Back to Journals » Lung Cancer: Targets and Therapy » Volume 13
ORIENT-31 as the Sakigake “Charging Samurai” Born of IMpower150 but Will MARIPOSA-2 IMPRESS in the “Meiji Modernization” of Post-3G EGFR TKI Progression?
Authors Nagasaka M , Ou SI
Received 9 January 2022
Accepted for publication 23 February 2022
Published 29 March 2022 Volume 2022:13 Pages 13—21
DOI https://doi.org/10.2147/LCTT.S355503
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Antonio Araujo
Misako Nagasaka,1– 3 Sai-hong Ignatius Ou1,2
1University of California Irvine School of Medicine, Department of Medicine, Division of Hematology-Oncology, Orange, CA, USA; 2Chao Family Comprehensive Cancer Center, Orange, CA, USA; 3St. Marianna University School of Medicine, Department of Medicine, Kawasaki, Japan
Correspondence: Misako Nagasaka, Associate Clinical Professor, University of California Irvine School of Medicine, Department of Medicine, Division of Hematology-Oncology, 101 the City Drive South, Building 200, Room 410, ZOT 4061, Orange, CA, 92868, USA, Email [email protected]
Abstract: Tyrosine kinase inhibitors (TKIs) have become the preferred first line therapy for those patients with non-small cell lung cancer (NSCLC) harboring epidermal growth factor receptor (EGFR) mutations. Given superior progression free survival (PFS) and overall survival (OS) when compared to earlier generations, third generation EGFR TKIs have become the first choice therapy in many parts of the world. Even though multiple strategies are in development to target both “on-target” and “off-target” resistance, the continuation of EGFR TKIs at the time of progression remains a controversial topic. This commentary focuses on both the ”classic” clinical trials of IMpower150 and IMPRESS and compares them to the recently reported ORIENT-31 and ongoing MARIPOSA-2 to discuss the future therapeutic strategies in the setting of progression post-third generation EGFR TKIs.
Keywords: sintilimab, ORIENT-31, EGFR+ NSCLC, bevacizumab biosimilar, EGFR TKI, third-generation EGFR TKI, T790M
Introduction
“Sakigake” literally means “forerunner”. Originating from the samurais who were the first to enter battle, sakigake is a term describing the frontrunners/pioneers and their courage towards the unknown. Sakigake is also the term used by the Japanese Pharmaceuticals and Medical Devices Agency (PMDA) to designate “breakthrough” therapy for drug approval.
This commentary reviews the evolving landscape of therapy in NSCLC patients harboring EGFR mutations who have progressed on EGFR TKIs. The current “frontier” includes different combinatory approaches both with or without continuation of EGFR TKIs.
EGFR TKI as the Standard of Care (SOC) in the First-Line (1L) Treatment of Advanced EGFR+ NSCLC
First/second-generation EGFR TKIs became the standard of care as first-line treatment of patients with advanced lung cancer harboring epidermal growth factor receptor (EGFR) mutation based on two retrospective randomized phase 3 trials1,2 and nine prospective randomized phase 3 studies.3–13 Given superior progression-free survival (PFS) over platinum-based chemotherapy in EGFR+ NSCLC as well as overall survival (OS) benefits with osimertinib over gefitinib or erlotinib, osimertinib, the prototypic 3rd-generation EGFR TKI, has now supplanted 1st-/2nd-EGFR TKI as the new SOC in EGFR+ NSCLC patients based on PFS and OS benefit from FLAURA.14,15
Resistance Mechanisms to EGFR TKIs
The main resistance mechanism to 1st generation EGFR TKI is the gatekeeper EGFR T790M mutation, while other mechanisms such as amplifications in cMET and amplifications/mutations in HER2, as well as small cell transformation exist.16,17
The mechanism of resistance to 3rd generation EGFR TKI can also be divided into EGFR dependent vs EGFR independent. On-target EGFR dependent mechanisms include the development of C797X, EGFR amplifications, and off-target or EGFR independent mechanisms include fusions (ie, RET), mutations (ie, BRAF V600E, HER2), cell cycle alterations, amplifications, and lineage plasticity.18 In certain circumstances, depending on the resistance mechanism, a targeted approach at the time of progression is technically possible, although these alterations tend to be a rare occurrence and a re-biopsy may not be possible in certain scenarios.
Continuation of EGFR TKI Post-Progression on 1G EGFR TKI: The Lesson of IMPRESS
For a while one of the important management questions upon disease progression on 1G/2G EGFR TKI was whether to continue the same EGFR TKI or not. This was based on the theoretical consideration that continual suppression of the oncogenic driver may be important given that resistance mechanisms are present in minor tumor clones and that the canonical founder EGFR mutation could still be present, although the activity of EGFR TKIs are generally limited in small cell lung cancer (SCLC) transformation.
The IMPRESS trial investigated this specific question where EGFR+ NSCLC participants were randomly assigned 1:1 to continue gefitinib 250 mg or placebo once daily in combination with six cycles of cisplatin-gemcitabine doublet chemotherapy. Surprisingly at that time, the median PFS was 5.4 months in both groups (95% CI=4.5–5.7 in the gefitinib group and 4.6–5.5 in the placebo group). Furthermore, continuation of gefitinib plus cisplatin and pemetrexed was detrimental to OS when compared with placebo plus cisplatin and pemetrexed (hazard ratio [HR]=1.44; 95% CI=1.07–1.94; P=0.016; median OS=13.4 vs 19.5 months) especially among EGFR+ NSCLC patients who harbored the EGFR T790M mutations as detected by plasma genotyping (HR=1.49; 95% CI=1.02–2.21; P=0.0432; median OS=10.8 vs 14.1 months), whereas statistical significance was not reached in T790M mutation-negative patients (HR=1.15; 95% CI=0.68–1.94; P=0.6093; median OS=21.4 vs 22.5 months).19,20 Based on the results of IMPRESS, platinum-based chemotherapy was considered the SOC while continuation of the same EGFR TKI in combination of platinum-based chemotherapy post-progression EGFR TKI was discouraged. However, with the widespread use of much more potent mutant-selective 3rd-generation EGFR TKIs that can overcome T790M mutation and have excellent CNS efficacy, and with the development of EGFR/MET bispecific antibodies,21 the results of IMPRESS need to be re-examined. IMPRESS was born of the era of 1G EGFR TKIs and lacks sophistication in the understanding of molecular features of EGFR+ NSCLC.
“On-Target” versus “Off-Target” Approaches to EGFR TKI Resistance
Now that osimertinib has been firmly established as the SOC for the first-line treatment of advanced EGFR+ NSCLC, the resistance patterns to first line EGFR TKIs have changed. Thus, in spite of the IMPRESS results, both “off-target” and “on-target”, non-biomarker and biomarker driven approaches are being vigorously tested.
“Off-Target” Approach
Clinical Signal from IMpower150, the First “Quad”
IMpower150 is a three-arm randomized phase 3 trial (1:1:1) comparing bevacizumab/carboplatin/paclitaxel (BCP) vs atezolizumab/carboplatin/paclitaxel (ACP) vs atezolizumab/bevacizumab/carboplatin/paclitaxel (ABCP) in treatment-naïve non-squamous NSCLC. The primary results indicated that ABCP conferred superior PFS and OS over BCP. Whether it is foresight or luck, the trial allowed EGFR+ and ALK+ patients who progressed on a TKI to enroll. Post-hoc analysis of the EGFR+/ALK+ population demonstrated an OS benefit with ABCP over BCP.22 This OS benefit was primarily thought to be due to patients with EGFR+ NSCLC.23 OS was NE (95% CI=NE–NE) with ABCP (N=26 of 400) vs 17.5 months (95% CI=11.7–NE) with BCP (N=32 of 400) which resulted in a HR of 0.31 (95% CI=0.11–0.83). However, although the updated analysis from the IMpower150 study24 showed that patients with EGFR mutations had numerically longer OS with the ABCP arm with a median of 26.1 months as compared to the BCP arm which reported a median of 20.3 months, the HR point estimate was 0.91 for ABCP vs BCP (95% CI=0.53–1.59), which may be a reflection of the limitations of sub-group analysis with a small number of patients or the results of patients crossing over to receive atezolizumab. Furthermore, no OS benefit was seen for atezolizumab + carboplatin + paclitaxel (ACP) compared with BCP in patients with sensitizing EGFR mutations who had received prior TKI therapy (21.4 vs 20.3 months; HR=1.16; 95% CI=0.71–1.89), hinting at the role of bevacizumab perhaps having synergistic effects to the combination of chemotherapy and ICI in this setting. Because of the post-hoc analysis of the nature, the US FDA did not approve ABCP combination for post-EGFR TKI progression, although this was approved by the European Medical Agency (EMA) for the treatment of these patients post-EGFR TKI progression. Although not officially approved by the FDA, some oncologists in the US have been using IMpower150 as the salvage therapy in the post-EGFR TKI progression setting. The adaptation of IMpower150 in many countries further solidify the concept of no continuation of EGFR TKI post-EGFR TKI progression.
ORIENT-31: The First Prospective Non-EGFR TKI Continuing “Quad”
ORIENT-31 is a phase 3 double-placebo-controlled trial that enrolled EGFR+ NSCLC patients who progressed on 1G/2G EGFR TKI without evidence of T790M or patients who progressed on osimertinib (one line of EGFR TKI). For patients who had 1G/2G TKIs who progressed due to an EGFR T790M mutation, they would have had to progress on osimertinib (two lines of EGFR TKIs). Eligible patients were randomized (1:1:1) to four cycles of cisplatin/pemetrexed, four cycles of cisplatin/pemetrexed/IBI305 (a bevacizumab biosimilar)/sintilmlimab (an PD-1 inhibitor) [arm A] vs four cycles of cisplatin/pemetrexed/sintilimab [arm B] vs four cycles of cisplatin/pemetrexed alone [arm C] (Figure 1). This was followed by maintenance pemetrexed, pemetrexed/sintilimab, or pemetrexed/sintilimab/IBI305 with single or double placebo as appropriate. Of the 444 patients enrolled, 118 (26.6%) were T790M+ and 125 (28.2%) had two prior lines of EGFR TKIs and 160 (26.0%) had brain metastasis. The majority of the patients had stage 4 disease (97.1%, 431/444) and a performance status of 1 (81.1%, 360/444).
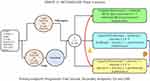 |
Figure 1 Study design of ORIENT-31. |
With a median follow-up time of 9.8 months, ORIENT-31 met its primary endpoint of PFS (by independent radiology review committee) with sintilimab + IBI305 + pemetrexed+ cisplatin (arm A) achieving a median PFS of 6.9 months (95% CI=6.0–9.3) vs 4.3 months (95% CI=4.1–5.4) for the pemetrexed + cisplatin (arm C) (HR=0.464; 95% CI=0.337–0.639; P<0.0001).25 ORR was also improved in arm A (ORR=43.9%; 95% CI=35.8–52.3) vs arm C (ORR=25.2%; 95% CI=18.5–2.9) with non-overlapping 95% confidence intervals.
The PFS benefit of the ORIENT-31 “quad” was observed irrespective of the presence (HR=0.480; 95% CI=0.291–0.793) or absence of brain metastasis (HR=0.548; 95% CI=0.375–0.803). Any PFS benefit of the ORIENT-31 “quad” among EGFR T790M+ patients was not reported but, given the small percentage of patients enrolled, this may not be interpretable one way or the other. However, the efficacy of the “quad” on T790M may be estimated from patients who received two lines of EGFR TKIs (as that means patients had prior T790M+ from 1G EGFR TKI and had to have received osimertinib as T790M should not appear if one had progressed on 3G EGFR TKI as the sole TKI). The “quad” did not achieve PFS benefit over chemotherapy among the patients who had two lines of EGFR TKIs (HR=0.719; 95% CI=0.432−.194), albeit the number of patients was limited.
The direct comparison between chemotherapy (arm C) alone vs chemotherapy + sintilimab (arm B) was not reported, which is an important result to follow up on. However, the median PFS of arm B was 5.6 months (95% CI=4.7–6.9), which was numerically higher than the median PFS of chemotherapy alone (arm C), although the 95% confidence intervals overlap.
Keynote-789 and Checkmate-722: Will the “Triples” Be Left Behind?
While we await the further maturation of the ORIENT-31 to allow for a meaningful comparison of median PFS between arm B and arm C to assess for any beneficial role of the addition of immune checkpoint inhibitors (ICI) to chemotherapy in the post-EGFR TKI setting, two global randomized phase 3 trials (Keynote-789 and ChecMate-722) have completed accrual.
The designs of Keynote-789 (N=492, NCT03515837) and Checkmate-722 (N=365, NCT02864251) are similar between themselves (Figure 2A and B) and to ORIENT-31 (Figure 1). Importantly, patients who had disease progression post-1G EGFR TKIs with the presence of T790M mutation would have to have received osimertinib. The principle is to investigate whether the addition of a PD-1 inhibitor to platinum-pemetrexed chemotherapy will improve the PFS in patients with EGFR+ NSCLC who have progressed on EGFR TKI(s). Given that these are large scale clinical trials, the results of these two trials will dictate if and how ICI will be used alone or in combination with anti-angiogenesis agents in the backbone of chemotherapy. Anti-angiogenesis agents have on-target side-effects such as hypertension, proteinuria, and potential increased risk of bleeding. However, based on the post-hoc IMpower150 analysis and the preliminary data of ORIENT-31, it is our expectation that the addition of ICI to platinum-based chemotherapy will likely result in a numerically longer but not statistically significant improvement in median PFS over platinum-based chemotherapy alone.
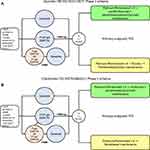 |
Figure 2 (A) Study design of Keynote-789 and (B) Checkmate-722. |
“On-Target” Non-Biomarker Driven Approach
MARIPOSA-2; the EGFR TKI Continuation Quad and “Meiji Restoration”?
MARIPOSA-2 is a randomized trial comparing platinum pemetrexed chemotherapy alone to addition of amivantamab, a bispecific EGFR/MET antibody and lazertinib, a third-generation EGFR TKI, in EGFR+ patients who had progressed on 1st or 2nd-generation EGFR TKI(s) without EGFR T790M or directly progressed from osimertinib with or without a preceding T790M mutation. The design of the trial is shown in Figure 3. While this study lacks ICI and anti-angiogenesis therapy, one can argue that amivantamab has promising efficacy data against all EGFR resistance as well as MET amplification, which is a common off-target resistance to osimertinib.21,26 Lazertinib is a potent, irreversible, mutant-selective, CNS-penetrant EGFR-TKI targeting both the T790M mutation and activating EGFR mutations while sparing wild-type EGFR and has demonstrated similar clinical efficacy including CNS activity as osimertinib.27 Furthermore, continuation of a third generation EGFR TKI (lazertinib) may have continual CNS treatment effects or may prevent/delay CNS metastases. Thus, the MARIPOSA-2 “quad” will utilize chemotherapy to overcome non-specific resistance. At the same time, amivantamab will target both on-target acquired EGFR mutations and off-target MET amplification, while lazertinib will continue to suppress pre-existing or future CNS metastasis.
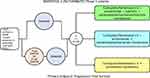 |
Figure 3 Study design of MARIPOSA-2. |
The Unanswered Question – The Role of Continuation of EGFR Blockade Post-Progression
Especially in an oligo-progressive situation, the continuation of EGFR TKI(s) beyond progression combined with localized therapy to the oligo-progressive site (ie, radiation therapy) has been accepted as a practical approach.28 TKI continuation beyond progression is supported by previous reports of patients with indolent and small volume progression benefiting from continuation of TKIs which are thought to be suppressing the majority of tumor cells.29,30 Furthermore, if progression of disease on EGFR TKI was extra-cranial, one could argue that the continuation of the same EGFR TKI could continue to have CNS protective effects.
“On-Target” Biomarker Driven Approach
As resistance to osimertinib is likely due to a minor clone of the EGFR+ NSCLC, continuing osimertinib with the addition of a specific inhibitor to inhibit the resistance mechanism has been performed and was successful in the TATTON trial (NCT02143466) where savolitinib, a MET TKI, was added to osimertinib in resistance cases where MET amplification was detected. Thus, a larger phase 2 study of osimertinib and savolitinib in the setting of osimertinib resistance (SAVANNAH; NCT03778229) is ongoing. A multi-cohort combination study of osimertinib with various inhibitors (EGFR, ALK, MET, RET) is also enrolling (ORCHARD; NCT03944772), given receptor tyrosine kinase fusions have been reported as resistance mechanisms to EGFR TKIs.31
Epilogue – ORIENT-31 as the “Last Samurai” Giving Way to the “Meiji Modernization” in the Era of Changing Landscape of 1L EGFR+ NSCLC Treatment (FLAURA-2 and MARIPOSA-1)?
As the history of modern Japan would show, the samurai of the shogunate eventually gave way to a central rule by Emperor Meiji with westernization and industrialization leading to the rise of modern Japan, the relevance of ORIENT-31 is questionable in the era of third-generation EGFR TKIs which are EGFR mutant selective with superior CNS penetration.27
While the numbers of patients who received two lines of EGFR TKIs (including osimertinib) were limited, ORIENT-31 did not show any PFS benefit in this patient subgroup. Furthermore, the benefit of ORIENT-31 in EGFR T790M+ was not reported but based on the results of two lines of EGFR TKIs as a surrogate for T790M+ in ORIENT-31. Thus, ORIENT-31 quad is unlikely to provide PFS benefit in this setting, although with the caveat of limited patients.
Furthermore, we do not know if the PFS benefit in ORIENT-31 will translate to an eventual OS benefit. Importantly, the IMpower150 “quad” did not confer OS advantage in the EGFR+ NSCLC patient subgroup. Finally, while the PFS HR benefit in ORIENT-31 was impressive at 0.46, would an absolute increase of 2.6 months in PFS justify the likely significant addition in cost by using a bevacizumab biosimilar and a PD-1 inhibitor both upfront and in the maintenance setting?
Furthermore, the current SOC of single agent osimertinib as 1L treatment of advanced EGFR+ NSCLC may be quickly evolving. FLAURA-2 is a randomized phase 3 trial investigating the addition of platinum-pemetrexed chemotherapy to osimertinib as first-line treatment of advanced EGFR+ NSCLC (Figure 4). The primary endpoint is PFS. MARIPOSA-1 is a three-arm randomized phase 3 trial comparing the combination of lazertinib + amivantamab vs osimertinib + placebo lazertinib vs lazertinib + placebo osimertinib. The third arm (lazertinib + placebo osimertinib) was requested by the FDA (Figure 5). If either FLAURA-2 or MARIPOSA-1 (or both trials) reads out positively, these combination therapies may possibly become the SOC first line therapy in the future. The magnitude of benefit, additional costs of long duration of therapy, the constant need for IV infusions, and the invariable increase in adverse events could all further complicate the selection of post-EGFR TKI progression therapy as platinum-based chemotherapy is used in FLAURA2 and the maximum on-target EGFR suppression would have already been used in MARIPOSA-1.
 |
Figure 4 Study design of FLAURA-2. |
 |
Figure 5 Study design of MARIPOSA-1. |
We are fortunate to be treating EGFR+ patients at a time when there are many potent treatment advances. We believe that anticipating how the next SOC will evolve would require an appreciation of history. Only time will tell how ORIENT-31 will place in the pantheon of clinical trials in EGFR+ NSCLC patients.
Disclosure
Dr Misako Nagasaka reports personal fees from AstraZeneca, Caris Life Sciences, Daiichi Sankyo, Takeda, Novartis, EMD Serono, Blueprint Medicines, Janssen, Pfizer, Lilly, Genentech, Mirati; non-financial support from AnHeart Therapeutics, outside the submitted work. Prof. Dr. Sai-Hong Ignatius Ou reports personal fees from Lilly, JNJ/Janssen, Pfizer, Daiichi Sankyo, BeiGene, and Dava Oncology, outside the submitted work. The authors report no other conflicts of interest in this work.
References
1. Mok TS, Wu YL, Thongprasert S, et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med. 2009;361(10):947–957. doi:10.1056/NEJMoa0810699
2. Han JY, Park K, Kim SW, et al. First-SIGNAL: first-line single-agent iressa versus gemcitabine and cisplatin trial in never-smokers with adenocarcinoma of the lung. J Clin Oncol. 2012;30(10):1122–1128. doi:10.1200/JCO.2011.36.8456
3. Maemondo M, Inoue A, Kobayashi K, et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med. 2010;362(25):2380–2388. doi:10.1056/NEJMoa0909530
4. Mitsudomi T, Morita S, Yatabe Y, et al. Gefitinib versus cisplatin plus docetaxel in patients with non-small-cell lung cancer harbouring mutations of the epidermal growth factor receptor (WJTOG3405): an open label, randomised phase 3 trial. Lancet Oncol. 2010;11(2):121–128. doi:10.1016/S1470-2045(09)70364-X
5. Zhou C, Wu YL, Gongyan C, et al. Erlotinib versus chemotherapy as first-line treatment for patients with advanced EGFR mutation-positive non-small-cell lung cancer (OPTIMAL, CTONG-0802): a multicentre, open-label, randomised, phase 3 study. Lancet Oncol. 2011;12(8):735–742. doi:10.1016/S1470-2045(11)70184-X
6. Rosell R, Carcereny E, Gervais R, et al. Erlotinib versus standard chemotherapy as first-line treatment for European patients with advanced EGFR mutation-positive non-small-cell lung cancer (EURTAC): a multicentre, open-label, randomised phase 3 trial. Lancet Oncol. 2012;13(3):239–246. doi:10.1016/S1470-2045(11)70393-X
7. Wu YL, Zhou C, Liam CK, et al. First-line erlotinib versus gemcitabine/cisplatin in patients with advanced EGFR mutation-positive non-small-cell lung cancer: analyses from the Phase III, randomized, open-label, ENSURE study. Ann Oncol. 2015;26(9):1883–1889. doi:10.1093/annonc/mdv270
8. Shi YK, Wang L, Han BH, et al. First-line icotinib versus cisplatin/pemetrexed plus pemetrexed maintenance therapy for patients with advanced EGFR mutation-positive lung adenocarcinoma (CONVINCE): a phase 3, open-label, randomized study. Ann Oncol. 2017;28(10):2443–2450. doi:10.1093/annonc/mdx359
9. Sequist LV, Yang JC, Yamamoto N, et al. Phase III study of Afatinib or cisplatin plus pemetrexed in patients with metastatic lung adenocarcinoma with EGFR mutations. J Clin Oncol. 2013;31(27):3327–3334. doi:10.1200/JCO.2012.44.2806
10. Wu YL, Zhou C, Hu CP, et al. Afatinib versus cisplatin plus gemcitabine for first-line treatment of Asian patients with advanced non-small-cell lung cancer harbouring EGFR mutations (LUX-Lung 6): an open-label, randomised phase 3 trial. Lancet Oncol. 2014;15(2):213–222. doi:10.1016/S1470-2045(13)70604-1
11. Wu YL, Cheng Y, Zhou X, et al. Dacomitinib versus gefitinib as first-line treatment for patients with EGFR-mutation-positive non-small-cell lung cancer (ARCHER 1050): a randomised, open-label, phase 3 trial. Lancet Oncol. 2017;18(11):1454–1466. doi:10.1016/S1470-2045(17)30608-3
12. Lee CK, Davies L, Wu YL, et al. Gefitinib or Erlotinib vs Chemotherapy for EGFR Mutation-Positive Lung Cancer: individual Patient Data Meta-Analysis of Overall Survival. J Natl Cancer Inst. 2017;109(6):958. doi:10.1093/jnci/djw279
13. Hasegawa Y, Ando M, Maemondo M, et al. The role of smoking status on the progression-free survival of non-small cell lung cancer patients harboring activating epidermal growth factor receptor (EGFR) mutations receiving first-line EGFR tyrosine kinase inhibitor versus platinum doublet chemotherapy: a meta-analysis of prospective randomized trials. Oncologist. 2015;20(3):307–315. doi:10.1634/theoncologist.2014-0285
14. Soria JC, Ohe Y, Vansteenkiste J, et al. Osimertinib in Untreated EGFR-Mutated Advanced Non-Small-Cell Lung Cancer. N Engl J Med. 2018;378(2):113–125. doi:10.1056/NEJMoa1713137
15. Ramalingam SS, Vansteenkiste J, Planchard D, et al. Overall Survival with Osimertinib in Untreated, EGFR-Mutated Advanced NSCLC. N Engl J Med. 2020;382(1):41–50. doi:10.1056/NEJMoa1913662
16. Sequist LV, Waltman BA, Dias-Santagata D, et al. Genotypic and histological evolution of lung cancers acquiring resistance to EGFR inhibitors. Sci Transl Med. 2011;3(75):75ra26. doi:10.1126/scitranslmed.3002003
17. Yu HA, Arcila ME, Rekhtman N, et al. Analysis of tumor specimens at the time of acquired resistance to EGFR-TKI therapy in 155 patients with EGFR-mutant lung cancers. Clin Cancer Res. 2013;19(8):2240–2247. doi:10.1158/1078-0432.CCR-12-2246
18. Schmid S, Li JJN, Leighl N. Mechanisms of osimertinib resistance and emerging treatment options. Lung Cancer. 2020;147:123–129. doi:10.1016/j.lungcan.2020.07.014
19. Soria JC, Wu YL, Nakagawa K, et al. Gefitinib plus chemotherapy versus placebo plus chemotherapy in EGFR-mutation-positive non-small-cell lung cancer after progression on first-line gefitinib (IMPRESS): a phase 3 randomised trial. Lancet Oncol. 2015;16(8):990–998. doi:10.1016/S1470-2045(15)00121-7
20. Mok TSK, Kim SW, Wu YL. Gefitinib Plus Chemotherapy Versus Chemotherapy in Epidermal Growth Factor Receptor Mutation-Positive Non-Small-Cell Lung Cancer Resistant to First-Line Gefitinib (IMPRESS): overall Survival and Biomarker Analyses. J Clin Oncol. 2017;35(36):4027–4034. doi:10.1200/JCO.2017.73.9250
21. Haura EB, Cho BC, Lee JS, et al. JNJ-61186372 (JNJ-372), an EGFR-cMet bispecific antibody, in EGFR-driven advanced non-small cell lung cancer (NSCLC). J Clin Oncol. 2019;37:9009. doi:10.1200/JCO.2019.37.15_suppl.9009
22. Socinski MA, Jotte RM, Cappuzzo F, et al. Overall survival (OS) analysis of IMpower150, a randomized Ph 3 study of atezolizumab (atezo) + chemotherapy (chemo) ± bevacizumab (bev) vs chemo + bev in 1L nonsquamous (NSQ) NSCLC. J Clin Oncol. 2018;36:9002. doi:10.1200/JCO.2018.36.15_suppl.9002
23. Reck M, Mok TSK, Nishio M, et al. Atezolizumab plus bevacizumab and chemotherapy in non-small-cell lung cancer (IMpower150): key subgroup analyses of patients with EGFR mutations or baseline liver metastases in a randomised, open-label phase 3 trial. Lancet Respir Med. 2019;7(5):387–401. doi:10.1016/S2213-2600(19)30084-0
24. Nogami N, Barlesi F, Socinski MA, et al. IMpower150 Final Exploratory Analyses for Atezolizumab Plus Bevacizumab and Chemotherapy in Key NSCLC Patient Subgroups With EGFR Mutations or Metastases in the Liver or Brain. J Thorac Oncol. 2021.
25. Lu S. ORIENT-31: phase III study of sintilimab with or without IBI305 plus chemotherapy in patients with EGFR mutated nonsquamous NSCLC who progressed after EGFR-TKI therapy.
26. Park K, Haura EB, Leighl NB, et al. Amivantamab in EGFR Exon 20 Insertion-Mutated Non-Small-Cell Lung Cancer Progressing on Platinum Chemotherapy: initial Results From the CHRYSALIS Phase I Study. J Clin Oncol. 2021;39(30):3391–3402. doi:10.1200/JCO.21.00662
27. Nagasaka M, Zhu VW, Lim SM, et al. Beyond Osimertinib: the Development of Third-Generation EGFR Tyrosine Kinase Inhibitors For Advanced EGFR+ NSCLC. J Thorac Oncol. 2021;16(5):740–763. doi:10.1016/j.jtho.2020.11.028
28. NCCN Guidelines Non-Small Cell Lung Cancer. Version 7.2021. Available from: https://www.nccn.org/professionals/physician_gls/pdf/nscl.pdf.
29. Park K, Yu CJ, Kim SW, et al. First-line erlotinib therapy until and beyond response evaluation criteria in solid tumors progression in Asian patients with epidermal growth factor receptor mutation-positive non-small-cell lung cancer: the ASPIRATION Study. JAMA Oncol. 2016;2:305–312. doi:10.1001/jamaoncol.2015.4921
30. Yap TA, Macklin-Doherty A, Popat S. Continuing EGFR inhibition beyond progression in advanced non-small cell lung cancer. Eur J Cancer. 2017;70:12–21. doi:10.1016/j.ejca.2016.10.014
31. Zhu VW, Klempner SJ, Ou SI. Receptor Tyrosine Kinase Fusions as an Actionable Resistance Mechanism to EGFR TKIs in EGFR-Mutant Non-Small-Cell Lung Cancer. Trends Cancer. 2019;5(11):677–692. doi:10.1016/j.trecan.2019.09.008
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
