Back to Journals » Clinical Ophthalmology » Volume 16
Outcomes from the Retrospective Multicenter Cross-Sectional Study on Lamellar Macular Hole Surgery
Authors Haave H, Petrovski BÉ, Zając M, Lumi X , Melekidou W, Lytvynchuk L , Ruban A, Znaor L, Nawrocki J, Nawrocka ZA , Petrovski G
Received 8 December 2021
Accepted for publication 27 April 2022
Published 8 June 2022 Volume 2022:16 Pages 1847—1860
DOI https://doi.org/10.2147/OPTH.S351932
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Hanna Haave,1,* Beáta Éva Petrovski,1,* Michał Zając,2 Xhevat Lumi,3 Wassiliki Melekidou,4 Lyubomyr Lytvynchuk,4,5 Andrii Ruban,6 Ljubo Znaor,7,8 Jerzy Nawrocki,2 Zofia Anna Nawrocka,2,* Goran Petrovski1,9,*
1Department of Ophthalmology, Institute for Clinical Medicine, Faculty of Medicine, University of Oslo, Oslo, Norway; 2Ophthalmic Clinic “Jasne Blonia”, Lodz, Poland; 3Eye Hospital, University Medical Centre, Ljubljana, Slovenia; 4Department of Ophthalmology, Justus Liebig University, University Hospital Giessen and Marburg GmbH, Giessen, Germany; 5Karl Landsteiner Institute for Retinal Research and Imaging, Vienna, Austria; 6Center of Clinical Ophthalmology, Kyiv, Ukraine; 7Department of Ophthalmology, University of Split School of Medicine, Split, Croatia; 8Department of Ophthalmology, University Hospital Centre, Split, Croatia; 9Center for Eye Research, Department of Ophthalmology, Oslo University Hospital, Oslo, Norway
*These authors contributed equally to this work
Correspondence: Goran Petrovski, Center for Eye Research, Department of Ophthalmology, Oslo University Hospital, Kirkeveien 166, Oslo, 0450, Norway, Tel +47 2301 5163, Email [email protected]
Purpose: To analyze the functional and anatomical parameters of lamellar macular hole (LMH) surgery with internal limiting membrane peeling and determine which surgical technique provides the best visual outcome.
Methods: This is a retrospective multicenter cross-sectional study on patients who underwent pars plana vitrectomy (PPV) for LMH with or without combined phaco-vitrectomy, as well as gas-, air- or BSS-tamponade. Pre- and postoperative examination included best corrected visual acuity (BCVA) measurements for functional comparison and optical coherence tomography (OCT) scans to determine the contributing anatomical parameters.
Results: A total of 66 consecutive patients were included (age: 71.79 ± 8.52 years), of which 47 (71.2%) were diagnosed as tractional type LMH, and 19 patients (28.8%) as degenerative type. An epiretinal membrane (ERM) was present in 63 of the patients (95.5%), LMH-associated epiretinal proliferation (LHEP) was present in 19 patients (28.8%), and 16 patients (24.2%) had concomitant ERM and LHEP. In the group of tractional LMH, the mean central foveal thickness (CFT) was 81.1% thicker (P < 0.05) than in the degenerative group. Thirty-one patients (47.0%) underwent a combined phaco-vitrectomy procedure, while the rest underwent 23G, 25G or 27G PPV. Seventeen of the 66 patients received gas-tamponade (25.7%)-either SF6 or C3F8, 26 received air-tamponade (39.4%), while the remaining 23 patients received balanced salt solution (BSS)-tamponade (34.9%) during vitrectomy. The total BCVA showed significant improvement postoperatively (p < 0.001) and accordingly in the following groups: tractional LMH type (p < 0.001), degenerative type (p < 0.001), simple PPV (p < 0.001), phaco-vitrectomy (p < 0.001), BSS injection (p < 0.01), gas-tamponade (p < 0.05). None of the patients included in the study developed a full thickness macular hole postoperatively.
Conclusion: PPV provided a high success rate and functional improvement for treating LMH for both tractional and degenerative types, as well as combined phaco-vitrectomy treatment when cataract was present.
Keywords: lamellar macular hole, surgical outcomes, tractional, degenerative, BCVA, OCT
Introduction
Any defect in the macula or the central fovea can lead to decreased visual acuity and/or metamorphopsia, which may reduce vision, as well as quality and capacity of life. Lamellar macular hole (LMH) is one such defect that can impair the visual acuity dependent upon the extent of the defect and the involvement of different retinal layers.1–5
The pathogenesis of LMH is not fully understood,6,7 but degenerative changes, vitreomacular traction, posterior vitreous detachment (PVD), epiretinal membranes (ERM), LMH-associated epiretinal proliferation (LHEP) and internal limiting membranes (ILM) appear to be involved in the majority of the patients.2,3,8–12 One hypothesis is that it could occur after PVD as an abortive process of a full thickness macular hole (FTMH) formation.13,14
LMH as a clinical entity was first described by Gass JD in 1975.15 Optical Coherence Tomography (OCT) has become the gold standard for detecting anatomical changes that affect and provoke LMH development.16–18
LMH has been defined by Witkin et al,19 Hubschman et al20 and The International Vitreomacular Traction Study group13 as a partial defect in the inner layers of the fovea, with the presence of irregular foveal contour, intraretinal splitting between the inner and outer retinal layers, loss of foveal tissue, but with intact photoreceptors and absence of a FTMH. Persistence of the outermost neuroretinal layer ensures a partially preserved visual acuity, often only with a mild metamorphopsia, compared to the less favorable vision that is usually the case in patients with FTMH.5
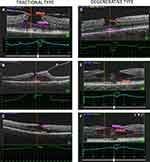
A more recent retrospective study by Govetto et al resulted in the classification of LMH into tractional and degenerative types.21 The tractional type is characterized by an associated ERM and/or vitreomacular traction (VMT), which cause a mechanical intraretinal separation, often between the outer plexiform- and the outer nuclear-layers (OPL and ONL).21 This leads to a schisis of the retinal layers – multiple narrow hyperreflective tissue bridges crossing wider hyporeflective spaces. Tractional LMH has a “moustache”-like morphology, with sharp intraretinal edges. The Ellipsoid Zone (EZ) is often intact.
The degenerative LMH is characterized by a “top hat” shape, with its appearance of a round-edged cavitation that could affect all the retinal layers.21 An EZ disruption is more common in the degenerative type,7,12,21 due to the central foveal bump. In general, the ratio between the inner and outer horizontal diameters of the hole is greater than 1:2. The degenerative type shows, similar to the tractional type, an epiretinal thickening, but in the degenerative type, this homogeneous intermediate reflective material seems to have less tractional property than the conventional ERM.20 This epiretinal material was named LHEP by Pang et al9,22 They theorized that LHEP is a result of migrating Müller glia cells, which hypothetically promote the closure of the LMH. Govetto21 also agreed in the Müller theory, and that the recruitment may also be responsible for the disruption of the EZ. The EZ disruption causes alterations in the photoreceptor layer, with a subsequent impairment of visual acuity (Figure 1).
Currently, the majority of the patients diagnosed with LMH are considered not to be candidates for vitrectomy during their first evaluation, and rather a follow-up examination approximately in half-a-year is being practiced. If the visual acuity is deteriorating during this period, vitrectomy is recommended according to an individual assessment. Patients with LMH do very often have some degree of visual impairment, despite the fact that the condition has been considered stable in several follow-up studies.2,5,21,23 Regardless of this stability, however, other studies have significantly proved that pars plana vitrectomy (PPV) can improve the visual acuity in this patient group, which certainly should be validated further.1,3,6–8,21–23
The aim of our study was to determine to what extent PPV with ILM-peeling has a beneficial effect upon visual acuity and anatomical parameters in patients with LMH. This retrospective multicentre study compared the pre- and post-operative Best Corrected Visual Acuity (BCVA) in the context of anatomical OCT parameters, to consider whether surgical intervention should be indicated to improve the patients’ vision and the quality of life.
LMH is a condition that correlates with aging, and with the anticipated increase in the aging population worldwide, it is appropriate to establish protocols for disease management such as that of LMH. We hereby compare the different surgical approaches including type of tamponade and vitreoretinal surgical steps in order to optimize the procedure and lay the foundation for future surgical protocols.
Patients and Methods
Study Design
This retrospective multicenter cross-sectional study is based on clinical records of 66 patients with LMH treated in the Department of Ophthalmology, Oslo University Hospital (OUS, Norway), Department of Ophthalmology, University Medical Centre Ljubljana (Slovenia), Department of Ophthalmology, University of Split (Croatia), Ophthalmic Clinic Jasne Blonia (Lodz, Poland), Center of Clinical Ophthalmology (Kyiv, Ukraine) and the Department of Ophthalmology, Justus Liebig University Giessen, UKGM (Giessen, Germany) in the period between April, 2016 and April, 2021. LMH was defined according to the following OCT parameters: presence of irregular foveal contour, separation of the neuroretinal layers, presence of at least one sign of loss of foveal tissue, and absence of FTMH.13,19,20 All LMH patients underwent PPV with ILM peeling, with or without combined phacoemulsification and intraocular lens (IOL) implantation. During the surgeries, different trocar sets were used (23G/25G/27G), as well as various tamponades (gas-air mixture (SF6/C3F8), air or balanced salt solution (BSS)) which were selected at the discretion of the surgeons. FTMHs, pseudoholes, foveoschisis and retinal detachment were excluded from the study.
Ethics
The present study adhered to the tenets of the Declaration of Helsinki and was multicenter registry study approved by the institutional data protection officers, thus, compliant to the individual institutional agreements on data confidentiality at each site: OUH, Norway and Kyiv, Ukraine; the National Medical Ethics Committee, Slovenia; the Institutional Ethics Committee of Ophthalmic Clinic Jasne Błonia, Poland; the Ethics Committee at University of Giessen, Germany; the Ethics Committee at University of Split, Croatia. Patient consent to review registry data where applicable was not required.
Diagnostic Methods
A large array of preoperative and intraoperative variables was collected regarding the surgical techniques, as well as anatomical and functional outcomes. Anatomical parameters were evaluated both pre- and post-operatively with the use of the following OCT machines: Norway (Zeiss Plex Elite (SS OCTA), Nidek RS-3000 OCTA); Poland, Ukraine and Slovenia (Triton, Topcon (SS OCT)); Germany (Heidelberg Engineering HRA+OCT Spectralis). The following preoperative parameters were analyzed: tractional versus degenerative type of LMH, Central Foveal Thickness (CFT), Minimal Foveal Thickness (MFT), Base and Top size of the holes, EZ disruption, presence of ERM and LHEP. The CFT was automatically measured by the OCT software, while the MFT was measured manually as the thinnest vertical distance from the base of the LMH down to the Bruch’s membrane, with the use of a software-based caliper. The Base and Top sizes of the hole were defined as the horizontal diameter (µm), respectively, at the outer and inner edges of the hole, and were also measured manually. Postoperative OCT findings were as follows: anatomical restoration of the foveal contour (symmetrical vs asymmetrical), intraretinal cysts, and FTMH development.
The primary outcome measured was an improvement in BCVA after PPV for LMH. The secondary outcomes being analyzed were postoperative anatomical restitution of foveal contour evaluated by OCT, complication rates, and analysis of impact of tamponade type on anatomical and functional outcomes.
Surgical Procedure
PPV is in general a standardized procedure but has some variations due to both the international and the individual surgeons’ preferences. Differences of the importance regarding the surgical procedure are noted to enable statistical comparison.
PPV was performed under retrobulbar or sub-Tenon’s anaesthesia (Xylocaine 20 mg/mL or Lidocaine 20 mg/mL: 2.5 mL + Marcaine 5 mg/mL: 2.5 mL), combined with phacoemulsification, if cataract was present. In the latter cases, the procedure of implantation of an acrylic foldable IOL in the capsular bag was first performed. Then, a standard three-port PPV with either 23-, 25- or 27-Gauge was performed in each patient, where the sclerotomies were placed 3.5 to 4 mm posterior to the limbus. Central core vitrectomy was performed followed by PVD using vacuum with the vitrectomy probe. The vitrectomy was completed with careful inspection of the retinal periphery.
The macula was further stained using ILM blue dye (Tissue Blue, 0.025%) to facilitate its peeling. If an ERM or LHEP was present, it was removed in advance or together with the ILM-peeling. Eventually, a complete fluid–air exchange (BSS) was performed, with or without a gas-tamponade at the surgeon’s discretion. The gas-air mixtures were either sulphur hexafluoride (SF6) or perfluoropropane (C3H8), and the respective concentrations of the gases were noted for each patient. The gas is intended to push and hold the retina back against the underlying choroid, to ensure an approximation of the LMH and prevent postoperative complications, such as retinal detachment or FTMH. Alternatively, the eyes were filled with air or only BSS. The cannulas were then removed, and the conjunctiva was repositioned to cover the sites of the sclerotomies. The sclerotomies were primarily closed without scleral sutures (self-sealed), but if leakage was apparent, the sclerotomies and conjunctiva were sutured accordingly.
Following surgery, the patients with gas tamponades were introduced to maintain face down position for 3 days postoperatively, to optimize the pressure of the gas against the macula. Topical antibiotics, topical corticosteroids (3 times daily for 3 weeks) and cycloplegics (Cyclopentolate 1%, 2 times daily for 10 days) were prescribed. Patients were examined on the first postoperative day at the hospital and were then either summoned back or told to schedule a follow-up appointment at their referring ophthalmologist, 3–4 weeks postoperatively. By that time, the gases would have resorbed for the applicable patients. All patients had follow-up appointments, and they completed both visual acuity assessment and OCT imaging of the macula.
Statistical Analysis
The data analysis was performed using descriptive statistical analysis: percentage distribution, mean and standard deviation (SD). In case of non-normality of continuous variables, median and interquartile range (IQR) and maximum/minimum ranges were calculated. Normality of continuous variables was tested on histogram and by the Shapiro–Wilk and Kolmogorov–Smirnov test. When the normality assumption was satisfied, the independent sample t-test was used to compare means of continuous- and numerical variables. Otherwise, the Wilcoxon signed rank test was used to compare repeated measurements (pre- and postoperative measurements) between the two groups. Chi-square test (χ2) was used to test the differences in the distribution of categorical variables, while the column proportions were compared using a z-test. The significance level was set as p < 0.05, and in case of the χ2 test, it was adjusted with Bonferroni correction to p < 0.05/n (where n is the number of analyses). Statistical Package for STATA (Stata version 14.0; College Station, TX, USA) and SPSS (SPSS version 24, IBM, Armonk, NY, USA) were used for the statistical analyses.
The Best Corrected Visual Acuity (BCVA) measured with the Snellen chart was converted into a logarithm of the minimal angle of resolution (logMAR) for statistical analysis purposes. The different gas tamponades, SF6 and C3H8, were collected into a common group in the statistical analysis, as each of them had a small number of patients and different injected concentrations within. Since the use of the gas tamponades has the same intention, to push and hold the retina back against the underlying choroid, it was considered appropriate to merge these patients’ groups for a more representative comparison. Furthermore, to study the relationship between the tamponades and the potential risk factors (baseline BCVA, hole type, EZ status, hole size and lens status), a simple multinomial (polytomous) logistic regression was fitted for each risk/confounding factor. Consequently, an adjusted multinomial (polytomous) logistic regression model was fitted with all of the risk/confounding factors. The crude and adjusted coefficients in terms of multinomial log-odds (logits) and their 95% confidence intervals (CIs) from these models were then reported. The multinomial logistic regression model was based on the data of 66 people who did not have missing values in any variable.
Results
Characteristics of the Studied Population
This retrospective study included 66 consecutive patients having LMH that had undergone PPV: 23 males (34.9%) and 43 females (65.1%). Their mean age at surgery was 71.8 ± 8.5 years (range: 59–87 years). Ten patients had diabetes mellitus, 8 patients had glaucoma, and 1 patient had both conditions.
Forty-seven (71.2%) of the total 66 eyes were diagnosed as tractional LMH, while the remaining 19 (28.8%) as degenerative type, without any significant differences in gender and age between the two LMH types. Thirty-one patients (47%) underwent a combined phaco-vitrectomy: 4 patients from the degenerative type, and 27 patients from the tractional type. Fifteen patients had undergone phacoemulsification for their cataract in advance of the PPV, while 15 others needed cataract surgery after the PPV procedure.
Seventeen of the 66 patients received gas-tamponade (25.7%), either SF6 or C3H8; 26 received air-tamponade (39.4%), while the remaining 23 patients (34.9%) received BSS-tamponade. The average time for the postoperative control was 14.1 months (range: 0.75–61). The characteristics of the studied group are summarized in Table 1.
 |
Table 1 Characteristics of the Studied Population |
Anatomical Pre- and Post-Operative Characteristics
ERM was present in 63 of the 66 patients (95.5%). The presence of an ERM showed significant statistical difference between the tractional (47 patients (100%)) and degenerative type (16 patients (84.2%)) of LMH (P < 0.05). LHEP was present in 19 patients (28.8%), and 16 patients (24.2%) had concomitant ERM and LHEP. An EZ disruption was present in 8 (17%) of the patients at the preoperative OCT scans in the tractional group, and 2 (10.5%) in the degenerative group.
In the tractional LMH group, the mean CFT was 81.1% thicker (P < 0.05) than the degenerative type of LMH (379.9 ± 117.7 µm, range 100–595 µm; vs 209.8 ± 60.4 µm, range 94–351 µm, respectively).
The other OCT parameters: MFT, Base size and Top size of the hole, showed no significant difference between the tractional and degenerative LMH groups. The values of these preoperative OCT parameters, as well as the CFT, EZ and ERM, are presented in Table 2.
 |
Table 2 Preoperative OCT Characteristics of the Studied Population |
In the degenerative group, the mean Top size of the hole was 472 µm (range 339–611 µm), and the mean Base size of the hole was 703 µm (range 530–910 µm). This was closer to the 1:2 ratio that is generally the case for the degenerative type of LMH, compared to the tractional type that had a mean Top size of 374 µm (range 283–571 µm) and a mean Base size of 483 µm (range 230–1019 µm). The ratios are thus 0.67 and 0.77, respectively, for the degenerative and tractional group.
Anatomical postoperative analysis was available for 54 patients. OCT showed a symmetrical foveal profile for 29 patients (54.5%), while asymmetrical for the remaining 25 patients. The postoperative foveal profile showed no significant difference between the tractional and degenerative LMH types. Intraretinal cysts appeared in 14 patients (25.5%) postoperatively, of whom 13 belonged to the tractional LMH group. None of our patients developed FTMH postoperatively. The postoperative OCT outcomes are presented in Table 3.
 |
Table 3 Postoperative Anatomical OCT Parameters |
The odds (relative log odds) of receiving a BSS- vs air-tamponade was significantly lower (Coeff: −3.11; CI 95%: −4.79—1.43) among those having degenerative LMH-type compared to those having tractional type, and this association did not change even after adjustment for the risk/confounding factors (baseline BCVA, hole type, EZ status, hole size and lens status). The risk/confounding factors showed no association with either the outcome in the crude- or the adjusted analysis (Coeff: −2.81; CI 95%: −4.62—1.00).
Functional Outcomes in the Studied Population
The mean preoperative BCVA in total was median: 0.3, IQR: 0.2–0.5, range: 0–1.7 on the logMAR scale. The mean preoperative BCVA in the tractional group was median: 0.3; IQR: 0.2–0.5; range: 0–1.7, while in the degenerative group it was median: 0.5; IQR: 0.3–0.6; range: 0.1–1.2 (Figure 2).
The total BCVA showed significant improvement postoperatively (p < 0.001). In the tractional group, the BCVA improved postoperatively to a median: 0.2; IQR: 0.1–0.3; range: 0–1, while in the degenerative group, it improved to a median: 0.2; IQR: 0.15–0.5; range: 0–0.6 (Figure 2).
The BCVA improved significantly in the postoperative period in the following groups: entire cohort, tractional type, degenerative type, BSS, gas-tamponade, simple PPV, and phaco-vitrectomy. The BCVA also improved in the subgroup with air tamponade, but not significantly. None of the groups showed any decrease in BCVA postoperatively. The functional values, BCVA (logMAR), are presented in Table 4 for each group.
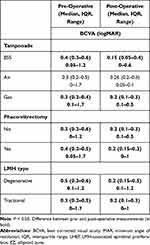 |
Table 4 Relationship Between Pre- and Postoperative BCVA (logMAR) in the Studied Groups |
The preoperative BCVA in the group with CFT <300µm was median: 0.4, IQR: 0.3–0.6, range: 0.1–1.2 logMAR, while the postoperative BCVA significantly improved to median: 0.2, IQR: 0.15–0.4, range: 0–0.6 logMAR (p = 0.008).
The preoperative BCVA in the group with CFT >300µm was median: 0.3, IQR: 0.2–0.5, range: 0–1.7 logMAR, and the postoperative BCVA significantly improved to median: 0.2, IQR: 0.1–0.3, range: 0–1 logMAR (p = 0.003). The functional improvement was significant in both subgroups. Additionally, the outcome of this study suggests that the visual improvement is best in patients with a better preserved preoperative CFT.
Discussion
Surgery in the treatment of LMH results in significant improvement of visual acuity. Combined phaco-vitrectomy should be performed if cataract is present. Tamponade might be avoided, as best results have been obtained in patients who had only BSS tamponade at the end of surgery.
To date, there has been no international consensus on the type of tamponade used during vitrectomy for LMH, nor about the optimal duration of postoperative prone positioning. Sun et al24 used 15% C3F8 and encouraged 3–5 days of such positioning, which provided a greater chance for restoration of the foveal configuration, compared to non-gas tamponade. Our study shows that BCVA significantly improves both with gas tamponade (p < 0.05) and BSS (p < 0.01). For patients with air tamponade, the BCVA also improved, but not significantly.
Since the visual improvement was best in the BSS group, as well as gases can increase the probability of postoperative cataract formation; furthermore, according to the fact that none of our patients developed FTMH postoperatively, one can suggest BSS to be a better initial tamponade in LMH surgery. However, if FTMH as a secondary complication does appear, then a gas tamponade could be the tamponade of choice. According to the pathological hypothesis of LMH as an abortive process of FTMH, such a complication can occur.13,14 It should be considered whether vitrectomy at an earlier stage can prevent FTMH development.
In our study, all patients diagnosed with tractional LMH had an ERM present as well. Only 3 patients had no presence of ERM and they belonged to the degenerative LMH group. Moreover, 19 patients had LHEP present, of whom 14 belonged to the tractional LMH group, which constitutes 21.2% in this subgroup, while the remaining 5 belonged to the degenerative ones (26.3%). The latter is considered to have a higher prevalence of LHEP instead of ERM, which commonly causes more permanent anatomical and functional changes. While the tractional LMH tensioned by an ERM tends to improve anatomically and functionally once the traction has been removed surgically. In our study, both the tractional and degenerative types showed a significant functional improvement postoperatively (p < 0.001), which are in favour of surgical intervention in both groups.
A tenth of our patients with degenerative LMH had an EZ disruption. The EZ is external to the ONL, and such ONL alterations are often present and difficult to differentiate from the EZ disruption, with the possibility of both conditions being actually present as well.
Thirty-one (47.0%) of our 66 patients had combined phaco-vitrectomy, which suggests that many had concomitant nuclear sclerosis of the lens, thus it is difficult to determine how much the age-related lens opacification contributes to their impaired visual acuity. However, it is in the patients’ advantage to perform combined surgery in order to achieve the full potential for visual improvement. The combined surgery patients showed a tendency for better improvement in the postoperative period than the PPV only group; however, this difference was not significant.
If one could affirm that the cataract impairs the visual acuity the most, it would indirectly imply that those patients would have a better preoperative BCVA in regard to LMH alone. However, if a better preoperative BCVA provides a better improvement postoperatively, these patients would achieve a greater improvement when combined phaco-vitrectomy is performed. This may imply it could be advantageous to intervene at an early stage, and not observe the patients half a year ahead in anticipation of a visual deterioration due to an eminent cataract. Phaco-vitrectomy is cost-saving for the patients, and cost-beneficial for the healthcare system.26 Nevertheless, future prospective studies should likely perform phacoemulsification initially, and then assess the necessity of PPV with ILM-peeling for an additional visual improvement. In fact, 15 of our patients did have phacoemulsification for their cataract in advance to PPV, which probably was not sufficient for a visual improvement itself. Additionally, 15 patients required phacoemulsification during the observational period after PPV. Assessing whether this is due to insufficient PPV only, or if the respective patients had a degree of cataract preoperatively; furthermore, if the patients developed cataract in the subsequent period, and if so, if these incidents belonged to the gas tamponade group – it is speculative in retrospect, but would certainly be of interest to know.
Furthermore, as many as 60 of 66 patients (90.9%) were pseudophakic at the end of the study. It is also conceivable that this number would be even higher with a longer follow-up time of the patients. However, these findings would be the rationale for performing a combined phaco-vitrectomy even at the early stages of cataract, to prevent a later intervention, as the fragility of the elderly patients increases in time.
Chois et al27 studied a sample of 34 patients, where 32 of them were pseudophakic at the final postoperative control; thus, similar to our study, it is difficult to determine to what extent the obscuration of the lens is responsible for the visual impairment. Coassin et al28 studied 106 symptomatic LMH patients that either underwent a simple PPV or phaco-vitrectomy in which the postoperative BCVA improved significantly (p < 0.001). When they excluded the phaco-vitrectomized patients from the analysis, the postoperative BCVA still improved significantly (p = 0.0036). In our study, both the PPV only and the phaco-vitrectomized patients had a significantly improved postoperative BCVA.
Theodossiadis et al5 conducted a long-term follow-up study of 41 LMH patients, with a mean follow-up period of 31.1 months. VA remained stable in 30 of the patients (75%), while the mean CFT decreased during the same period (p < 0.001), which correlated with the patients that experienced a deteriorated VA (p = 0.002). Although the majority maintained a stable VA, as many as 39 patients complained of metamorphopsia at the final examination. That was 8 times more than at the first examination. Based on this study, the VA can be relatively stable, so to claim that vitrectomy is not indicated for LMH, it might inflict upon the remaining 25% who had unstable VA a restricted quality of life.
ERMs were identified in 63 of our patients at baseline (95%). This is in agreement with previous reports, which reported it in 100% of the patients.3,8,11,12,25 Theodossiadis et al5 found that ERM participated in the enlargement of the LMH and that deteriorating VA for these patients should be an indication for vitrectomy.
A correlation between a low MFT and poor preoperative VA has been detected in a study by Holland1 on 89 eyes. The observation of patients with relatively well-preserved VA and MFT will thus be followed and observed according to current procedures. On the contrary, Holland et al1 also found a significant correlation between the level of pre- and post-operative VA: the better preoperative VA, the better the postoperative VA gets. In such cases, it will be beneficial to perform PPV on the majority of these patients, to ensure the VA improves as much as possible.
It has been reported that LHEPs, described as yellowish pigmented and soft material over the retina, turn the ERM/ILM-peeling into a more difficult task to perform surgically than “conventional ERM”. The robustness of the LHEP certainly explains its ability to induce permanent changes, which result in poorer preoperative BCVA and lack of improvement postoperatively.27 LHEP is commonly present in the degenerative type of LMH, and confirms that this is a more permanent damaging condition than the tractional type. The result by Coassin et al28 concluded that BCVA significantly improved postoperatively in the tractional group (p < 0.0001) but not in the degenerative group (p = 0.27).
This is in agreement with the recently published meta-analysis by Xu et al,29 which included 8 studies that have been investigating whether LHEP can be used to predict the VA outcome postoperatively. The meta-analysis confirmed that patients without LHEP had a better postoperative VA than patients with LHEP. In our study, a subgroup of pre- and post-operative BCVA comparison between ERM and LHEP would have provided limited utility due to the low number of cases. However, both the degenerative and tractional LMH types benefited from the ILM/ERM-peeling, with a significant improvement of the BCVA in both groups (p < 0.001).
In our study, anatomical postoperative analysis was available for 54 patients. OCTs showed symmetrical foveal profile for 29 of them (54.5%), while asymmetrical for the remaining 25 patients. The postoperative foveal profile did not show significant difference between the tractional and degenerative LMH types. Considering that 25 patients had asymmetrical foveal profile postoperatively, this anatomical outcome is either less important or too small to affect the functional one, since BCVA significantly improved in both the tractional and degenerative types (p < 0.001). This is in agreement with both Sun et al24 and Michalewska et al,25 who found that improvement in the foveal configuration was not essential for the improvement of the visual acuity, but rather depended on the release of the tractional ERM and on the continuity of the EZ.
Intraretinal cysts appeared in 14 patients (25.5%) postoperatively, of whom 13 belonged to the tractional LMH group. It is difficult to conclude whether the appearance of postoperative intraretinal cysts is an indicator of the release of the traction, since 13 of the 14 patients with postoperative intraretinal cysts belonged to the tractional LMH type; furthermore, it cannot be excluded that the cysts are due to an inflammatory postoperative process.
FTMH, as already mentioned, is a feared but not unpreventable complication of LMH, both in the natural pathophysiological course and as a postoperative complication. A recent study published in 2021 by Chehaibou et al30 performed a centripetally oriented ILM-peeling, where they left some proliferative material at the edges of the hole, in order to not impair its connection with the underlying retinal layers. The peri-hole peeling technique was used to reduce the risk of postoperative FTMH, and none of their limited number of 11 patients developed this complication. Furthermore, Takahashi et al31 reported favorable outcomes of embedding LHEP into the retinal cleavage during surgical treatment of degenerative LMH, a technique intended to promote LMH closure, and thereby avoid postoperative FTMH development. New and possible more preferred techniques, which may result in better postoperative outcomes and simultaneously minimize the risk of complications, are important to determine for future treatment purposes. In addition to peri-hole peeling and embedding the LHEP into the retinal cleavage, this especially applies to the double inverted flap technique studied by Frisina et al,32 the foveal sparing ILM-peeling by Morescalchi et al,33 and the implantation of the highly concentrated autologous Platelet-Rich Plasma used by Hagenau et al.34
Our study is in agreement with that of Choi et al27 in their conclusion that different combinations of parameters may explain the wide variability of VA that has been reported after vitrectomy for LMH. Consequently, one cannot apply the same yardstick to all the LMH patients, and the intervention should be individualized in the same direction as any other precision medicine procedures. OCT parameters may predict whether a surgical intervention can improve the VA. However, this should not prevent an individual with poor potential for improvement, based on the predicting parameters, to be offered vitrectomy when one’s condition is progressing. We are in agreement with Choi et al27 in their recommendation of vitrectomy for patients with progressive, disabling visual loss and an increase in EZ disruption. Additionally, Takahashi et al31 emphasize that external limiting membrane integrity is an anatomical parameter important for the functional recovery, which should be included in the pre- and post-operative examination.
To date, vitrectomy for FTMH has given extensive and successful evidence, as well as established surgical approach worldwide. To the contrary, the surgical procedure for LMH has been disputed during the last decade, with studies claiming LMH to be in a stable condition,2 while other studies disagreeing, and additionally showing that vitrectomy has a beneficial effect.6,7,28
Our study has some limitations: it is a retrospective multicentre study, which made it difficult to ascertain the exact time for postoperative examinations was performed for each patient. The length of the postoperative follow-up is an important factor, as well as the time until a possible improvement, which can be used to inform the patients at what time in the postoperative period they might expect a noticeable effect. Another limitation of our study is that the patients at the Oslo University Hospital could not have their postoperative examinations at the hospital, but instead at their referring ophthalmologist – the latter had no opportunity to send the postoperative OCT-scans for each case, but a journal description. Therefore, the pre- and post-operative OCT parameters could not be compared, and rather used the data about the presence or absence of postoperative FTMH as a complication. A comparison of the pre- and postoperative anatomical parameters is useful in obtaining information about the CFT, MFT, Base size, Top size, EZ-continuity, PVD, and Macular Edema. An anatomical improvement may indeed prevent a deteriorating visual acuity, that otherwise can occur in the absence of a surgical intervention, although the functional outcome would not significantly improve postoperatively.
Furthermore, the study did not exclude patients with other eye diseases, since the retrospective nature made it difficult to collect a sufficient cohort of patients without current or previous eye diseases. Different clinical entities can mutually influence each other, and ageing is a factor of risk for the majority. PVD is an example of an eye condition that can contribute to pathological development of LMH. Myopia is a risk factor for PVD, and possibly indirectly for LMH as well. Dividing the patients into myopic and hypermetropic subgroups could have also determined whether myopia increases the probability of LMH development.
The strength of our study is the multicentre sample size, which includes patients from 5 different countries.
Several, similar studies have been previously reported; however, in regard to a future protocol for treatment of LMH, it is important to determine which surgical technique provides the best functional and anatomical outcomes for the patients.
Conclusions
Visual and anatomical outcomes of PPV for LMH have until now shown inconsistent results, where some have proven a beneficial improvement, while others found no statistical significance postoperatively. This discrepancy could be explained by the use of different surgical approaches. This study, which includes the use of different endotamponades during PPV, has taken this into account so that the pre- and postoperative outcomes could be used for a representative comparison.
The study results conclude that the procedure, which appears to best improve the functional outcomes in LMH surgery uses BSS tamponade, and preferably with combined phaco-vitrectomy, if cataract is present. The surgical intervention improves both the BCVA in the tractional (p < 0.001) and the degenerative group (p < 0.001). The use of BSS may also be favored, since it does not dispose of any postoperative cataract development, which is the case with the use of air/gas tamponades. Additionally, none of our patients experienced LMH reopening or FTMH development, which are complications one previously thought could have been prevented by gas-tamponade.
Author Information
These authors are shared first (Hanna Haave and Beáta Éva Petrovski) and last (Zofia Anna Nawrocka and Goran Petrovski) authors.
Disclosure
The authors report no conflicts of interest in relation to this work.
References
1. Holland L, Chen JC, Lee LR. Anatomical and functional outcomes of pars plana vitrectomy for lamellar macular defects. Asia Pac J Ophthalmol. 2015;4:134–139. doi:10.1097/APO.0000000000000065
2. Purtskhvanidze K, Balken L, Hamann T, et al. Long-term follow-up of lamellar macular holes and pseudoholes over at least 5 years. Graefes Arch Clin Exp Ophthalmol. 2018;256:1067–1078. doi:10.1007/s00417-018-3972-2
3. Lee CS, Koh HJ, Lim HT, et al. Prognostic factors in vitrectomy for lamellar macular hole assessed by spectral-domain optical coherence tomography. Acta Ophthalmol. 2012;90(8):e597–e602. doi:10.1111/j.1755-3768.2012.02456.x
4. Yeo JH, Oh R, Lee JY, et al. Optical coherence tomography angiographic findings of lamellar macular hole: comparisons between tractional and degenerative subtypes. Sci Rep. 2020;10:13331.
5. Theodossiadis PG, Grigoropoulos VG, Emfietzoglou I, et al. Evolution of lamellar macular hole studied by optical coherence tomography. Graefes Arch Clin Exp Ophthalmol. 2009;247:13–20. doi:10.1007/s00417-008-0927-z
6. Sanisoglu H, Elbay A, Sevim S, et al. Surgical therapy versus observation for lamellar macular hole: a retrospective comparison study. Clin Ophthalmol. 2013;7:1843–1848.
7. Figueroa MS, Govetto A, Steel DH, et al. Pars plana vitrectomy for the treatment of tractional and degenerative lamellar macular holes: functional and anatomical results. Retina. 2019;39(11):2090–2098. doi:10.1097/IAE.0000000000002326
8. Figueroa MS, Noval S, Contreras I. Macular structure on optical coherence tomography after lamellar macular hole surgery and its correlation with visual outcome. Can J Ophthalmol. 2011;46:491–497. doi:10.1016/j.jcjo.2011.09.011
9. Pang CE, Spaide RF, Freund KB. Comparing functional and morphologic characteristics of lamellar macular holes with and without lamellar hole-associated epiretinal proliferation. Retina. 2015;35:720–726. doi:10.1097/IAE.0000000000000390
10. Casparis H, Bovey EH. Surgical treatment of lamellar macular hole associated with epimacular membrane. Retina. 2011;31:1783–1790. doi:10.1097/IAE.0b013e31820a6818
11. Garretson BR, Pollack JS, Ruby AJ, et al. Vitrectomy for a symptomatic lamellar macular hole. Ophthalmology. 2008;115(5):884–886.e881. doi:10.1016/j.ophtha.2007.06.029
12. Michalewska Z, Michalewski J, Odrobina D, Nawrocki J. Non-full-thickness macular holes reassessed with spectral domain optical coherence tomography. Retina. 2012;32:922–929. doi:10.1097/IAE.0b013e318227a9ef
13. Duker JS, Kaiser PK, Binder S, et al. The International Vitreomacular Traction Study Group classification of vitreomacular adhesion, traction, and macular hole. Ophthalmology. 2013;120:2611–2619. doi:10.1016/j.ophtha.2013.07.042
14. Nava U, Cereda MG, Bottoni F, et al. Long-term follow-up of fellow eye in patients with lamellar macular hole. Graefes Arch Clin Exp Ophthalmol. 2017;255:1485–1492. doi:10.1007/s00417-017-3652-7
15. Gass JD. Lamellar macular hole: a complication of cystoid macular edema after cataract extraction: a clinicopathologic case report. Trans Am Ophthalmol Soc. 1975;73:231–250.
16. Haouchine B, Massin P, Tadayoni R, et al. Diagnosis of macular pseudoholes and lamellar macular holes by optical coherence tomography. Am J Ophthalmol. 2004;138(5):732–739. doi:10.1016/j.ajo.2004.06.088
17. Hee MR, Puliafito CA, Wong C, et al. Optical coherence tomography of macular holes. Ophthalmology. 1995;102:748–756. doi:10.1016/S0161-6420(95)30959-1
18. Puliafito CA, Hee MR, Lin CP, et al. Imaging of macular diseases with optical coherence tomography. Ophthalmology. 1995;102:217–229. doi:10.1016/S0161-6420(95)31032-9
19. Witkin AJ, Ko TH, Fujimoto JG, et al. Redefining lamellar holes and the vitreomacular interface: an ultrahigh-resolution optical coherence tomography study. Ophthalmology. 2006;113:388–397. doi:10.1016/j.ophtha.2005.10.047
20. Hubschman JP, Govetto A, Spaide RF, et al. Optical coherence tomography-based consensus definition for lamellar macular hole. Br J Ophthalmol. 2020;104:1741–1747. doi:10.1136/bjophthalmol-2019-315432
21. Govetto A, Dacquay Y, Farajzadeh M, et al. Lamellar macular hole: two distinct clinical entities? Am J Ophthalmol. 2016;164:99–109. doi:10.1016/j.ajo.2016.02.008
22. Pang CE, Spaide RF, Freund KB. Spaide RF and Freund KB. Epiretinal proliferation seen in association with lamellar macular holes: a distinct clinical entity. Retina. 2014;34(8):1513–1523. doi:10.1097/IAE.0000000000000163
23. Bottoni F, Deiro AP, Giani A, et al. The natural history of lamellar macular holes: a spectral domain optical coherence tomography study. Graefes Arch Clin Exp Ophthalmol. 2013;251(2):467–475. doi:10.1007/s00417-012-2044-2
24. Sun JP, Chen SN, Chuang CC, et al. Surgical treatment of lamellar macular hole secondary to epiretinal membrane. Graefes Arch Clin Exp Ophthalmol. 2013;251:2681–2688. doi:10.1007/s00417-013-2364-x
25. Michalewska Z, Michalewski J, Odrobina D, et al. Surgical treatment of lamellar macular holes. Graefes Arch Clin Exp Ophthalmol. 2010;248:1395–1400. doi:10.1007/s00417-010-1400-3
26. Hertzberg SNW, Veiby N, Bragadottir R, et al. Cost-effectiveness of the triple procedure - phacovitrectomy with posterior capsulotomy compared to phacovitrectomy and sequential procedures. Acta Ophthalmol. 2020;98:592–602. doi:10.1111/aos.14367
27. Choi WS, Merlau DJ, Chang S. Vitrectomy for macular disorders associated with lamellar macular hole epiretinal proliferation. Retina. 2018;38:664–669. doi:10.1097/IAE.0000000000001591
28. Coassin M, Mastrofilippo V, Stewart JM, et al. Lamellar macular holes: surgical outcome of 106 patients with long-term follow-up. Graefes Arch Clin Exp Ophthalmol. 2018;256:1265–1273. doi:10.1007/s00417-018-3989-6
29. Xu H, Qin L, Zhang Y, et al. Surgery outcomes of lamellar macular eyes with or without lamellar hole-associated epiretinal proliferation: a meta-analysis. BMC Ophthalmol. 2020;20:345. doi:10.1186/s12886-020-01617-4
30. Chehaibou I, Philippakis E, Mané V, et al. Surgical outcomes in patients with lamellar macular holes selected based on the optical coherence tomography consensus definition. Int J Retina Vitreous. 2021;7(1):31. doi:10.1186/s40942-021-00297-6
31. Takahashi K, Morizane Y, Kimura S, et al. Results of lamellar macular hole-associated epiretinal proliferation embedding technique for the treatment of degenerative lamellar macular hole. Graefes Arch Clin Exp Ophthalmol. 2019;257:2147–2154. doi:10.1007/s00417-019-04425-9
32. Frisina R, Parrozzani R, Pilotto E, Midena E, Double Inverted A. Flap surgical technique for the treatment of idiopathic lamellar macular hole associated with atypical epiretinal membrane. Ophthalmologica. 2019;242:49–58. doi:10.1159/000496297
33. Morescalchi F, Russo A, Gambicorti E, et al. Peeling of the internal limiting membrane with foveal sparing for treatment of degenerative lamellar macular hole. Retina. 2020;40:1087–1093. doi:10.1097/IAE.0000000000002559
34. Hagenau F, Nobl M, Vogt D, et al. Highly concentrated autologous platelet-rich plasma restores foveal anatomy in lamellar macular hole surgery. Klin Monbl Augenheilkd. 2021;238:885–892. doi:10.1055/a-1409-9268
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The
full terms of this license are available at https://www.dovepress.com/terms.php
and incorporate the Creative Commons Attribution
- Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted
without any further permission from Dove Medical Press Limited, provided the work is properly
attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.