Back to Journals » Clinical Ophthalmology » Volume 17
Outcomes of Medical and Surgical Management in Aqueous Misdirection Syndrome
Authors AlQahtani RD, Al Owaifeer AM, AlShahwan S, AlZaben K, AlMansour R
Received 15 August 2022
Accepted for publication 13 December 2022
Published 9 March 2023 Volume 2023:17 Pages 797—806
DOI https://doi.org/10.2147/OPTH.S385864
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Scott Fraser
Reham Dakam AlQahtani,1 Adi Mohammed Al Owaifeer,1,2 Sami AlShahwan,1 Khawlah AlZaben,1 Raghad AlMansour3
1King Khaled Eye Specialist Hospital, Riyadh, Saudi Arabia; 2Ophthalmology Unit, Department of Surgery, College of Medicine, King Faisal University, Al-Ahsa, Saudi Arabia; 3College of Medicine, Imam Mohammed bin Saud University, Riyadh, Saudi Arabia
Correspondence: Adi Mohammed Al Owaifeer, Ophthalmology Unit, Department of Surgery, College of Medicine, King Faisal University, P.O. Box 400, Al-Ahsa, 31982, Saudi Arabia, Tel +966135808573, Fax +966135800820, Email [email protected]
Purpose: To report the outcomes of medical and surgical management in patients diagnosed with aqueous misdirection syndrome (AMS).
Patients and Methods: A retrospective chart review of all cases diagnosed with AMS at a single tertiary care eye center during the period from 2014 to 2021. Outcome measures were anatomical success (deepening of the anterior chamber (AC)), functional success (improvement in visual acuity), and treatment success (control of intraocular pressure (IOP)).
Results: A total of 26 eyes with AMS from 24 patients were included. The patients were followed for a mean duration of 24 ± 18 months. Although some patients initially responded to medical and laser therapy, all but one (3.8%) eventually required surgery during the first 3 months after presentation. The mean duration from presentation until surgery was 45.9 ± 45.8 days (range: 2– 119 days). The majority of cases (69.2%) were managed by pars plana vitrectomy. At the last follow-up visit, anatomical success was achieved in 20 (76%) eyes, 15 (57%) eyes had a final visual acuity that was either similar to or better than baseline, and successful control of IOP was achieved in 17 (65%) eyes. Univariate analysis revealed that a history of trabeculectomy as a cause of AMS was a risk factor for treatment failure (OR, 7.8; 95% CI, 1.16– 52.35; P, 0.02).
Conclusion: Our findings indicate that medical and laser management of AMS provide temporary control, and almost all patients eventually require surgery within the first 3 months. A history of trabeculectomy was found to be a risk factor for treatment failure.
Keywords: aqueous misdirection syndrome, malignant glaucoma, angle-closure glaucoma, hyaloidotomy
Introduction
Aqueous misdirection syndrome (AMS) or malignant glaucoma is a relatively uncommon cause of secondary angle-closure glaucoma that can threaten vision if not treated early.1 It occurs due to anterior rotation of the ciliary body resulting in a misdirected pathway of aqueous humor posteriorly and blockage of the movement into the anterior chamber (AC) at the level of the equator of the lens, vitreous face, and ciliary processes.
Clinically, it presents as axial shallowing of the AC despite a patent peripheral iridotomy (PI) with elevated or normal intraocular pressure (IOP). It is considered a diagnosis of exclusion, after ruling out mimickers, ie, pupillary block, serous and hemorrhagic choroidal detachment.2 AMS may occur following any type of intraocular surgery, but it is most commonly associated with glaucoma surgery.3,4
The initial management is medical by topical drops (eg, cycloplegics, sympathomimetics, beta-blockers), in addition to systemic hyperosmotic therapy. The aim of the treatment is to shrink the vitreous, displace the lens–iris diaphragm and decrease aqueous production. In addition to medical treatment, Nd-Yag laser hyaloidotomy and capsulotomy are laser interventions that are used to treat AMS.
Surgical management is indicated in the event of a failure in the aforementioned treatment modalities, and the aim of surgery is to increase the aqueous flow into the AC by either disrupting the vitreous face via trans-corneal needling or removing the vitreous by anterior or pars plana vitrectomy (PPV).3,5–8
The majority of AMS reports in the literature focus on a single treatment modality (eg, PPV)9–12; thus, there is a limited amount of data on the combined outcomes of AMS treatment following different types of interventions. Therefore, the aim of our current study is to report the treatment outcomes of a serial cohort of AMS patients managed at a tertiary care eye hospital.
Materials and Methods
Study Design and Sample Size
We performed a retrospective chart review of all patients diagnosed with AMS (ICD code: H40.839) from 2014 to 2021 at a single tertiary eye care center. The diagnostic criteria of AMS were elevated IOP associated with a uniform (central and peripheral) shallowing of the AC in the presence of a patent PI and the absence of any posterior segment pathology (eg, choroidal effusion, suprachoroidal hemorrhage, posterior segment tumor).
Demographics and Clinical Information
At presentation, the following demographic and clinical details were obtained from the electronic medical records: age, sex, systemic disease, laterality of the affected eye, best-corrected visual acuity, IOP, refraction, axial length, lens status, cup-to-disc ratio, number of glaucoma medications used, history of any ocular disease and past surgical history. We also documented whether the patient was diagnosed before presentation at our institute, and if so, the period from diagnosis until presentation at our hospital.
In each subsequent follow-up visit, we documented the following: best-corrected visual acuity, IOP, number of glaucoma medications used, AC depth, complications, recurrence, and the need for additional laser and/or surgical intervention.
Outcome Measures
The three outcome measures in our study were as follows: anatomical success, functional outcome, and treatment success. Anatomical success was defined as further deepening of the AC compared to presentation. The functional outcome was assessed by comparing baseline best-corrected visual acuity and acuity at the final follow-up visit. Finally, treatment success was defined as success in achieving an IOP level between 5 and 21 mmHg either with or without glaucoma medications, and without loss of light perception.
Data Analysis
Statistical analysis was performed using R (RStudio version 1.1.463 Mac, RStudio Inc., Boston, MA). Quantitative variables were reported in mean ± standard deviation (SD) and evaluated using the Student’s t-test, while categorical variables were reported in percentages and numbers. Kaplan–Meier survival analysis was used to estimate the success rate in cases that underwent PPV. P-values <0.05 were considered as statistically significant.
Results
Baseline characteristics are outlined in Table 1. Twenty-six eyes of 24 patients were included. The mean age of patients was 57.8 ± 22.9 years (range: 4–87 years), and 15 (62%) were female. Five patients (20%) were diagnosed with AMS prior to presentation at our institute with a mean duration of 28.2 ± 20.4 days (range: 7–80 days) from diagnosis until presentation. Eighteen (69%) patients were previously diagnosed with glaucoma and the most common type was primary angle-closure glaucoma (72%). At presentation, the mean IOP was 27.2 ± 10.3 mmHg (range: 9–45 mmHg) and the mean number of glaucoma medications used was 2.4 ± 1.8 (range: 0–4). The surgeries that had been performed prior to the diagnosis of AMS were identified in detail in Table 1.
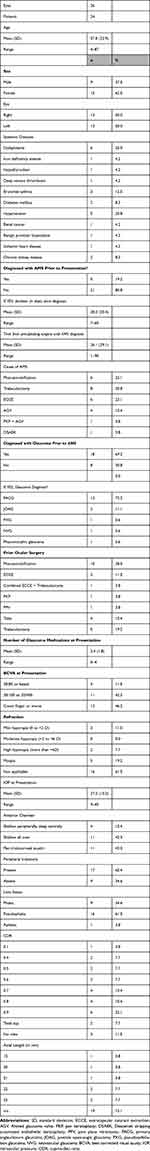 |
Table 1 Baseline Characteristics |
The majority (76%) of patients were initially managed with medical treatment, whereas the remaining (24%) underwent laser treatment. Almost all (96.2%) patients eventually required surgery to control AMS. The mean duration from presentation until the first surgery was 45.9 ±45.8 days (range: 2–119 days).
Regarding surgical interventions, three (12%) patients underwent AC reformation combined with a surgical PI, all of which failed and eventually required PPV. One (4%) patient underwent AC reformation combined with phacoemulsification, and the IOP was controlled in this patient without any further intervention. Four (16%) patients were managed by cyclophotocoagulation (CPC) that was opted due to poor visual potential. Out of these, the IOP remained controlled in one patient, two patients progressed to no light perception, and one patient required an additional session of CPC. Finally, glaucoma drainage device surgery and goniosynechiolysis were performed in one (4%) patient each, both of these patients did not require further intervention.
Eighteen (69%) patients eventually required PPV to control IOP. PPV was combined with phacoemulsification in 3 (12%) patients and glaucoma drainage device surgery in 2 (8%) patients. A Kaplan–Meier curve was plotted to assess the survival probability of patients who underwent PPV (Figure 1). The overall success rate of achieving IOP between 5 and 21 mmHg at 5 years was 60%.
 |
Figure 1 Survival analysis of cases that underwent pars plana vitrectomy (PPV) for the management of aqueous misdirection syndrome (AMS). |
The mean duration of follow-up was 24.5 ± 18.2 months (range: 8–62 months). At the last follow-up visit, the mean IOP was 17.2 ± 9.5 mmHg (compared to 27.2 ± 10.3 at baseline, P < 0.001), and the mean number of glaucoma medications used was 1.8 ± 1.5 (compared to 3.1 ± 1.4 at baseline, P = 0.005).
Table 2 outlines the results of the outcome measures of our study (anatomical success, functional outcome, and treatment success). Furthermore, a univariate analysis was performed to identify the risk factors for treatment failure (Table 3). The only factor that was significantly associated with failure was a history of trabeculectomy as the cause of AMS (OR: 7.8, 95% CI: 1.16–52.35, P-value: 0.02).
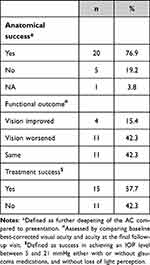 |
Table 2 Final Outcomes of the Study Measures (Anatomical Success, Functional Outcome, and Treatment Success) |
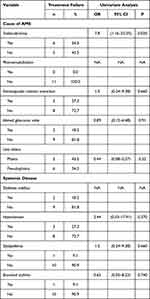 |
Table 3 Analysis of Risk Factors for Failure of Treatment |
Discussion
In our current study, we sought to retrospectively review all cases diagnosed with AMS from presentation until the last follow-up documenting all interventions undergone during management, both medical and surgical. We believe that this would provide more insight into the effectiveness of different treatment modalities and the outcomes of AMS management in general.
AMS can be precipitated by many factors including miotics, YAG laser treatment, diode laser CPC, trauma, intraocular surgeries especially glaucoma surgery. In our study, the most common cause of AMS was trabeculectomy (30.8%) as in previous studies.5,9,10 Other less common causes were cataract surgery, glaucoma drainage device surgery, and keratoplasty.
In our study, the initial treatment of AMS was medical in 76.9% of the patients. Although some initially responded, almost all patients (96%) eventually required surgery. This finding is in concordance with previously reported papers in the literature.5,13–15 This supports the evidence that medical management in AMS is a temporizing measure. Cycloplegics act by tightening the lens zonules to pull the lens posteriorly and reverse shallowing of the AC and subsequently improve the flow of aqueous humor; however, it is evident that the cycloplegic effect does not lead to reversal of the underlying pathology. The IOP-lowering effect of glaucoma drops could also explain the short-term control of AMS in our study by medical treatment.
Laser therapy is another treatment approach in AMS management. An anterior hyaloidotomy disrupts the posterior capsule and anterior vitreous face to allow free flow of aqueous between the AC and vitreous. However, in our study, it was only effective in one (3.8%) patient. Contrary to our findings, Greenfield et al16 reported a series of 10 eyes with AMS, out of which four (40%) responded after laser treatment with deepening of the anterior chamber and normalization of IOP. A possible reason for a better response to laser in their study compared to ours is that half of their patients were open-angle glaucoma. In contrast, the majority (72.2%) of our patients were angle-closure.
Four patients (16%) in our study were initially treated with AC reformation. Three of these were combined with a surgical PI, and one was combined with phacoemulsification. In the three cases that were combined with a surgical PI, IOP was not controlled postoperatively, and the patients eventually required a PPV, whereas in the case that had AC reformation combined with phacoemulsification, the IOP was controlled without further intervention. Our findings imply that AC reformation alone is not a sufficient intervention to control AMS; however, this does not eliminate the utility of AC reformation. A previous report by Thompson et al17 showed that patients undergoing AC reformation at presentation, followed by surgery, had a higher chance of achieving IOP control; this could be explained by the effect of immediate AC reformation on reducing the chances of synechiae formation.
Although CPC can be an inciting factor to AMS, some reports have suggested CPC as a treatment option. Thomas et al18 reported five hyperopic eyes with AMS that were successfully managed with CPC achieving AC deepening and control of IOP over a follow-up period ranging from 1 to 8 years after a single CPC session in four cases and two sessions, 1 year apart, in one patient. The postulated benefit of CPC in AMS is that it may aid in reversing the rotation of the ciliary body and lead to a disruption of the anterior vitreous phase either via laser energy or the inflammation associated with the procedure.9,19,20 In our study, CPC was opted as an intervention in four patients (16%). Among these, two patients ended up with no light perception, so no further intervention was performed, one required another session of CPC that was enough to provide adequate IOP control, and the last patient was controlled after the first CPC treatment and did not need any further intervention.
PPV is considered the treatment of last resort in refractory cases of AMS. The success rate of PPV in our study was 80%, 80%, and 60% at 1, 3, and 5 years, respectively. The high success of PPV in AMS reflects its utility in counteracting the underlying mechanism by removing the vitreous, which is congested by the shunted aqueous, and subsequently contributing to the posterior displacement of the iris-lens-diaphragm, which in turn leads to deepening of the AC. Our success rate echoes evidence from previous studies that prove the efficacy of PPV in providing adequate IOP control in patients with AMS.12,21
A history of trabeculectomy as the precipitating factor of AMS was significantly associated with treatment failure in our study (OR: 7.8, 95% CI: 1.16–52.35, P-value: 0.02). This observation was also noticed in the study reported by Thompson et al,17 in which trabeculectomy was a significant factor associated with a delay in AMS recovery. It is possible that the fistulous tract diverting aqueous into the subconjunctival space post-trabeculectomy impedes AC reformation and control of AMS. This mechanism is supported by Karolina et al, who studied the risk factors of malignant glaucoma and found AMS was higher among patients post penetrating glaucoma surgeries.22
Our study is subject to limitations inherent to retrospective studies. Furthermore, due to failure in documentation, our results lack several objective parameters (eg, refraction and axial length) that could have added more value to our findings.
Conclusion
In conclusion, medical and laser treatment were only helpful in providing short-term control of AMS, and almost all patients eventually required surgical intervention. AC reformation with a surgical PI may temporarily reconstruct the anterior chamber; however, it does not reverse AMS. Although some cases showed good response following either phacoemulsification or CPC, most patients eventually required a PPV. Finally, trabeculectomy was a predictor of treatment failure in our study.
Ethics Statement
The study was approved by the Institutional Review Board (IRB) at King Khalid Eye Specialist Hospital. The protocol of the study was followed by the Declaration of Helsinki. Given the nature of retrospective study reviewing the files for data collection and without any identity to any patient in our project, a written informed consent was impossible and impracticable to obtain.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Grzybowski A, Kanclerz P. Acute and chronic fluid misdirection syndrome: pathophysiology and treatment. Graefes Arch Clin Exp Ophthalmol. 2017;256(1):135–154. doi:10.1007/s00417-017-3837-0
2. Chandler PA, Grant WM. Mydriatic-cycloplegic treatment in malignant glaucoma. Arch Ophthalmol. 1962;68:353–359. doi:10.1001/archopht.1962.00960030357010
3. Dave P, Senthil S, Rao HL, Garudadri CS. Treatment outcomes in malignant glaucoma. Ophthalmology. 2013;120(5):984. doi:10.1016/j.ophtha.2012.10.024
4. Little BC, Hitchings RA. Pseudophakic malignant glaucoma: Nd:YAG capsulotomy as a primary treatment. Eye. 1993;7(Pt 1):102–104. doi:10.1038/eye.1993.21
5. Debrouwere V, Stalmans P, Van Calster J, Spileers W, Zeyen T, Stalmans I. Outcomes of different management options for malignant glaucoma: a retrospective study. Graefes Arch Clin Exp Ophthalmol. 2012;250:131–141. doi:10.1007/s00417-011-1763-0
6. Ali GY, Al-Mahmood AM, Khandekar R, Abboud EB, Edward DP, Kozak I. Outcomes of pars plana vitrectomy in the management of refractory aqueous misdirection syndrome. Retina. 2017;37(10):1916–1922. doi:10.1097/iae.0000000000001430
7. Francis BA, Wong RM, Minckler DS. Slit-lamp needle revision for aqueous misdirection after trabeculectomy. J Glaucoma. 2002;11(3):183–188. doi:10.1097/00061198-200206000-00004
8. Mardelli PG, Mardelli ME. Slit-lamp needling of the anterior capsule for aqueous misdirection after hyaloido-zonulectomy and iridectomy. J Glaucoma. 2018;27(4):e77–e79. doi:10.1097/ijg.0000000000000877
9. Muqit MMK, Mj M. Malignant glaucoma after phacoe- mulsification: treatment with diode laser cyclophotocoagulation. J Cataract Refract Surg. 2007;33:130–132. doi:10.1016/j.jcrs.2006.07.041
10. Dumont HB, Lehoux BA, Santiago PY. Le laser diode dans le traitement du glaucome malin. J Fr Ophtalmol. 2006;29(2):2573–2577.
11. Sharma A, Sii F, Shah P, Kirkby GR. Vitrectomy– phacoemulsification–vitrectomy for the management of aqueous misdirection syndromes in phakic eyes. Ophthalmology. 2006;113:1968–1973. doi:10.1016/j.ophtha.2006.04.031
12. Harbour JW, Rubsamen PE, Palmberg P. Pars plana vitrectomy in the management of phakic and pseudophakic malignant glaucoma. Arch Ophthalmol. 1996;114:1073–1078. doi:10.1001/archopht.1996.01100140275003
13. Zhou C, Qian S, Yao J, et al. Clinical analysis of 50 Chinese patients with aqueous misdirection syndrome: a retrospective hospital-based study. J Int Med Res. 2012;40:1568–1579. doi:10.1177/147323001204000437
14. Simmons RJ. Malignant glaucoma. Br J Ophthalmol. 1972;56(3):263–272. doi:10.1136/bjo.56.3.263
15. Trope GE, Pavlin CJ, Bau A, et al. Malignant glaucoma. Clinical and ultrasound biomicroscopic features. Ophthalmology. 1994;101(6):1030–1035. doi:10.1016/S0161-6420(94)31222-X
16. Greenfield DS, Tello C, Budenz DL, et al. Aqueous misdirection after glaucoma drainage device implantation. Ophthalmology. 1999;106(5):1035–1040. doi:10.1016/S0161-6420(99)00530-8
17. Thompson A, Vu D, Postel E, Challa P. Factors impacting outcomes and the time to recovery from malignant glaucoma. Am J Ophthalmol. 2020;209:141–150. doi:10.1016/j.ajo.2019.07.023
18. Stumpf T, Austin M, Bloom P, McNaught A, Morgan J. Transscleral cyclodiode laser photocoagulation in the treatment of aqueous misdirection syndrome. Ophthalmology. 2008;115(11):2058–2061. doi:10.1016/j.ophtha.2008.05.026
19. Muqit MM, Menage MJ. Malignant glaucoma after phacoemulsification: treatment with diode laser cyclophotocoagulation. J Cataract Refract Surg. 2007;33(1):130.
20. Massicotte EC, Schuman JS. A malignant glaucoma-like syndrome following pars plana vitrectomy. Ophthalmology. 1999;106(7):1375–1379. doi:10.1016/S0161-6420(99)00727-7
21. Dugel PU, Heuer DK, Thach AB, et al. Annular peripheral choroidal detachment simulating aqueous misdirection after glaucoma surgery. Ophthalmology. 1997;104(3):439–444. doi:10.1016/S0161-6420(97)30294-2
22. Krix-Jachym K, Żarnowski T, Rękas M. Risk Factors of Malignant Glaucoma Occurrence After Glaucoma Surgery. National Library Medicine; 2017. Available from: https://www.ncbi.nlm.nih.gov/pmc/articles/PMC5613646/.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
