Back to Journals » Open Access Emergency Medicine » Volume 15
Outcome of Rodenticide Poisoning and Its Associated Factors Among Adult Patients Admitted with Rodenticide Poisoning at the Emergency Unit of Debre Tabor Comprehensive Specialized Hospital, Debre Tabor, North Central Ethiopia
Authors Tassew SF , Haile BA, Amera Birlie T
Received 1 February 2023
Accepted for publication 20 May 2023
Published 26 May 2023 Volume 2023:15 Pages 189—197
DOI https://doi.org/10.2147/OAEM.S405970
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Hans-Christoph Pape
Sheganew Fetene Tassew,1 Betlhem Amha Haile,2 Tekalign Amera Birlie3
1Department of Emergency Medicine and Critical Care Nursing, College of Health Science, Debre Tabor University, Debre Tabor, Ethiopia; 2Department of Human Nutrition, Institute of Public Health, College of Medicine and Health Science, University of Gondar, Gondar, Ethiopia; 3Department of Adult Health Nursing, College of Health Science, Debre Tabor University, Debre Tabor, Ethiopia
Correspondence: Sheganew Fetene Tassew, Department of Emergency Medicine and Critical Care Nursing, Debre Tabor University, P.O. Box 272, Debre Tabor, Amhara Regional State, Ethiopia, Tel +251 918511768, Email [email protected]
Introduction: Rodenticides are pesticides used to eradicate rodents. It is a common reason for visits to the emergency department and hospitalization, and it is a major public health concern. Intentional or unintentional intoxication can result in severe consequences with a high fatality rate. In Ethiopia, studies on the outcome of rodenticide poisoning are scarce. The goal of this study was to assess the outcomes of rodenticide poisoning and its associated factors in adult patients admitted to the emergency unit of Debre Tabor Comprehensive Specialized Hospital in North Central Ethiopia.
Methods: A retrospective record review institutional-based cross-sectional study design was used on 156 adult patients treated with rodenticide poisoning at Debre Tabor Comprehensive Specialized Hospital’s emergency unit between May 1, 2017 and April 30, 2022. To collect data from patient medical documents and the Health Management Information System, an abstraction sheet was employed. The information was entered into EPI data version 4.6, then exported and analyzed using STATA version 14 software. To analyze the relationship between the dependent and independent variables, bivariable and multivariable regression were used.
Results: A total of 156 participants were involved in the study. The majority of them 55.13% were in the age group of 19– 37 years with the median age of 23 years. Three-fourth of the cases were suicidal poisoning. Overall, 49.35% patients presented to Debre Tabor Specialized Hospital had poor outcome. Having suicidal poisoning (AOR = 10.64; 95% CI: 2.43, 46.53), having tachycardia (AOR = 5.41; 95% CI: 1.54, 18.98), being referred from other health center (AOR = 5.78; 95% CI: 1.97, 16.95) were factors associated with poor outcome.
Conclusion: Rodenticide poisoning had a poor overall outcome. Suicidal poisoning, tachycardia, and referral from other health facilities were all important predictors in poor rodenticide poisoning outcomes.
Keywords: rodenticide, poisoning, emergency
Introduction
Poisoning is a major global public health issue, and occurrences are growing due to changes in lifestyle and social behavior. According to the World Health Organization (WHO), unintentional poisoning resulted in 106,683 deaths and the loss of 6.3 million years of healthy life worldwide (disability adjusted life years, DALYs).1,2 Rodenticides are pesticides that are used to kill rodents. Because they destroy insects, they are also used as a grain preservative. It is a broad term that covers a vast range of compounds. The drugs’ chemical makeup, mode of action, hazardous dosages, and lethal impact differ. It is categorized according to whether the agent is anticoagulant or non-anticoagulant. They are commonly used as self-harm and suicide agents in several parts of the poor world.3
Rodenticide poisoning causes severe symptoms involving several essential organs, which is lethal. They are among the most toxic substances regularly found in homes. The varieties of rodenticides used over the years are legion.4
According to the American Association of Poison Control Centers (AAPCC), rodenticides are responsible for 75,514 cases, 66,362 of which are unintentional and 1598 of which are intentional, with 29 persons dying as a result.5
Despite the fact that rodenticide poisoning is a major public health risk in Africa, only ten of 58 nations (17.2%) have poisons information centers (PICs).6
In Ethiopia, there is a scarcity of epidemiological data on the consequences of rodenticide poisoning. According to a study conducted in central Ethiopia, poisoning is the most common agent for self-poisoning and is associated with significant mortality.7
There are only two studies done on the outcome of rodenticide poisoning in Ethiopia, so the current study is intended to assess outcome and associated factors among adult patients admitted with rodenticide poisoning at the emergency unit of DTCSH, Debre Tabor, North Central Ethiopia, 2022.
Methods
Study Design and Study Period
Institutional-based cross-sectional study was used. The data were collected from June 1 through June 30, 2022.
Study Area
Debre Tabor Comprehensive Specialized Hospital was founded in 1923 E.C. in Debre Tabor Town. Debre Tabor is the capital city of South Gondar Zone; it is 666 kilometers from Addis Ababa and 103 kilometers from Bahir Dar, the capital city of Amhara Regional State. DTCSH has provided preventative, delivery, and curative health services to about 2.7 million people in the Zone and surrounding regions. It contains around 275 inpatient beds in six disciplines, three laboratory sites, five emergency OPDs, and more than 12 outpatient departments (OPDs). Debre Tabor University also uses it as a teaching hospital. Every year, around 8000 emergency patients are seen at the emergency department.
Source Population
During the study period, all rodenticide-poisoned adult patients were included, whereas patients with inadequate medical records and patients who took rodenticide mixed with other poisons were excluded. This study included the records/charts of all patients who came in medical emergency DTCSH with rodenticide poisoning in the specified study period.
Variables of the Study
The dependent variable in the study was patient outcome after rodenticide poisoning, while the independent variables were socio-demographic variable, time of arrival, mode of poison, admission status, referral status, mode of hospital arrival, co-morbidities, length of stay, treatment given, blood pressure, and pulse rate at admission. A data abstraction sheet was constructed using information from patients’ medical records and was adapted from several sources.8–11
Operational Definition
Good outcome: Refers when the physician declared the status improved (recovered) at discharge, transfer out to higher institution, against medical advice.
Poor outcome: Refers when the physician declared death summary or survived with disability.
Recovered: When the rodenticide poisoned patient was discharged without disability.
Disability: Either physical or psychological disability leading to a new morbidity, dialysis, respiratory, or another organ failure, following rodenticide poisoning, which was confirmed by laboratory investigation or suspected by clinicians, based on the clinical complaints of patients.
Data Collection Procedure and Tool
After retrieving the patient card from the card store using the medical record number and cross-referencing it with the registration book, the essential information was recorded using a structured checklist format. The cases were chosen if and only if they met the inclusion criteria. The sampling procedure of this study was census method with included all patients presented with rodenticide poisoning. The data abstraction format was created in English and included variables such as sex of patients, age of patients, residence, route of poisoning, mode of poisoning, clinical presentations, the status of patients when they arrived at the hospital, reason of patients for taking the poison, time of arrival to the hospital’s emergency department, length of hospital stay, treatment given for rodenticide poisoning, and outcome. Data were collected from the records by two well-trained BSc nurses.
Data Quality Control
The pre-test on 5% of total study population were conducted in DTCSH. Any errors discovered throughout the pretesting process were corrected, and changes were made to the final version of the data abstraction format. As data collectors, two BSc nurses were assigned. One day of training was provided on the goal of the study, the structured checklist, and ensuring the confidentiality of the charts to the data collectors. The data was supervised by a competent supervisor to ensure its completeness and consistency. During and after data collection, all acquired data was checked for completeness and consistency.
Data Analysis
The data was entered into EPI data version 4.6, then exported and analyzed using STATA version 14 software. Frequency tables, text, and figure were used to present the data. The association of factors with the outcome was investigated using bivariable and multivariable analysis. If the p-value was less than 0.25, variables were transferred from bivariable to multivariable. The strength of the statistical link was quantified using the odds ratio and 95% confidence intervals, and statistical significance was determined using p-values of 0.05.
Results
Socio-Demographic Characteristics
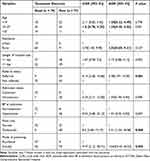
The study included 156 adult patients admitted to Debre Tabor Comprehensive Specialized Hospital (DTCSH) with rodenticide poisoning from May 1, 2017 to April 30, 2022. One hundred fifty-six (156) patient’s medical records had complete information and were included in the final analysis making the response rate 100%. The majority, 86 (55.13%) were in the age group of 20–40 years. The median age of patients was found to be 23 with IQR of (19, 30.5) years. More than half, 83 (53.21%), were females. The majority, 131 (83.97%), of the admitted patients were from rural residence (Table 1).
 |
Table 1 Socio-Demographic Characteristics of Adult Patients Admitted with Rodenticide Poisoning at the Emergency Unit of DTCSH, Debre Tabor, North Central Ethiopia, 2022 (N = 156) |
Baseline Information of the Patients
Three-fourth of the cases were related to suicidal poisoning. Commonly reported reason for taking the poison was quarrel with family members, which accounts for 46 (36.8%). Two-third, 102 (65.38%), clients arrived at the hospital by public transport (Table 2).
 |
Table 2 Base Line Information of the Adult Patients Admitted with Rodenticide Poisoning at the Emergency Unit of DTCSH, Debre Tabor, North Central Ethiopia, 2022 (N = 156) |
Clinical Characteristics
At the time of admission, 123 (78.85%) of the adult patients were conscious, and 113 (72.44%) were normotensive.
More than half of the cases, 90 (57.69%), arrived within two hours. Only 27 (17.37%) of the cases had comorbidities. Complications such as psychiatric illness, dyspepsia, acute respiratory distress syndrome, acute renal failure, and electrolyte imbalance occurred in more than half of the cases, 89 (57.05%). All of the poisoning routes were ingestion and non-anticoagulant in type (Table 3).
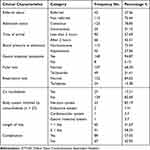 |
Table 3 Clinical Characteristics of Adult Patients Admitted with Rodenticide Poisoning at the Emergency Unit of DTCSH, Debre Tabor, North Central Ethiopia, 2022 (N = 156) |
Treatment Given
Based on the patient’s case, the client received both pharmacological and non-pharmacological care for rodenticide poisoning. Gastric lavage was used as a non-pharmacologic treatment to prevent hazardous substance absorption in 90 (57.69%) of the cases. Metoclopramide 148 (94.87%), cimetidine 146 (93.59%), and magnesium sulphate 116 (74.36%) were the most commonly used pharmacologic treatments (Table 4).
 |
Table 4 Treatment Given for Adult Patients Admitted with Rodenticide Poisoning at the Emergency Unit of DTCSH, Debre Tabor, North Central Ethiopia, 2022 (n = 156) |
Patient Outcome
Among 156 records of adult patients with the diagnosis of rodenticide poisoning included in the study, 77 (49.56%) had poor outcome, from those who had poor outcome (43.8%) were died and 5.76% were survived with disability (Figure 1).
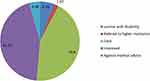 |
Figure 1 Treatment outcome of adult patients admitted with rodenticide poisoning at the emergency units of DTCSH, Debre Tabor, North Central Ethiopia, 2022 (n = 156). |
Factors Associated with Poor Treatment Outcome of Rodenticide Poisoning
Both bi-variable and multivariable logistic regression were undertaken. In the multivariable analysis, age, residence, mode of poisoning, referral status, admission status, blood pressure at admission, pulse rate, and length of hospital stay were fitted for multivariable analysis. However, in bi-variable analysis, only modes of poisoning, referral status and pulse rate were significantly associated with poor outcomes of rodenticide poisoning. Patients with suicidal poisoning were 10.64 times more likely to have poor outcome than those with accidental poisoning (AOR = 10.64; 95% CI: 2.43, 46.53). Patients who referred from other health facility were 5.78 times more likely to have poor outcome than self-referred (AOR = 5.78; 95% CI: 1.97, 16.95). Patient with tachycardia at admission were 5.41 times more likely to have poor outcome than those with normal pulse rate (AOR = 5.41; 95% CI: 1.54, 18.98) (Table 5).
Discussion
The purpose of this research was to assess the treatment outcome of rodenticide poisoning and its associated factors in adult patients admitted to the DTCSH emergency unit. According to this study, the overall magnitude of poor outcome in rodenticide poisoning was 49.56% (95% CI) (41.5, 57.2). This finding was reinforced by investigations in Morocco and Egypt, which reported a death rate of 49% and 45.5%, respectively.12,13 However, a study conducted at Felege Hiwot Referral Hospital Bahir Dar and Tehran-Iran found a lower mortality rate of 31.2% and 24.06%, respectively.4,14 This disparity could be attributed to differences in study period, study population, medication availability, and both studies explain that most of the tablets were exposed to air or expired tablets, which have lower phosphide content due to gradual release of phosphine gas.
However, when we compared this prevalence to a comparable study done on 418 patients by Chugh et al, the overall mortality from rodenticide poisoning was 77.2%, 58.9% Nehru Hospital Chandigarh in Northwest India, and 61% in Hospital Avicenna, Rabat.15–17 This variation can be attributed to differences in sample size, study population, study area, study design, dose eaten, time of arrival, kind of poisoning, and vomiting, which accounts for 94.87% of the total in our study because vomiting boosts the survival rate.18 Other factors, such as financial background, cultural differences (traditional versus medical counsel), and religious beliefs, may contribute to those results outside of Ethiopia.
Suicidal poisoning patients were 10.64 times more likely to have a poor outcome than accidental poisoning patients (AOR = 10.64; 95% CI: 2.43, 46.53). The finding was supported by research conducted in eastern Ethiopia.19 The explanation for this could be because if a person consumes the poison for suicide reasons, they may swallow it all because it is intended to kill them.
Patients referred from another health facility were 5.78 times more likely than non-referred patients to have a poor outcome (AOR = 5.78; 95% CI: 1.97, 16.95). This could be attributed to a late presentation to the hospital beginning with poison intake, as well as a lack of ambulances or facilities in that rural health center.
Patients who had tachycardia at the time of admission were 5.41 times more likely to have a poor outcome than those who had a normal pulse rate (AOR = 5.41; 95% CI: 1.54, 18.98). This was also the conclusion of an Egyptian study.13,20 This is because rodenticide poisoning disrupts electrolytes, resulting in cardiac arrhythmia and cardiogenic shock. Hypotension, on the other hand, will cause tachycardia as a compensatory strategy. As a result, most common cause of mortality in rodenticide poisoning was cardiac arrest.
Limitation of the Study
Use of cross-sectional study was the limitation. Because of financial scarcity, we used cross-sectional study; it was better if we used cohort study.
Conclusions
Rodenticide poisoning had a bad overall outcome. Suicidal poisoning, having tachycardia, and being referred from another health center all contributed to poor rodenticide poisoning outcomes. As a recommendation, the health care providers should better organize to generate information on the mortality of rodenticide poisoning and suicidal act. Health care providers and institutions should maintain counseling and family-centered services that allow adults to develop coping mechanisms and pay special attention to the vital signs of rodenticide-poisoned patients.
Abbreviations
AlP, aluminum phosphide; AAIPP, acute aluminum phosphide poisoning; AKI, acute kidney injury; AOR, adjusted odds ratio; CI, confidence interval; DTCSH, Debre Tabor Comprehensive Specialized Hospital; INR, International Normalized Ratio; ICU, intensive care unit; PT, prothrombin time; RR, relative risk; WHO, World Health Organization; ZnP, zinc phosphide.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The study was approved by the ethical review committee at Debre Tabor University’s college of health science with the ethical approval number 3547. Following that, an official approval letter was received from DTCSH authorities. Because this is a retrospective medical record study, patient informed permission was waived, and personal identifiers such as name and medical registration number were not included, as approved by Debre Tabor University’s College of Health Science ethical review committee. The study was conducted per the declaration of Helsinki. The information was kept completely confidential and was only used for research purposes.
Acknowledgments
We would like to thank Debre Tabor University’s College of Health Sciences for allowing us to conduct this study. We would also like to thank DTCSH for providing us with a permission letter to perform the study, as well as hospital administrators, emergency unit, and patient medical record room staff members for their cooperation in carrying out the study.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
Debre Tabor University provided financial assistance for this study, from proposal development to report writing. Otherwise, the research would not have been funded for publication.
Disclosure
The authors declare that they have no competing interests.
References
1. Global health estimates: leading causes of death; 2022. Available from: https://www.who.int/data/gho/data/themes/mortality-and-global-health-estimates/ghe-leading-causes-of-death.
2. Suicide worldwide in 2019; 2022. Available from: https://www.who.int/publications-detail-redirect/9789240026643.
3. Paul Prabhakar Abhilash K, Jayakaran J. Rodenticide poisoning literature review and management. Curr Med Issues. 2020;17:129–133.
4. Bogale DE, Ejigu BD, Muche TA. Clinical profile and treatment outcome of aluminum phosphide poisoning in Felege Hiwot Referral Hospital, Northwest Ethiopia: a retrospective study. Open Access Emerg Med. 2021;13:239–248. doi:10.2147/OAEM.S313181
5. 2020 annual report of the American Association of Poison Control Centers’ National Poison Data System (NPDS): 38th annual report – PubMed; 2022. Available from: https://pubmed.ncbi.nlm.nih.gov/34890263/.
6. A promising poison information centre model for Africa – pubMed; 2022. Available from: https://pubmed.ncbi.nlm.nih.gov/30456069/.
7. Desalew M, Aklilu A, Amanuel A, Addisu M, Ethiopia T. Pattern of acute adult poisoning at Tikur Anbessa specialized teaching hospital, a retrospective study, Ethiopia. Hum Exp Toxicol. 2011;30(7):523–527. doi:10.1177/0960327110377520
8. Bilics G, Héger J, Pozsgai É, et al. Successful management of zinc phosphide poisoning—a Hungarian case. Int J Emerg Med. 2020;13:48. doi:10.1186/s12245-020-00307-8
9. Gopalakrishnan S, Kandasamy S, Iyyadurai R. Rodenticide poisoning: critical appraisal of patients at a tertiary care center. Indian J Crit Care Med Peer Rev off Publ Indian Soc Crit Care Med. 2020;24(5):295–298.
10. Ng WY, Ching CK, Chong YK, Ng SW, Cheung WL, Mak TWL. Retrospective study of the characteristics of anticoagulant-type rodenticide poisoning in Hong Kong. Journal of Medical Toxicology. 2018;14(3):218–228. doi:10.1007/s13181-018-0660-x
11. Woyessa AH, Palanichamy T. Patterns, associated factors, and clinical outcomes of poisoning among poisoning cases presented to selected hospitals in Western Ethiopia: hospital-based study. Emerg Med Int. 2020;2020:5741692. doi:10.1155/2020/5741692
12. Farzaneh E, Ghobadi H, Akbarifard M, et al. Prognostic factors in acute aluminium phosphide poisoning: a risk-prediction nomogram approach. Basic Clin Pharmacol Toxicol. 2018;123(3):347–355. doi:10.1111/bcpt.13005
13. Abdelrahim MKM. Supportive measures in treatment of aluminum phosphide poisoning as a trial to reduce mortality at Assiut University Hospital. Report No.: NCT03879356; 2019. Available from: https://clinicaltrials.gov/ct2/show/NCT03879356.
14. Soltaninejad K, Nelson LS, Bahreini SA, Shadnia S. Fatal aluminum phosphide poisoning in Tehran-Iran from 2007 to 2010. Indian J Med Sci. 2012;66(3–4):66–70. doi:10.4103/0019-5359.110909
15. Chugh SN, Arora B, Arora B, Malhotra KC, Malhotra KC. Incidence & outcome of aluminium phosphide poisoning in a hospital study. Indian J Med Res. 1991;94:232–235.
16. Singh S, Singh D, Wig N, Jit I, Sharma BK. Aluminum phosphide ingestion--a clinico-pathologic study. J Toxicol Clin Toxicol. 1996;34(6):703–706. doi:10.3109/15563659609013832
17. Hajouji Idrissi M, Oualili L, Abidi K, Abouqal R, Kerkeb O, Zeggwagh AA. Facteurs de gravité de l'intoxication aiguë au phosphure d'aluminium (Phostoxin®) [Severity factors of aluminium phosphide poisoning (Phostoxin)]. Ann Fr Anesth Reanim. 2006;25(4):382–385. French. doi:10.1016/j.annfar.2005.12.004
18. Mehrpour O, Jafarzadeh M, Abdollahi M. A systematic review of aluminium phosphide poisoning. Arh Hig Rada Toksikol. 2012;63(1):61–73. doi:10.2478/10004-1254-63-2012-2182
19. Treatment outcome and associated factors among patients admitted with acute poisoning in a tertiary hospital in Eastern Ethiopia: a cross-sectional study - Shambel Nigussie, Fekade Demeke, Melaku Getachew, Firehiwot Amare; 2022. Available from: https://journals.sagepub.com/doi/full/10.1177/20503121221078155.
20. Aluminium and zinc phosphide poisoning: clinical Toxicology: vol 47, No 2; 2022. Available from: https://www.tandfonline.com/doi/abs/10.1080/15563650802520675?journalCode=ictx20.
 © 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2023 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.