solution (BSS) for the prevention of postoperative VH in PDR patients undergoing PPV. In one randomized clinical trial study of PDR patients undergoing PPV for non-clearing VH, postoperative VH was less frequent in eyes that received SF6 compared to BSS.29 The current study did not find significant differences in postoperative outcomes between tamponade agents. Further investigation is needed to determine the efficacy of different tamponade agents in patients with PDR complicated with non-clearing VH requiring PPV.
Limitations of the current study include its retrospective nature. Future investigation may aim to prospectively examine the outcomes of PPV with endolaser PRP and may seek to compare it with other methods of treatment for PDR. Furthermore, this study had a relatively small sample size with only 57% of the cohort followed for more than 6 months. Future studies may seek to employ a larger sample size to more accurately characterize short- and long-term outcomes and complications after PPV with endolaser PRP. Additionally, this study carries relevant selection bias in surgeon’s preference (ie, surgical technique of different sized vitrectors, ILM peeling, and tamponade agents). Lastly, this study carries bias in decisional standards for injections and lasers (ie, decision to perform preoperative or intraoperative IVA, treatment algorithm for DME, clinic-based PRP, number of laser spots, and area of laser ablation).
Conclusion
The current study demonstrates that PPV with endolaser PRP for patients with PDR results in significant and prolonged improvements in visual acuity with a relatively low rate of postoperative VH. Hemoglobin A1c and duration of diabetes and PDR may be prognostic indicators for postoperative outcomes after PPV with PRP. Intraoperative subtenon triamcinolone acetonide and preoperative anti-VEGF and PRP therapy are associated with improved postoperative outcomes. As surgical techniques evolve, continued investigation will be necessary to improve clinical outcomes in patients with PDR.
Abbreviations
BCVA, Best corrected visual acuity; CPT, current procedural terminology DME, diabetic macular edema; HbA1c, hemoglobin A1c; ILM, internal limiting membrane; logMAR, Logarithm of the Minimum Angle of Resolution, NVD, neovascularization of the disc; NVE, neovascularization elsewhere; OCT, optical coherence tomography; PRP, panretinal photocoagulation; PPV, pars plana vitrectomy; PDR, proliferative diabetic retinopathy; RD, retinal detachment; VEGF, vascular endothelial growth factor; VH, vitreous hemorrhage.
Acknowledgments
This paper was presented at the Association for Research in Vision and Ophthalmology as an abstract presentation with interim and preliminary findings. The poster’s abstract was published in “Poster Abstracts” in Investigative Ophthalmology & Visual Science June 2022, Vol.63, 2212: https://iovs.arvojournals.org/article.aspx?articleid=2780520.
Funding
Bascom Palmer Eye Institute received funding from the NIH Core Grant P30EY014801, Department of Defense Grant #W81XWH-13-1-0048, and a Research to Prevent Blindness Unrestricted Grant. The sponsors or funding organizations had no role in the design or conduct of this research.
Disclosure
Dr. Yannuzzi is a consultant for Genentech, Alcon, and REGENXBIO. Dr. Sridhar is a consultant for Alcon, Allergan, Apellis, Dorc, Genentech, Ocuterra, and Regeneron. The authors report no other conflicts of interest in this work.
References
1. Shimura M, Yasuda K, Nakazawa T, Shiono T, Nishida K. Panretinal-photocoagulation before pars plana vitrectomy influences vitreous level of interleukin-6 but not of vascular endothelial growth factor in patients with diabetic retinopathy. Int J Biomed Sci. 2007;3(1):31–37.
2. Gupta A, Bansal R, Gupta V, Dogra MR. Six-month visual outcome after pars plana vitrectomy in proliferative diabetic retinopathy with or without a single preoperative injection of intravitreal bevacizumab. Int Ophthalmol. 2012;32(2):135–144. doi:10.1007/s10792-012-9541-5
3. The Diabetic Retinopathy Study Research Group. Photocoagulation treatment of proliferative diabetic retinopathy. Clinical application of Diabetic Retinopathy Study (DRS) findings, DRS report number 8. Ophthalmology. 1981;88(7):583–600.
4. Kaiser RS, Maguire MG, Grunwald JE, et al. One-year outcomes of panretinal photocoagulation in proliferative diabetic retinopathy. Am J Ophthalmol. 2000;129(2):178–185. doi:10.1016/s0002-9394(99)00322-0
5. Chappelow AV, Tan K, Waheed NK, Kaiser PK. Panretinal photocoagulation for proliferative diabetic retinopathy: pattern scan laser versus argon laser. Am J Ophthalmol. 2012;153(1):137–42 e2. doi:10.1016/j.ajo.2011.05.035
6. Parikh R, Shah RJ, VanHouten JP, Cherney EF. Ocular findings at initial pan retinal photocoagulation for proliferative diabetic retinopathy predict the need for future pars plana vitrectomy. Retina. 2014;34(10):1997–2002. doi:10.1097/IAE.0000000000000192
7. The Diabetic Retinopathy Vitrectomy Study Research Group. Early vitrectomy for severe vitreous hemorrhage in diabetic retinopathy. Two-year results of a randomized trial. Diabetic Retinopathy Vitrectomy Study report 2. Arch Ophthalmol. 1985;103(11):1644–1652. doi:10.1001/archopht.1985.01050110038020
8. Gupta B, Sivaprasad S, Wong R, et al. Visual and anatomical outcomes following vitrectomy for complications of diabetic retinopathy: the DRIVE UK study. Eye. 2012;26(4):510–516. doi:10.1038/eye.2011.321
9. Gupta B, Wong R, Sivaprasad S, Williamson TH. Surgical and visual outcome following 20-gauge vitrectomy in proliferative diabetic retinopathy over a 10-year period, evidence for change in practice. Eye. 2012;26(4):576–582. doi:10.1038/eye.2011.348
10. The Diabetic Retinopathy Vitrectomy Study Research Group. Early vitrectomy for severe proliferative diabetic retinopathy in eyes with useful vision. Results of a randomized trial--Diabetic Retinopathy Vitrectomy Study Report 3. Ophthalmology. 1988;95(10):1307–1320. doi:10.1016/s0161-6420(88)33015-0
11. Yorston D, Wickham L, Benson S, Bunce C, Sheard R, Charteris D. Predictive clinical features and outcomes of vitrectomy for proliferative diabetic retinopathy. Br J Ophthalmol. 2008;92(3):365–368. doi:10.1136/bjo.2007.124495
12. Khuthaila MK, Hsu J, Chiang A, et al. Postoperative vitreous hemorrhage after diabetic 23-gauge pars plana vitrectomy. Am J Ophthalmol. 2013;155(4):757–63, 63 e1–2. doi:10.1016/j.ajo.2012.11.004
13. Bach M. The Freiburg visual acuity test-variability unchanged by post-hoc re-analysis. Graefes Arch Clin Exp Ophthalmol. 2007;245(7):965–971. doi:10.1007/s00417-006-0474-4
14. Schulze-Bonsel K, Feltgen N, Burau H, Hansen L, Bach M. Visual acuities “hand motion” and “counting fingers” can be quantified with the Freiburg visual acuity test. Invest Ophthalmol Vis Sci. 2006;47(3):1236–1240. doi:10.1167/iovs.05-0981
15. Gross JG, Glassman AR, Liu D, et al. Five-year outcomes of panretinal photocoagulation vs intravitreous ranibizumab for proliferative diabetic retinopathy: a randomized clinical trial. JAMA Ophthalmol. 2018;136(10):1138–1148. doi:10.1001/jamaophthalmol.2018.3255
16. Antoszyk AN, Glassman AR, Beaulieu WT, et al. Effect of intravitreous aflibercept vs vitrectomy with panretinal photocoagulation on visual acuity in patients with vitreous hemorrhage from proliferative diabetic retinopathy: a randomized clinical trial. JAMA. 2020;324(23):2383–2395. doi:10.1001/jama.2020.23027
17. Glassman AR, Beaulieu WT, Maguire MG, et al. Visual acuity, vitreous hemorrhage, and other ocular outcomes after vitrectomy vs aflibercept for vitreous hemorrhage due to diabetic retinopathy: a secondary analysis of a randomized clinical trial. JAMA Ophthalmol. 2021;139(7):725–733. doi:10.1001/jamaophthalmol.2021.1110
18. Hamanaka T, Akabane N, Yajima T, Takahashi T, Tanabe A. Retinal ischemia and angle neovascularization in proliferative diabetic retinopathy. Am J Ophthalmol. 2001;132(5):648–658. doi:10.1016/s0002-9394(01)01108-4
19. Liao M, Wang X, Yu J, et al. Characteristics and outcomes of vitrectomy for proliferative diabetic retinopathy in young versus senior patients. BMC Ophthalmol. 2020;20(1):416. doi:10.1186/s12886-020-01688-3
20. Ryan A, Saad T, Kirwan C, Keegan DJ, Acheson RW. Maintenance of perioperative antiplatelet and anticoagulant therapy for vitreoretinal surgery. Clin Exp Ophthalmol. 2013;41(4):387–395. doi:10.1111/ceo.12017
21. Lauermann P, Klingelhöfer A, Mielke D, et al. Risk factors for severe bleeding complications in vitreoretinal surgery and the role of antiplatelet or anticoagulant agents. Ophthalmol Retina. 2021;5(8):e23–e29. doi:10.1016/j.oret.2021.04.013
22. Narendran N, Williamson TH. The effects of aspirin and warfarin therapy on haemorrhage in vitreoretinal surgery. Acta Ophthalmol Scand. 2003;81(1):38–40. doi:10.1034/j.1600-0420.2003.00020.x
23. Degenring RF, Kreissig I, Jonas JB. Intraokulare Triamcinolongabe bei diffusem diabetischen Makulaödem [Intraocular triamcinolone for diffuse diabetic macular edema]. Ophthalmology. 2004;101(3):251–254. German. doi:10.1007/s00347-003-0862-7
24. Koga T, Mawatari Y, Inumaru J, Fukushima M, Tanihara H. Trans-Tenon’s retrobulbar triamcinolone acetonide infusion for refractory diabetic macular edema after vitrectomy. Graefes Arch Clin Exp Ophthalmol. 2005;243(12):1247–1252. doi:10.1007/s00417-005-0045-0
25. Cheema RA, Mushtaq J, Al-Khars W, Al-Askar E, Cheema MA. Role of intravitreal bevacizumab (Avastin) injected at the end of diabetic vitrectomy in preventing postoperative recurrent vitreous hemorrhage. Retina. 2010;30(10):1646–1650. doi:10.1097/IAE.0b013e3181d6def0
26. Nakamura A, Shimada Y, Horio N, Horiguchi M. Vitrectomy for diabetic macular edema with posterior subtenon injection of triamcinolone acetonide. Folia Ophthalmologica Japonica. 2004;55(12):958–962.
27. Novak MA, Rice TA, Michels RG, Auer C. Vitreous hemorrhage after vitrectomy for diabetic retinopathy. Ophthalmology. 1984;91(12):1485–1489. doi:10.1016/s0161-6420(84)34099-4
28. Schachat AP, Oyakawa RT, Michels RG, Rice TA. Complications of vitreous surgery for diabetic retinopathy. II. Postoperative complications. Ophthalmology. 1983;90(5):522–530. doi:10.1016/s0161-6420(83)34540-1
29. Rush RB, Velazquez JC, Rosales CR, Rush SW. Gas tamponade for the prevention of postoperative vitreous hemorrhaging after diabetic vitrectomy: a randomized clinical trial. Am J Ophthalmol. 2022;242:173–180. doi:10.1016/j.ajo.2022.06.015
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
