Back to Journals » Clinical Ophthalmology » Volume 17
Outcomes of Non-Penetrating Deep Sclerectomy for Primary Congenital Glaucoma Performed by Experienced versus Trainee Surgeons: A Cohort Study
Authors Khan OA, Sesma G , Alawi A , AlWazae M
Received 29 December 2022
Accepted for publication 2 March 2023
Published 16 March 2023 Volume 2023:17 Pages 897—906
DOI https://doi.org/10.2147/OPTH.S403016
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Omar Abdallah Khan, Gorka Sesma, Abeer Alawi, Manal AlWazae
Pediatric Ophthalmology and Strabismus Division, King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia
Correspondence: Gorka Sesma, Pediatric Ophthalmology and Strabismus Division, King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia, Tel +966114849700, Fax +966114821908, Email [email protected]
Purpose: To compare the outcomes of non-penetrating deep sclerectomy (NPDS) for primary congenital glaucoma (PCG) performed by experienced vs trainee surgeons.
Patients and Methods: This retrospective cohort study was conducted in 2022 in Saudi Arabia. Consultants (Gr-1) and trainee pediatric ophthalmologists (Gr-2) performed NPDS on pediatric patients with PCG. Success was defined as an intraocular pressure (IOP) less than 21 mmHg at 6 months after surgery. Complications, glaucoma medications, and additional procedures were also observed in the two groups.
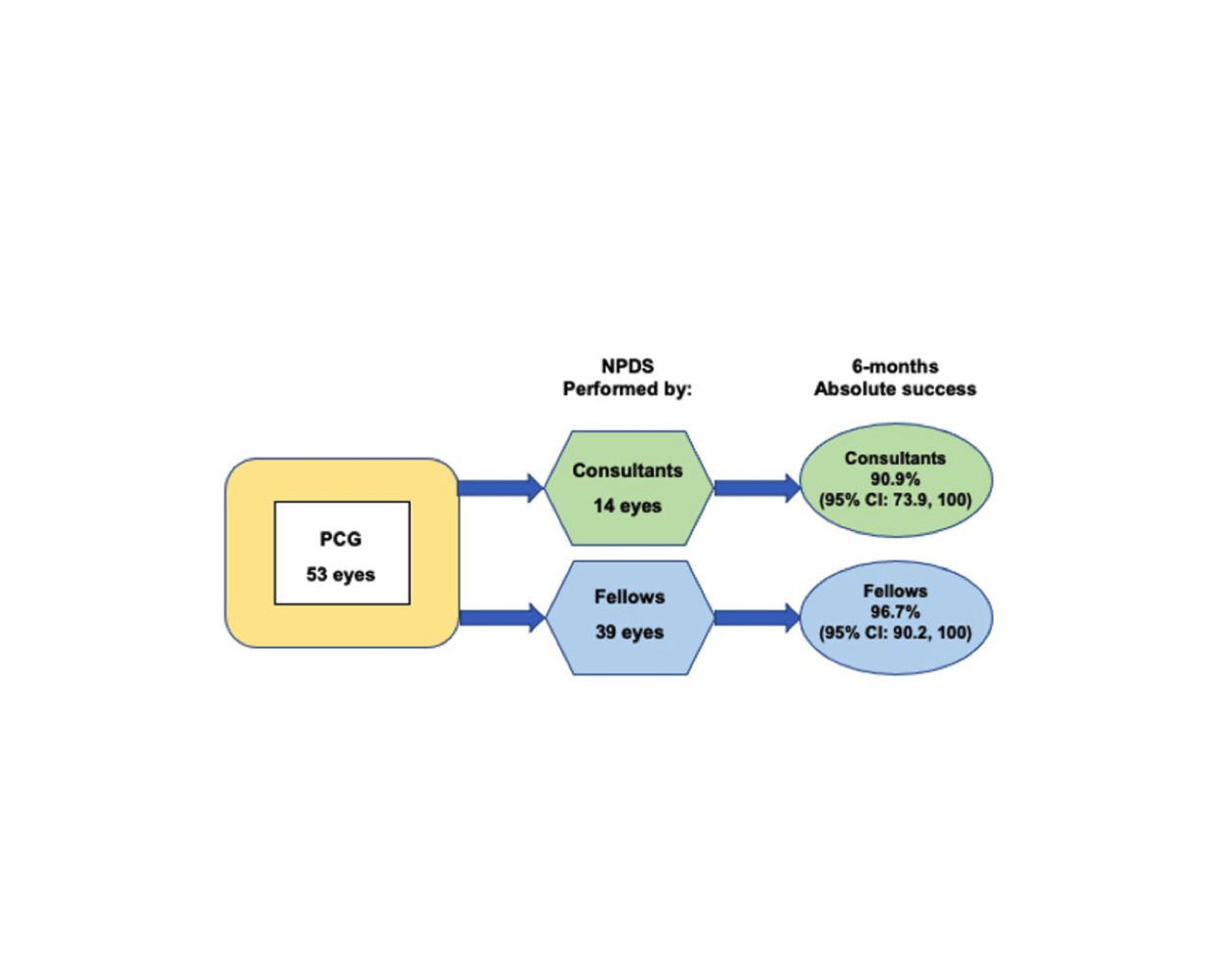
Results: Gr-1 and Gr-2 operated on 14 and 39 eyes with PCG, respectively. The absolute success rates were 90.9% (95% confidence interval [CI]: 73.9, 100) in Gr-1 and 96.7% (95% CI: 90.2, 100) in Gr-2 (odds Ratio=1.1; 95% CI: 0.87, 1.3; P=0.54). Survival analysis suggested that the failure rate in the first 6 months after NPDS was not significantly different between the two groups (hazard ratio=1.45; 95% CI: 0.13, 16.0; P=0.767). The complications included hypotony (2 cases), vitreous hemorrhage (1 case), and total flap penetration (1 case). Only one eye in Gr-2 needed glaucoma medication after surgery. There was no significant difference in the success rates of one surgeon before and after training (P=0.43). The age (P=0.59) and sex (P=0.77) of patients, type of surgeon (P=0.94), and preoperative IOP (P=0.59) were not significant predictors of a stable IOP at 6 months after NPDS.
Conclusion: At 6 months after NPDS surgery performed by experienced and trainee pediatric ophthalmologists, the outcomes (stabilization of IOP) were similar between the two groups.
Keywords: pediatric glaucoma, learning curve, medical education, surgical training, intraocular pressure, glaucoma surgery
Graphical Abstract:
Plain Language Summary
Primary congenital glaucoma is one of the leading causes of childhood blindness worldwide, particularly in Saudi Arabia, where the disease is common. The goal of the treatment is to lower the intraocular pressure. The gold standard therapies are goniotomy, trabeculotomy, and trabeculectomy; however, these operations have the possibility of frequent and serious complications. The procedure for glaucoma at we prefer to do is called NPDS. The procedure allows fluid to drain out the eye through a filtration membrane. The eye is not surgically opened during the procedure making it safer than other glaucoma procedures.
In this study, we use this as the first treatment option in our hospital for patients with primary congenital glaucoma, with good results. This is a teaching hospital where fellows learn about this procedure before putting it into practice in other parts of the country.
We compared the outcomes of NPDS performed by experienced vs trainee surgeons for primary congenital glaucoma. Fourteen eyes were operated on by experienced surgeons, while 39 eyes were operated on by trainee surgeons under direct supervision. Patients were monitored for 6 months. Without antiglaucoma treatment, an intraocular pressure of <21 mmHg was considered complete success. Our results indicated that the failure rate after the procedure was not statistically different between both groups, with good intraocular pressure results and low rates of complications. NPDS is a safe method for treating primary congenital glaucoma. The learning curve of the technique can be reached rapidly and safely under competent supervision, hence providing a therapeutic option for primary congenital glaucoma.
Introduction
Glaucoma is one of the top three causes of visual impairment in children in the United Kingdom.1 In industrialized countries, the incidence of primary congenital glaucoma (PCG) is approximately 1 in 10,000 to 20,000 live births.2 However, the incidence of PCG is much higher in the Arab population, where the disease has a confirmed genetic etiology.3 A systematic approach has been proposed to control PCG in Saudi Arabia, including universal screening for the responsible gene among prospective parents and surgical treatment of children with PCG.4 Approximately 70 new cases of PCG are reported annually in our tertiary eye hospital, where experts train pediatric ophthalmologists in addition to providing treatment.5 Since 1993, two pediatric ophthalmologists have been trained per year at this institute, and up to 50 pediatric ophthalmologists may be available to serve the 22 million people in the Saudi population.6 In the coming years, local ophthalmologists will also be trained to manage children with eye diseases (including PCG).
Although modern technologies and simulators have made it easier to train subspecialists, the constant supervision of expert subspecialist ophthalmologists is critical for maintaining the delivery of high-quality service. Globally, ophthalmologists have been successfully trained for cataract and glaucoma surgeries using wet laboratories,7–9 which are mainly used to manage the eyes of adult patients. However, children with PCG are managed with goniotomy, trabeculotomy, and trabeculectomy (a commonly performed surgery with serious complications).10
Non-penetrating glaucoma surgeries aim to reduce the intraocular pressure (IOP) with avoiding the complications associated with penetrating glaucoma procedures.10,11 Non-penetrating deep sclerectomy (NPDS) has high efficacy and a good safety profile for adults with open-angle glaucoma.11,12 However, it is a challenging surgery that requires supervised training.13 Training surgeons for NPDS in pediatric patients is also complicated because children with PCG are less cooperative and have longer life expectancies, which makes long-term follow-up difficult for patients who travel from distant locations.14
The outcomes of NPDS have been compared with those of other procedures to treat PCG.15,16 However, to our knowledge, no studies have compared the outcomes of NPDS between surgeries performed by trainees vs experienced ophthalmologists. In this study, we compared the success rates of NPDS performed by trainees vs experienced ophthalmologists, and evaluated the determinants of success 6 months after NPDS in Saudi pediatric patients.
Materials and Methods
Ethical Statements
This retrospective two-armed cohort study was conducted at the King Khaled Eye Specialist Hospital after obtaining the approval of the ethics and research committee (approval no. 22109-R). The requirement for informed consent was waived due to the retrospective nature of the study. However, Before any medical exams, studies, or treatment, all patients or their legal representatives completed a general consent form granting the institution permission to utilize their medical data for research purposes if necessary. All medical records and other personally identifiable health information will always be kept confidential, safeguarding the patient’s privacy, and will only be disclosed with the patient’s express written consent. No other details will be revealed. All the tenets of the Helsinki Declaration were strictly followed.
Sample Size Calculation
To calculate the sample size for this study, we tested the null hypothesis that there were no differences in the 6-month absolute success rates of NPDS between surgeries performed by the trainees vs consultants (alternative hypothesis: there is a difference in the 6-month absolute success rate). To achieve a 95% confidence interval and 80% power with a 1:3 ratio of consultant to trainee and an assumed success rate of 96% in consultants (Gr-1) and 60% in trainees (Gr-2), we required at least 14 cases in Gr-1 and 42 in Gr-2. We used the open-source Epi software to calculate the sample size.17
Study Design
Pediatric patients with PCG who underwent NPDS between January 2020 and June 2022, completed 6 months of follow-up, and had not undergone glaucoma surgeries previously were included in this study. Patients with secondary congenital glaucoma (anterior segment dysgenesis, aniridia, prior surgery, trauma, microphthalmia, or any other anomalies) or a history of glaucoma surgery were excluded.
Gr-1 and Gr-2 comprised eyes with PCG managed by NPDS performed by a pediatric consultant ophthalmologist and trainee pediatric ophthalmologists, respectively. If the surgery was performed by a pediatric ophthalmologist consultant, the case was placed in Gr-1. If the surgery was performed by fellow ophthalmologists as the first operating surgeon and supported by a consultant, the case was placed in Gr-2. Surgeries were assigned to the consultant or trainee based on the number of surgeries booked on a specific date. When the number of patients booked allowed the trainer to teach, the fellow performed the surgery under direct supervision. If six or more pediatric surgeries were booked on a single day, the consultant performed the NPDS surgery alone. Three ophthalmologists were involved in the eye care of children with PCG and performed the NPDS.
Clinical ocular examinations were performed with a head-mounted ophthalmic magnifier (Topcon, Oakland, NJ, USA). The eye was evaluated under general anesthesia, during which the IOP was measured with an iCare tonometer (iCare PRO tonometer; iCare Finland Oy, Vantaa, Finland), the corneal diameter was measured with a caliper, and the axial length was measured using a Tomey-AL-2000 ultrasound device (model AL-2000; Tomey, Nürnberg, Germany). The anterior segment (cornea, Haab striae, depth of the anterior chamber, iris configuration, pupil size and configuration, and chamber angle) and posterior part (the optic nerve head, macula, and retina) of the eye were examined under a microscope. The cup-to-disc ratio (C:D) was measured by direct examination to check whether the posterior segment was visible. In the presence of corneal haze, we used optical coherence tomography to measure the C:D value. The grades of corneal haze included none, mild (visible with a direct focal ophthalmoscope but causing difficulties in refraction), moderate (partially obscured details of the iris), and severe (completely obscured details of the iris).18 These data were extracted from the Health Information Management System of the hospital. The demographic information of patients included their age (in months) and sex. The preoperative ocular data included information related to laterality, presence of a family history of congenital glaucoma, number of antiglaucoma medications, IOP, corneal diameter, axial length, presence of corneal haze, and presence of Haab striae.
Surgical Procedure
The surgery was performed under general anesthesia under the constant supervision of an experienced anesthesia team. Using aseptic measures, an eyelid speculum and a 6–0 polyglactin traction suture were placed on the upper limb of the cornea to expose the upper quadrant. A fornix-based conjunctival incision was made to expose the sclera. The Tenon’s capsule was removed with the help of a Tooke knife. A 5×4-mm scleral flap was created (scleral thickness: 40–50%). A sponge soaked with 0.02% mitomycin was applied to the bare sclera and held for 3 minutes. Immediately afterward, the area was rinsed with at least 100 mL of balanced saline solution. A second 4×3-mm deep scleral flap was created under the previous one, starting posteriorly and advancing toward the limbus. This deep flap (90–95% scleral depth) was extended to reach the suprachoroidal space. A few scleral collagen fibers were retained above this space, and a suprachoroidal lake was created. The traction sutures were released, and anterior chamber paracentesis was performed with a 30-gauge needle. This helped decompress the globe and avoid sudden decompression of the eye in the event of perforation. When the Schlemm’s canal was reached in the corneal limbus and spontaneous drainage of the aqueous humor was observed, we carefully continued dissecting along this surgical plane to reach at least 1.5 mm of clear stromal cornea. Thereafter, we cut the flap (including the external wall of the Schlemm’s canal) with great caution to avoid perforation. The first scleral flap was closed with two loose 10–0 nylon stitches. The conjunctiva was closed with running 8-0 polyglactin sutures.
Outcomes Measured
Follow-up examinations were performed on day 1, week 1, and months 1, 3, and 6. At each follow-up visit, the eye was examined under sedation, and the IOP, corneal diameter, axial length, and C:D ratio were measured. Postoperative complications—such as choroidal detachment, shallow anterior chamber, hyphema, cataract formation, and posterior synechia—were documented. If antiglaucoma medication was prescribed or other procedures were performed to control IOP, the relevant details were documented. The outcome variable was IOP at follow-up. Absolute success was indicated by an IOP of less than 21 mmHg without medication, whereas qualified success was indicated by the IOP remaining unchanged when antiglaucoma medication was used (according to the guidelines of the World Glaucoma Association).19
Statistical Analysis
The above data were entered into an Excel (Microsoft Corp., Redmond, WS, USA) spreadsheet, checked for consistency, transferred to SPSS (version 25; IBM Corp., Armonk, NY, USA), and analyzed using nonparametric univariate analyses. Qualitative data are presented as numbers and percentages, whereas quantitative data are presented as the median and interquartile range. To compare qualitative variables in Gr-1 and Gr-2, we calculated the odds ratio, 95% confidence interval (CI), and two-sided P-values. For analyses involving >2 subgroups, we calculated the chi-squared (χ2) value, degree of freedom, and P-value. Quantitative variables were compared using the Mann–Whitney U-test (two-sided P-values). A P-value of <0.05 was considered statistically significant. Kaplan–Meier analysis was used to evaluate between-group differences in the success rates of IOP control by the time of follow-up after NPDS.
Results
Our cohort consisted of 14 and 39 eyes with PCG operated upon by a consultant (Gr-1) and by trainee pediatric ophthalmologists (Gr-2), respectively. Most demographic characteristics and preoperative ocular parameters of patients were similar between groups (Table 1). Patients with a family history of glaucoma were more common in Gr-2 than in Gr-1. Patients with preoperative corneal haze (severe) were more common in Gr-1 than in Gr-2, although the difference was not statistically significant. The IOP declined after surgery in both groups, although the differences were not statistically significant (Figure 1). Moreover, the IOP was not significantly different between groups at the follow-up visits (Table 2). The 6-month absolute success rate was 90.9% (95% CI: 73.9, 100) in Gr-1 and 96.7% (95% CI: 90.2, 100) in Gr-2 (risk ratio [RR]=1.1; 95% CI: 0.87, 1.3; P=0.54). When cases that were lost to follow-up at 6 months (3 in Gr-1 and 9 in Gr-2) were considered failures, the success rates declined to 71.4% (95% CI: 47.8, 95.1) in Gr-1 and 74.4% (95% CI: 60.7, 88.1) in Gr-2 (RR=0.96; 95% CI: 0.66, 1.4; P=0.82). When these patients were considered absolute successes (IOP: ≤21 mmHg at the 6-month follow-up), the success rates increased to 92.9% and 97.4% in Gr-1 and Gr-2, respectively. The failure rates in the first 6 months after NPDS were not significantly different between groups (hazard ratio=1.45; 95% CI: 0.13, 16.0; P=0.767). The Kaplan–Meier survival graph (Figure 2) indicated that the 6-month failure rate of NPDS was similar between groups.
The complications included hypotony (2 cases), vitreous hemorrhage (1 case), and total flap penetration (1 case). A failed surgery in Gr-1 resulted in a mild corneal haze in the eye. Two eyes with failed surgery in Gr-2 had mild and moderate corneal hazes, respectively. Regression analysis suggested that none of the factors (including expert vs trainee surgeons) were significant predictors of success in IOP control at 6 months after NPDS (Table 3). We did not compare qualified success between the two groups because only one eye in Gr-2 required one medication at the 1-month follow-up. A trainee who operated on 18 eyes with PCG under supervision had a success rate of 94.4% (17/18). The same surgeon, after gaining experience, became a consultant who operated on five eyes with PCG independently and had a success rate of 80% (4/5). For this surgeon, there was no significant difference in success rate between surgeries performed before and after training (P=0.43).
 |
Table 3 Predictors of Success in Maintaining an Intraocular Pressure (IOP) of Less Than 21 mmHg After Non-Penetrating Deep Sclerectomy for Primary Congenital Glaucoma |
Discussion
NPDS is not commonly performed to treat PCG worldwide. Nevertheless, it showed promising results in our study, even when performed by trainees under the supervision of experienced experts. One eye operated upon by the consultant had an accidental full penetration of the scleral flap; however, the postoperative complications of hypotony and vitreous bleeding could be managed effectively. None of the demographic characteristics or preoperative ocular parameters of patients were predictors of success in IOP control after NPDS.
This is perhaps the first study to highlight the usefulness of NPDS and the ease of hands-on training for pediatric ophthalmology trainees to perform this surgery. The overall short-term success of NPDS did not differ significantly between trainees and trainers. Therefore, NPDS can be implemented as a training module to manage PCG in a teaching hospital. Our literature search did not reveal any other studies comparing trainee vs trainer outcomes for NPDS in a similar pediatric age group. Therefore, we compared the success rates of NPDS in our study with those reported for glaucoma surgeries in adults and other eye surgeries in pediatric patients. Karaconji et al19 reported no difference in the results of deep sclerectomy performed by trainees vs trainers. Although the sample size in that study was substantial, the mean age of patients with glaucoma was 76 years. Another study on a small number of adults with glaucoma reported that the success rate of IOP control after non-penetrating glaucoma surgery was similar between surgeries performed by resident ophthalmologists (who were competent in the phacoemulsification procedure) vs consultants.19 Although the indicators (such as a review of the video recording of the surgery) used in that study differed from those used in previous studies, it was unique in that it compared the outcomes of two groups operated upon by different types of surgeons.9
The eyes of children with glaucoma differ from those of adults with glaucoma. Children have a thin sclera, an enlarged eyeball, and an abnormal angle of the anterior chamber.20 These features negatively affect the surgeon’s attempt to create a flap, which often carries a risk of total penetration and incomplete removal of the abnormal trabecular meshwork at the angle of the anterior chamber. Therefore, NPDS is more challenging in children than in adults and may result in lower success rates. In addition, handling these delicate structures and thin sclera requires a high level of skill in the early stages of training. Although simulators and residual corneoscleral rims have been used to improve the skills of pediatric glaucoma surgeons, it is difficult for such simulators to mimic NPDS surgeries in PCG eyes.8,21 Therefore, hands-on training under expert supervision is the only option available for medical education, and the results can be periodically evaluated from the perspective of the patient. In this study, NPDS showed high success rates during the first 6 months of follow-up in both groups. Another study investigating the long-term outcomes of NPDS in 74 eyes of 63 children with glaucoma reported success rates of 86.5% and 79.7% at 6 months and 3 years, respectively.16 However, before NPDS can be routinely recommended to pediatric ophthalmologists, it is important to compare it with other conventional surgeries for managing PCG.
The complications observed during surgery and at the 1-month follow-up included deep flap puncturing, vitreous hemorrhage, and hypotony. Aslan et al9 noted that perforation of the trabeculo-Descemet membrane occurred in 15.5% of patients who underwent NPDS. In our study, this complication was observed in only one eye in the consultant group, and this could be related to factors associated with the eye rather than with the skills of the surgeon. In our study, the age or sex of the patient, IOP before surgery, laterality, and type of surgeon did not affect the short-term stabilization of the IOP after NPDS. This is consistent with the results of Al-Obeidan et al,16 who reviewed the factors affecting the success of penetrating deep sclerectomy and NPDS to treat childhood glaucoma in Saudi Arabia. In a small series of adult glaucoma cases managed by NPDS, Aslan et al9 noted that the success rate of the surgery was influenced by the type of glaucoma and the surgical method, although there were no between-group differences in age or sex. A study with a larger sample size and longer follow-up period is needed to review the predictors of success after NPDS in children with PCG.
We did not find a significant impact of the learning curve on NPDS surgeries performed by a trainee who subsequently became a consultant. This is in contrast with the results of a study in Turkey, where the authors reported absolute success rates of 49.2% and 84.9% in early and late cases operated upon by experienced surgeons, respectively.9 This suggests that although experience improves the ability of a surgeon to provide better outcomes and minimize complications, factors related to the glaucomatous eye can also influence the results.
Although the World Health Organization recommends one pediatric ophthalmologist per 10 million people,22 this ratio is declining alarmingly in industrialized nations.23 Hospitals providing surgical care for PCG are recommended to have the facilities to accommodate at least 20 new cases per year and host surgeons with substantial experience in adult glaucoma surgery.24 Approximately 70 new cases of PCG present annually to our tertiary eye hospital.5 Therefore, building the capacity for more pediatric ophthalmologists to perform glaucoma surgery for PCG will help reduce childhood blindness. Over the years, surgeons have tried several surgeries and their modifications to manage eyes with PCG. However, the search for an ideal surgery—with fewer complications, long-term IOP control, and improved quality of life—is still ongoing.25 Even if NPDS surgeries have a high success rate and low rate of complications, additional research is required to determine their long-term efficacy.26
This study had a few limitations. First, our comparisons were based solely on short-term outcomes. Second, Aslan et al9 detailed a process for performing repeated video reviews of surgeries by masked examiners; however, this review was not performed in our study. Finally, this research was conducted in a teaching institution, and the random allocation of cases to trainees vs trainers was not possible. However, this selection bias had a minimal effect on our results.
Conclusion
The quality of training and efficiency of NPDS was reflected by the stabilization of the IOP without additional medication or surgical procedures within 6 months after the surgery. Reviewing the long-term results and establishing standard indicators for monitoring the surgical skills of trainees during NPDS will help promote this surgery among pediatric ophthalmologists, hospital administrators, and parents. This can help improve the vision-related quality of life of children with PCG.
Abbreviations
PCG, primary congenital glaucoma; IOP, intraocular pressure; NPDS, non-penetrating deep sclerectomy; C:D ratio, cup-to-disc ratio; CI, confidence interval; RR, risk ratio.
Data Sharing Statement
The data that support the findings of this study are available on request from the corresponding author, GS, and after approval of the IRB Committee of King Khaled Eye Specialist Hospital. The data are not publicly available due to restrictions, their containing information that could compromise the privacy of research participants.
Acknowledgments
We thank the staff of the pediatric ophthalmology division, operation theatre, research department, and Health Information Management System (IT department) of King Khaled Eye Specialist Hospital and Rajiv Khandekar (Rajivkhandekar.com) for extending their support to this research. We would like to thank Editage for English language editing.
Author Contributions
Omar Abdallah Khan: First author; study conceptualization; acquisition of data; supervision; data curation; formal analysis; writing – original draft.
Gorka Sesma: Corresponding and Senior author; study conception; methodology; formal analysis; writing – original draft; critical reviewing; writing-reviewing editing; supervision.
Abeer Alawi: Co-author; investigation; acquisition of data; data curation; execution; writing – original draft.
Manal AlWazae: Co-author; investigation; acquisition of data; data curation; execution; formal analysis; writing – original draft.
All authors contributed to data analysis, drafting, or revising the article, have agreed on the journal to which the article will be submitted, reviewed and agreed on all versions of the article before submission, during revision, gave final approval of the version to be published, and agree to be accountable for all aspects of the work.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Solebo AL, Teoh L, Sargent J, Rahi JS; British Childhood Visual Impairment and Blindness Study Interest Group. Avoidable childhood blindness in a high-income country: findings of the British childhood visual impairment and blindness study 2. Br J Ophthalmol. 2022:
2. Badawi AH, Al-Muhaylib AA, Al Owaifeer AM, Al-Essa RS, Al-Shahwan SA. Primary congenital glaucoma: an updated review. Saudi J Ophthalmol. 2019;33(4):382–388. doi:10.1016/j.sjopt.2019.10.002
3. Jemmeih S, Malik S, Okashah S, Zayed H. Genetic epidemiology of primary congenital glaucoma in the 22 Arab countries: a systematic review. Ophthalmic Epidemiol. 2022;29(1):1–12. doi:10.1080/09286586.2021.1883676
4. Malik R, Khandekar R, Boodhna T, et al. Eradicating primary congenital glaucoma from Saudi Arabia: the case for a national screening program. Saudi J Ophthalmol. 2017;31(4):247–249. doi:10.1016/j.sjopt.2017.08.002
5. Alanazi FF, Song JC, Mousa A, et al. Primary and secondary congenital glaucoma: baseline features from a registry at King Khaled eye specialist hospital, Riyadh, Saudi Arabia. Am J Ophthalmol. 2013;155(5):882–889. doi:10.1016/j.ajo.2012.12.006
6. Khan AO, Al-Mesfer S. Pediatric ophthalmology and strabismus in the Kingdom of Saudi Arabia. J AAPOS. 2004;8(6):513–514. doi:10.1016/j.jaapos.2004.05.001
7. Lin JC, Yu Z, Scott IU, Greenberg PB. Virtual reality training for cataract surgery operating performance in ophthalmology trainees. Cochrane Database Syst Rev. 2021;12(12):CD014953. doi:10.1002/14651858.CD014953.pub2
8. Dean WH, Buchan J, Gichuhi S, et al. Simulation-based surgical education for glaucoma versus conventional training alone: the GLAucoma Simulated Surgery (GLASS) trial. A multicentre, multicountry, randomised controlled, investigator-masked educational intervention efficacy trial in Kenya, South Africa, Tanzania, Uganda and Zimbabwe. Br J Ophthalmol. 2022;106(6):863–869. doi:10.1136/bjophthalmol-2020-318049
9. Aslan F, Yuce B, Oztas Z, Ates H. Evaluation of the learning curve of non-penetrating glaucoma surgery. Int Ophthalmol. 2018;38(5):2005–2012. doi:10.1007/s10792-017-0691-3
10. Sood D, Rathore A, Sood I, Singh G, Sood NN. Long-term outcome of combined trabeculotomy-trabeculectomy by a single surgeon in patients with primary congenital glaucoma. Eye. 2018;32(2):426–432. doi:10.1038/eye.2017.207
11. El Sayyad F, Helal M, El-Kholify H, Khalil M, El-Maghraby A. Nonpenetrating deep sclerectomy versus trabeculectomy in bilateral primary open-angle glaucoma. Ophthalmology. 2000;107(9):1671–1674. doi:10.1016/S0161-6420(00)00263-3
12. Slagle G, Groth SL, Montelongo M, Sponsel WE. Nonpenetrating deep sclerectomy for progressive glaucoma: long-term (5-year) follow-up of intraocular pressure control and visual field survival. J Curr Glaucoma Pract. 2020;14(1):3–9. doi:10.5005/jp-journals-10078-1273
13. Eldaly MA, Bunce C, Elsheikha OZ, Wormald R. Non-penetrating filtration surgery versus trabeculectomy for open-angle glaucoma. Cochrane Database Syst Rev. 2014;2:CD007059.
14. Tan YL, Chua J, Ho CL. Updates on the surgical management of pediatric glaucoma. Asia Pac J Ophthalmol. 2016;5(1):85–92. doi:10.1097/APO.0000000000000182
15. Elhofi A, Helaly HA. Non-penetrating deep sclerectomy versus trabeculectomy in primary congenital glaucoma. Clin Ophthalmol. 2020;14:1277–1285. doi:10.2147/OPTH.S253689
16. Al-Obeidan SA, Osman EE, Dewedar AS, Kestelyn P, Mousa A. Efficacy and safety of deep sclerectomy in childhood glaucoma in Saudi Arabia. Acta Ophthalmol. 2014;92(1):65–70. doi:10.1111/j.1755-3768.2012.02558.x
17. Dean AG, Sullivan KM, Soe MM. OpenEpi: open source epidemiologic statistics for public health; 2013. Available from: http://www.OpenEpi.com.
18. Shaarawy T, Grehn F, eds. Guidelines on Design and Reporting of Glaucoma Surgical Trials. Amsterdam: Kugler Publications; 2009.
19. Karaconji T, Mercieca K, Romera P, McNaught A, Anand N. A comparison of deep sclerectomy trainer versus trainee outcomes. J Glaucoma. 2019;28(5):427–432. doi:10.1097/IJG.0000000000001195
20. Chan JY, Choy BN, Ng AL, Shum JW. Review on the management of primary congenital glaucoma. J Curr Glaucoma Pract. 2015;9(3):92–99. doi:10.5005/jp-journals-10008-1192
21. Pujari A, Rakheja V, Dada T, et al. Gonioscopy and angle-based glaucoma surgical training on human eyes in the wet lab. J Glaucoma. 2022;31(10):839–845. doi:10.1097/IJG.0000000000002075
22. WHO. Programme for the prevention of blindness and deafness. A five-year project for the prevention of childhood blindness: report of a WHO Consultation. [WHO/PBL/02.88]. Geneva: WHO; 2002. Available from: https://apps.who.int/iris/handle/10665/67848.
23. Lee KE, Sussberg JA, Nelson LB, Thuma T. The economic downturn of pediatric ophthalmology and its impact on access to eye care. J Pediatr Ophthalmol Strabismus. 2023;60(1):18–24.
24. Moore DB, Tomkins O, Ben-Zion I. A review of primary congenital glaucoma in the developing world. Surv Ophthalmol. 2013;58(3):278–285. doi:10.1016/j.survophthal.2012.11.003
25. Mocan MC, Mehta AA, Aref AA. Update in genetics and surgical management of primary congenital glaucoma. Turk J Ophthalmol. 2019;49(6):347–355. doi:10.4274/tjo.galenos.2019.28828
26. Hoffmann EM, Aghayeva F, Schuster AK, et al. Results of childhood glaucoma surgery over a long-term period. Acta Ophthalmol. 2022;100(2):e448–e454. doi:10.1111/aos.14985
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.




