Back to Journals » Lung Cancer: Targets and Therapy » Volume 10
Outcomes Following Stereotactic Body Radiotherapy with Intensity-Modulated Therapy versus Three-Dimensional Conformal Radiotherapy in Early Stage Non-Small Cell Lung Cancer
Authors Mix M, Tanny S , Nsouli T, Alden R , Chaudhari R , Kincaid R, Rosenbaum PF, Bogart JA , Aridgides P
Received 23 October 2019
Accepted for publication 29 November 2019
Published 20 December 2019 Volume 2019:10 Pages 151—159
DOI https://doi.org/10.2147/LCTT.S235713
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Sai-Hong Ignatius Ou
Michael Mix,1 Sean Tanny,1 Tamara Nsouli,1 Ryan Alden,1 Rishabh Chaudhari,2 Russell Kincaid,1 Paula F Rosenbaum,3 Jeffrey A Bogart,1 Paul Aridgides1
1Department of Radiation Oncology, SUNY Upstate Medical University, Syracuse, NY 13210, USA; 2Department of Radiation Oncology, University of Cincinnati/University Hospital Barrett Cancer Center, Cincinatti, OH 45267, USA; 3Department of Public Health and Preventive Medicine, SUNY Upstate Medical University, Syracuse, NY, 13210, USA
Correspondence: Michael Mix
Department of Radiation Oncology, SUNY Upstate Medical University, 750 E Adams St, Syracuse, NY 13210, USA
Tel +1 315 464-5276
Email [email protected]
Introduction: The treatment techniques used for stereotactic body radiation therapy (SBRT) for early-stage lung cancer continue to evolve. In this study, clinical outcomes following SBRT were evaluated according to the use of either 3D conformal radiotherapy (3DCRT) or intensity-modulated radiation therapy (IMRT).
Patients and methods: Patients with stage I NSCLC who received SBRT from 2007 to 2015 were retrospectively reviewed. Disease control and survival were assessed using Kaplan-Meier estimates. Dosimetric analyses for target dose heterogeneity and coverage were performed.
Results: A total of 297 patients with 351 lesions were included. 3DCRT was used in 52% and IMRT in 48%. IMRT was utilized at a higher rate in more recent years. The most common regimens were 48 Gy in 4 fractions and 54–60 Gy in 3 fractions. With a median follow up of 22.7 months, there were 17 local failures for a crude relapse rate of 5.7%. Local failure did not differ in patients treated with 3DCRT and IMRT (4.9% vs 6.5%, p=0.573). Mean dose to gross tumor volume (GTV) as a percent of prescription dose was higher with 3DCRT compared with IMRT (107.7% vs 103.6%, p < 0.0001). Tumor stage, histology, and SBRT regimen did not correlate with local tumor control. Overall survival for the entire population approximated 72% at 2 years. Treatment was well tolerated with 6 documented grade 3+ events.
Conclusion: In this single-institution cohort of SBRT for early-stage NSCLC, there was no discernible difference in clinical outcomes between those treated with 3DCRT and IMRT.
Keywords: SBRT, IMRT, stereotactic ablative radiation therapy, SABR, radiotherapy
Introduction
Over the past decade, stereotactic body radiotherapy (SBRT) has revolutionized the treatment of early-stage, node-negative non-small cell lung cancers (NSCLC).1 While lobectomy with lymph node sampling is the standard of care for patients medically fit for surgery,2 many patients have comorbidities such as cardiopulmonary disease that render them medically inoperable.3 For these patients, as well as patients with limited metastatic disease to the lung, SBRT represents a well-tolerated and efficacious treatment modality with rates of local control ranging from 85% to 95%.4–9
SBRT generally refers to external beam treatments using rigid immobilization, five or fewer fractions, large dose per fraction, highly conformal treatment, and image guidance. At its inception, SBRT for lung tumors was a complex endeavor combining physics and physician oversight to ensure proper immobilization and accurate target localization to ensure safe delivery of ultra-high doses of radiation to small targets. Treatment consisted of multiple conformal fields that were non-coplanar requiring couch adjustments.10 The time required for set-up, image-guided patient positioning verification, and delivery of 8–12 fields was appreciable. As clinical experience with SBRT-developed, intensity-modulated radiation therapy (IMRT) has become more common in SBRT planning.11,12 The use of IMRT likewise improved SBRT efficiency by decreasing treatment time.13 However, despite the widespread adoption of IMRT, rigorous comparison to 3D conformal radiation therapy 3DCRT has not occurred. The current investigation sought to evaluate outcomes according to SBRT treatments with either 3DCRT or IMRT in a single institution cohort of patients with early-stage NSCLC.
Materials and Methods
The records of all patients with Stage I NSCLC treated with SBRT in SUNY Upstate Medical University between 2007 and 2015 were collected and de-identified. The SUNY Upstate Medical University Institutional Review Board (IRB) for the Protection of Human Subjects approved this study with a determination that patient consent was not required by the IRB, since patient data confidentiality was maintained and all records were retrospective (approval ID: 910050–1). Following institutional policy, all research was in compliance with the Declaration of Helsinki. Beginning in 2016, our institution began implementing respiratory-gated SBRT with volumetric-modulated radiotherapy therapy (VMAT), as well as 3DCRT with the use of flattening filter-free beams. Patients in this era were excluded and will be the focus of a separate analysis. Baseline patient and tumor characteristics, SBRT treatment details, and clinical outcomes were extracted from the medical record and/or the treatment planning systems. In cases where patients received metachronous treatments, patient characteristics are ascribed to initial time point, while effort was made to ascribe tumor characteristics to individually treated lesions. Although management and follow up were at the discretion of the treating physician, patients were generally seen with follow up CT imaging 1 month after completion of therapy, at 3–6 month intervals for the first 5 years, and annually thereafter. Surveillance positron emission tomography (PET) imaging was not routine and performed only in cases where there was suspicion for recurrence or second primary cancer.
Immobilization was generally achieved with whole-body fixation devices. Abdominal compression was commonly utilized in those who could tolerate it to dampen the effect of the respiratory excursion. Use of four-dimensional CT (4DCT) was routine to assess target motion, and was acquired on a four-slice CT scanner (Lightspeed; GE Medical Systems, Chicago, IL, USA) with an external respiratory monitoring system (Real-time Position Management [RPM] System, Varian Medical Systems, Palo Alto, CA, USA). Patients were treated either with an internal target volume (ITV) approach, where the entirety of motion was encompassed in planning target volume, or advanced respiratory motion management techniques (Phase-based respiratory gating, dynamic tumor tracking). Effect of respiratory management method was previously reported to have no appreciable influence on clinical outcomes.14 Patients were treated on one of four treatment machines: Varian 21EX, Varian TrueBeam, Accuray Tomotherapy, and Brainlab Vero. On-board volumetric imaging was used for patient alignment. Cine mode portal image verification for treatment fields was routinely used with 3DCRT treatments. IMRT techniques included static gantry sliding window, VMAT, and helical tomotherapy.
Outcomes assessed include local failure, nodal/distant failure, overall survival, and toxicity. Statistical analyses were conducted using SPSS (versions 24 and 25). Means of continuous variables were compared with t-tests. Chi-square tests were used to assess possible associations across pairs of categorical variables. Kaplan-Meier analyses were undertaken to assess survival and time to local failure, as well as provide median time to event statistics. Log-rank tests provided p-values for these comparisons. Cox proportional hazards modeling was used to assess the effects of treatment type on overall survival with control for age, gender, maximum lesion diameter, number of lesions, and respiratory motion management. Hazard ratios and 95% confidence intervals (CI) were reported in the Cox model. Given that all local failures occurred in the first chronologically treated lesion for patients with multiple lesions, survival analyses (Kaplan Meier and Cox modeling) were limited to the first treated lesion. Comparisons of tumor characteristics (e.g., histology, size) and dosimetry across SBRT treatment modalities at the lesion level (n=351 lesions in n=297 patients) were undertaken using generalized linear mixed models to account for correlation within individuals. Pearson correlation coefficient was calculated to analyze the effect of increasing number of fractions and local control. Statistical significance was defined as p ≤ 0.05 in all analyses.
Results
Baseline Characteristics
Between 2007 and 2015, 297 patients with 351 lesions were treated at our institution. The treatment paradigm for subsequent (secondary or tertiary) lung lesions was repeat SBRT, if the disease was found to be localized. Forty-eight patients received SBRT to two lesions, and six patients received SBRT to three lesions. Median follow up was 22.7 months (range 0.5–107 months). Baseline patient characteristics are shown in Table 1. Median age was slightly higher for patients treated with IMRT. Tumor characteristics according to treatment modality are shown in Table 2. Overall 96.9% of treated lesions were biopsy proven. Tumors treated with IMRT were more likely to be squamous cell carcinoma compared with those treated with a 3DCRT (41.2% vs 29%, p<0.019). A higher percentage of patients receiving IMRT were not pathologically confirmed (14.9% vs 4.5%, p=0.001). Tumor size did not vary significantly between treatment modality (p=0.488).
 |
Table 1 Patient Characteristics According to Treatment Modality at Time of Initially Treated Lesion with Stereotactic Body Radiation Therapy (n=297) |
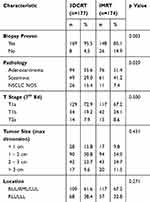 |
Table 2 Tumor Characteristics According to Treatment Modality for Each Lesion Treated with Stereotactic Body Radiation Therapy (n=351) |
Treatment and Planning Details
The majority of patients were treated on the Varian 21EX (44%) and Accuray Tomotherapy (42%). Other treatment machines utilized were BrainLab Vero (8%) and Varian Truebeam (6%). Treatment details are summarized in Table 3. Most lesions treated with 3DCRT utilized the Varian 21EX (79.1%) as compared to helical tomotherapy for the majority of IMRT (85.6%). The most common dose fractionations were 48 Gy in 4 fractions (45%) and 54–60 Gy (54 Gy with heterogeneity corrections, 60 Gy without) in 3 fractions (30%). Higher number of fraction SBRT schemes (4 or 5) were more common with IMRT than 3DCRT (68% vs 46%, p<0.0001). The use of IMRT increased over time, as it was used for only 6.3% of treatments in 2009 vs 47.8% in 2015 (p < 0.01). The use of 4 or 5 fraction regimens increased with time, where from 2007 to 2008 was 48.3% of SBRT and by year 2015 comprised 67% (p=0.033).
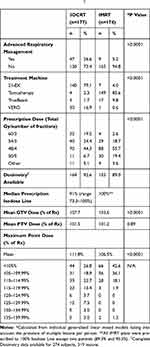 |
Table 3 Treatment Details (n=351)† |
Mean GTV, max GTV, and max PTV doses as a percentage of prescription dose were significantly higher with 3DCRT (Table 3), indicating more heterogeneity. Mean PTV dose was similar for 3DCRT and IMRT, however.
There were 24 patients (8.1%) treated with mixed 18MV and 6MV beams, all of which were 3D-planned. All other treatments were 6 MV only. The fraction of high energy treatment varied, but only two patients had > 50% 18MV beam usage (0.80 and 0.625 18MV utilization).
Clinical Outcomes
There were 17 local failures among the 351 lesions treated (4.8%). There were no significant differences observed in local failure rate according to treatment type - 4.9% for 3DCRT and 6.5% for IMRT, p = 0.573 (see Figures 1 and 2). There were no differences in proportions of patients with either nodal/distant only failure (19.4% and 12%), those who died without evidence of recurrence (31% and 28.9%), or those who were alive without recurrence (43.2% and 53.5%) according to 3DCRT and IMRT SBRT, respectively (p=0.186). Local failure was not associated with treatment machine (21-EX, Tomotherapy, Truebeam, or Vero, p=0.407). There were no local failures observed within the 24 patients with the inclusion of high energy (18MV) treatments. Maximum tumor diameter approached significance for risk of local failure (p=0.075). Local failure highly correlated with inferior overall survival (p<0.001).
 |
Figure 1 Freedom from to local failure according to treatment modality with either three-dimensional radiotherapy (3D) or intensity-modulated radiotherapy (IMRT). |
 |
Figure 2 Freedom from local failure for the entire patient cohort. |
Absolute risk of local failure was not affected by fractionation schedule, occurring in 4% of patients treated with < 4 fractions and 6.6% with 4 or more (p=0.440). There was however an inverse correlation with number of fractions and time to local failure, with a shorter time to local failure with larger number of fraction treatment schemes. Overall 95.6% of lesions were treated with either 54–60Gy (3 fraction), 48Gy (4 fraction), or 50Gy (5 fraction) regimens (see Table 3). This may indicate a correlation with a higher biologically effective dose (54–60Gy in 3 fractions) with improved local control, however a detailed investigation into this possible relationship will be the subject of a future analysis. Histology was not a predictor for local failure (p=0.239). There was no increased risk of local failure observed for lower lobe lesions, which are potentially associated with increased tumor motion, compared to all other locations (p=0.638).
For the entire cohort, median overall survival (OS) was 43.4 months. The median OS was 42.8 months for IMRT and 46.7 for 3D (see Figures 3 and 4). By log-rank testing, treatment modality was not associated with differences in OS (p=0.427). Cox proportional hazard analyses to assess variables predicting overall survival showed no association with treatment modality and survival (see Table 4). Increasing tumor diameter was predictive of inferior survival (p=0.048). Treatment of multiple lesions was associated with longer survival when dating from initial therapy (p=0.017). There was too few toxicity to permit meaningful conclusions on the impact of treatment type. Overall, there were four grade 3 toxicities (2 dyspnea, 1 pneumonitis, 1 rib fracture). There were no grade 4 or 5 toxicities. On Pearson-Chi square testing there was no difference between 3DCRT or IMRT for the development of either dyspnea post-treatment (p=0.657) or radiation pneumonitis (p=0.686).
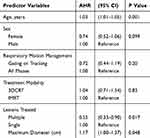 |
Table 4 Cox Proportional Hazard Model Assessing Overall Survival (n=297); Adjusted hazard Ratios (AHR) and 95% Confidence Intervals (CI) |
 |
Figure 3 Overall survival according to treatment modality with either three-dimensional radiotherapy (3D) or intensity-modulated radiotherapy (IMRT). |
 |
Figure 4 Overall survival for the entire patient cohort. |
Discussion
The use of SBRT to treat lung tumors (primary or metastatic) has benefited from rapid adoption while also incorporating significant technological advancements. Early on, small retrospective series suggested comparable clinical outcomes with IMRT.11 Most prospective studies have focused on optimal dose and fractionation – aiming to both maintain excellent local control while mitigating the risk of toxicity. These protocols were led largely by the RTOG/NRG, and with the exception of RTOG 0236, allowed both 3DCRT and IMRT. There have been no comparisons of these modalities from these prospective trials, perhaps given the small number of failure events. Similar questions exist in conventionally fractioned lung cancer treatment, however. In a secondary analysis of RTOG 0617, there was no difference in outcomes between those treated with 3D and IMRT, although the latter cohort had patients with more advanced disease.15 Both methods of treatment planning and delivery continue to be widely used for both conventionally fractionated and SBRT lung cancer treatments. Whether the addition of immune checkpoint inhibition to SBRT will improve outcomes for stage I NSCLC remains unknown and is the subject of several accruing prospective studies.16
The series we report herein represents one of the largest experiences analyzing clinical outcomes by 3DCRT vs IMRT planning/delivery. The local failure rate overall (4.8%) is consistent with published literature.4,6–8,10 In 297 patients and 351 lesions, there was not an appreciable difference in local failure or survival rates between those treated with 3DCRT and IMRT. This is consistent with a publication from Kumar et al, who reported no difference in outcomes of 176 SBRT treatments between static field volumetric modulated arc therapy (VMAT) or helical tomotherapy.17 Recent data have suggested inferior rates of local control with tumors of squamous histology, compared with adenocarcinoma.18 Interestingly, there was no noted difference in failure rates based on histology in our analysis, however there were more patients with squamous cell carcinoma treated with IMRT – thus representing a potential confounder. The association noted between improved survival and multiple lesions treated may be an artefact of increased follow-up and sufficient health to warrant additional treatment in such patients.
There were too few recorded toxicity events in our cohort for conclusions regarding effect of treatment type on pneumonitis rates. A Dutch series aiming to compare clinical and radiographic rates of pneumonitis between 3DCRT and VMAT did not uncover differences in early follow-up.19 A similar analysis focused on radiographic changes following SBRT of either 3DCRT or VMAT likewise was not able to appreciate a difference after a median follow up of 20.5 months.20
While clinical outcome comparisons of IMRT and 3DCRT are relatively sparse, there have been several studies of potential effects on treatment delivery and planning. With VMAT, the ability to deliver from multiple incident angles with arcs has been shown to consistently shorten treatment times when compared to static field delivery (IMRT or 3D), and achieve comparable plan quality.21–24 This fact benefits both clinic throughput, leads to patient satisfaction, and shorter treatment times limit the potential for intrafraction motion.25 Moreover, VMAT has been suggested to have increased conformity to target, as well as shaper dose falloff – leading to lower lung doses.26 The benefit of superiority for PTV coverage and organ sparing seems to hold up regardless of tumor location within the lungs.27
Despite these dosimetric advantages, there have been concerns raised about the use of modulated fields with regard to the interplay of leaf motion and tumor motion with respiration. As targets move with the breathing cycle, there exists a theoretical risk of GTV under treatment. Fortunately, an analysis by Rao and colleagues suggested only negligible interplay effects between lung tumor motion and multi-leaf collimator.28 Similar studies have been done in liver tumors also suggesting only insignificant effects.29
Several prospective studies have investigated varying dose fractionation schemes, with varying utilization of IMRT. After the Indiana experience uncovered potential increased toxicity of three fraction regimen, the initial cooperative group experience, RTOG 0236 did not allow IMRT. The centrally located study, RTOG 0813, did allow IMRT, and patients on the entire study were reportedly similar, with slight predominance for IMRT at the 12 Gy x 5 fraction dose level.30 In the prospective randomized phase II RTOG 0915 study evaluating 34 Gy in a single fraction and 48 Gy in 4 fractions, IMRT was allowed, but breakdown of treatment was not reported. In the currently enrolling PACIFIC-4 (RTOG Foundation 3515) and JoLT STABLE-MATES trials, IMRT is allowed, along with 3DCRT.
In the aforementioned cooperative group trials, as well as retrospective analyses, it is typical for nominal dose and fractionation to be reported, yet heterogeneity is less commonly noted. Our series reports both prescription dose, prescription isodose line, and mean GTV dose relative to marginal dose (PTV prescription dose). We believe this sort of heterogeneity reporting is vital to report SBRT literature moving forward – as local control rates may be tied to target heterogeneity. In our IMRT cohort, plans were more likely to be homogenous with IDL prescriptions of 100%, while 3DCRT plans tended to prescribe to lower lines, leading to higher mean GTV doses. Caution is warranted when interpreting data across studies, in that dose and fractionation does not tell the whole story.
This study has several limitations worth noting. First, its retrospective design renders it vulnerable to typical criticisms, including potential underreporting of toxicity, local failure, and biases in patient management. Second, median follow-up is relatively short, and although the majority of local failures occur within 2 years,31 robustness of clinical outcomes could be questioned. Finally, a paucity of both local failure and toxicity events makes complex multivariate analyses difficult, and the possibility exists for confounding between tumor and treatment factors and the 3D vs IMRT investigational aim.
Conclusion
In this single-institution series of patients undergoing SBRT for early-stage NSCLC, there were no appreciable differences in local control or survival in those treated with 3DCRT and IMRT and further study will be required to draw definitive conclusions regarding the comparative effectiveness. Future reporting of prescription isodose and heterogeneity with SBRT series will be valuable.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Aridgides P, Bogart J. Stereotactic body radiation therapy for stage I non-small cell lung cancer. Thorac Surg Clin. 2016;26(3):261–269. doi:10.1016/j.thorsurg.2016.04.008
2. Ginsberg RJ, Rubinstein LV. Randomized trial of lobectomy versus limited resection for T1 N0 non-small cell lung cancer. Lung cancer study group. Ann Thorac Surg. 1995;60(3):615–622. [discussion 22-3]. doi:10.1016/0003-4975(95)00537-U.
3. Bogart JA, Wallen J. Management of patients with stage I lung cancer. J Oncol Pract. 2017;13(2):69–76. doi:10.1200/JOP.2016.019711
4. Timmerman R, Paulus R, Galvin J, et al. Stereotactic body radiation therapy for inoperable early stage lung cancer. JAMA. 2010;303(11):1070–1076. doi:10.1001/jama.2010.261
5. Timmerman RD, Hu C, Michalski J, et al. Long-term results of RTOG 0236: a phase II trial of Stereotactic Body Radiation Therapy (SBRT) in the treatment of patients with medically inoperable stage I non-small cell lung cancer. Int J Radiat Oncol Biol Phys. 2014;90(1):S30. doi:10.1016/j.ijrobp.2014.05.135.
6. Videtic GMM, Donington J, Giuliani M, et al. Stereotactic body radiation therapy for early-stage non-small cell lung cancer: executive summary of an ASTRO evidence-based guideline. Pract Radiat Oncol. 2017;7(5):295–301. doi:10.1016/j.prro.2017.04.014
7. Nyman J, Hallqvist A, Lund JA, et al. SPACE – a randomized study of SBRT vs conventional fractionated radiotherapy in medically inoperable stage I NSCLC. Radiother Oncol. 2016;121(1):1–8. doi:10.1016/j.radonc.2016.08.015
8. Ball D, Mai GT, Vinod S, et al. Stereotactic ablative radiotherapy versus standard radiotherapy in stage 1 non-small-cell lung cancer (TROG 09.02 CHISEL): a phase 3, open-label, randomised controlled trial. Lancet Oncol. 2019;20(4):494–503. doi:10.1016/S1470-2045(18)30896-9
9. Laliscia C, Fabrini MG, Delishaj D, et al. Clinical outcomes of stereotactic body radiotherapy in oligometastatic gynecological cancer. Int J Gynecol Cancer. 2017;27(2):396–402. doi:10.1097/IGC.0000000000000885
10. Timmerman R, Papiez L, McGarry R, et al. Extracranial stereotactic radioablation: results of a phase I study in medically inoperable stage I non-small cell lung cancer. Chest. 2003;124(5):1946–1955. doi:10.1378/chest.124.5.1946
11. Videtic GM, Stephans K, Reddy C, et al. Intensity-modulated radiotherapy-based stereotactic body radiotherapy for medically inoperable early-stage lung cancer: excellent local control. Int J Radiat Oncol Biol Phys. 2010;77(2):344–349. doi:10.1016/j.ijrobp.2009.05.004
12. Moustakis C, Blanck O, Ebrahimi Tazehmahalleh F, et al. Planning benchmark study for SBRT of early stage NSCLC : results of the DEGRO Working Group Stereotactic Radiotherapy. Strahlenther Onkol. 2017;193(10):780–790. doi:10.1007/s00066-017-1151-8.
13. Roa DE, Schiffner DC, Zhang J, et al. The use of RapidArc volumetric-modulated arc therapy to deliver stereotactic radiosurgery and stereotactic body radiotherapy to intracranial and extracranial targets. Med Dosim. 2012;37(3):257–264. doi:10.1016/j.meddos.2011.09.005
14. Aridgides P, Nsouli T, Chaudhari R, et al. Clinical outcomes following advanced respiratory motion management (respiratory gating or dynamic tumor tracking) with stereotactic body radiation therapy for stage I non-small-cell lung cancer. Lung Cancer (Auckl). 2018;9:103–110. doi:10.2147/LCTT.S175168
15. Chun SG, Hu C, Choy H, et al. Impact of intensity-modulated radiation therapy technique for locally advanced non-small-cell lung cancer: a secondary analysis of the NRG oncology RTOG 0617 randomized clinical trial. J Clin Oncol. 2017;35(1):56–62. doi:10.1200/JCO.2016.69.1378
16. Vansteenkiste J, Wauters E, Reymen B, Ackermann CJ, Peters S, De Ruysscher D. Current status of immune checkpoint inhibition in early stage NSCLC. Ann Oncol. 2019;30(8):1244–1253. doi:10.1093/annonc/mdz175
17. Kumar SS, Hall L, Li X, et al. Comparison of outcomes of stereotactic body radiation therapy delivered with three different technologies to the lung. J Radiosurg SBRT. 2018;5(3):209–216.
18. Shiue K, Cerra-Franco A, Shapiro R, et al. Histology, tumor volume, and radiation dose predict outcomes in NSCLC patients after stereotactic ablative radiotherapy. J Thorac Oncol. 2018;13(10):1549–1559. doi:10.1016/j.jtho.2018.06.007
19. Palma DA, Senan S, Haasbeek CJ, Verbakel WF, Vincent A, Lagerwaard F. Radiological and clinical pneumonitis after stereotactic lung radiotherapy: a matched analysis of three-dimensional conformal and volumetric-modulated arc therapy techniques. Int J Radiat Oncol Biol Phys. 2011;80(2):506–513. doi:10.1016/j.ijrobp.2010.02.032
20. Badellino S, Muzio JD, Schivazappa G, et al. No differences in radiological changes after 3D conformal vs VMAT-based stereotactic radiotherapy for early stage non-small cell lung cancer. Br J Radiol. 2017;90(1078):20170143. doi:10.1259/bjr.20170143
21. Herbert C, Kwa W, Nakano S, et al. Stereotactic body radiotherapy: volumetric modulated arc therapy versus 3D non-coplanar conformal radiotherapy for the treatment of early stage lung cancer. Technol Cancer Res Treat. 2013;12(6):511–516. doi:10.7785/tcrt.2012.500338
22. Holt A, van Vliet-vroegindeweij C, Mans A, Belderbos JS, Damen EM. Volumetric-modulated arc therapy for stereotactic body radiotherapy of lung tumors: a comparison with intensity-modulated radiotherapy techniques. Int J Radiat Oncol Biol Phys. 2011;81(5):1560–1567. doi:10.1016/j.ijrobp.2010.09.014
23. McGrath SD, Matuszak MM, Yan D, Kestin LL, Martinez AA, Grills IS. Volumetric modulated arc therapy for delivery of hypofractionated stereotactic lung radiotherapy: a dosimetric and treatment efficiency analysis. Radiother Oncol. 2010;95(2):153–157. doi:10.1016/j.radonc.2009.12.039
24. Ong CL, Verbakel WF, Cuijpers JP, Slotman BJ, Lagerwaard FJ, Senan S. Stereotactic radiotherapy for peripheral lung tumors: a comparison of volumetric modulated arc therapy with 3 other delivery techniques. Radiother Oncol. 2010;97(3):437–442. doi:10.1016/j.radonc.2010.09.027
25. Rossi MM, Peulen HM, Belderbos JS, Sonke JJ. Intrafraction motion in stereotactic body radiation therapy for non-small cell lung cancer: intensity modulated radiation therapy versus volumetric modulated arc therapy. Int J Radiat Oncol Biol Phys. 2016;95(2):835–843. doi:10.1016/j.ijrobp.2016.01.060
26. Zhang GG, Ku L, Dilling TJ, et al. Volumetric modulated arc planning for lung stereotactic body radiotherapy using conventional and unflattened photon beams: a dosimetric comparison with 3D technique. Radiat Oncol. 2011;6:152. doi:10.1186/1748-717X-6-152
27. Rauschenbach BM, Mackowiak L, Malhotra HK. A dosimetric comparison of three-dimensional conformal radiotherapy, volumetric-modulated arc therapy, and dynamic conformal arc therapy in the treatment of non-small cell lung cancer using stereotactic body radiotherapy. J Appl Clin Med Phys. 2014;15(5):4898. doi:10.1120/jacmp.v15i5.4898
28. Rao M, Wu J, Cao D, et al. Dosimetric impact of breathing motion in lung stereotactic body radiotherapy treatment using intensity modulated radiotherapy and volumetric modulated arc therapy [corrected]. Int J Radiat Oncol Biol Phys. 2012;83(2):e251–6. doi:10.1016/j.ijrobp.2011.12.001
29. Hubley E, Briscoe M, Ploquin N, Pierce G. Technical note: a novel quality assurance test to identify gantry angle inaccuracies in respiratory-gated VMAT treatments. Med Phys. 2017;44(10):5075–5080. doi:10.1002/mp.2017.44.issue-10
30. Bezjak A, Paulus R, Gaspar LE, et al. Safety and efficacy of a five-fraction stereotactic body radiotherapy schedule for centrally located non-small-cell lung cancer: NRG oncology/RTOG 0813 trial. J Clin Oncol. 2019;37(15):1316–1325. doi:10.1200/JCO.18.00622
31. Senthi S, Lagerwaard FJ, Haasbeek CJ, Slotman BJ, Senan S. Patterns of disease recurrence after stereotactic ablative radiotherapy for early stage non-small-cell lung cancer: a retrospective analysis. Lancet Oncol. 2012;13(8):802–809. doi:10.1016/S1470-2045(12)70242-5
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
