Back to Journals » Research and Reports in Urology » Volume 12
Organ-Specific Therapeutic Effect of Paclitaxel and Carboplatin Chemotherapy After Platinum-Based Chemotherapy and Pembrolizumab for Metastatic Urothelial Carcinoma
Authors Furubayashi N , Negishi T, Miura A, Nakamura N, Nakamura M
Received 2 July 2020
Accepted for publication 12 September 2020
Published 9 October 2020 Volume 2020:12 Pages 455—461
DOI https://doi.org/10.2147/RRU.S270495
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jan Colli
Nobuki Furubayashi, Takahito Negishi, Akihiro Miura, Nobutaka Nakamura, Motonobu Nakamura
Department of Urology, National Hospital Organization Kyushu Cancer Center, Fukuoka, Japan
Correspondence: Nobuki Furubayashi Tel +81-92-541-3231
Fax +81-92-551-4585
Email [email protected]
Background: To evaluate the organ-specific therapeutic effect of paclitaxel and carboplatin (TC) chemotherapy in patients who failed platinum-based chemotherapy and pembrolizumab for metastatic urothelial carcinoma (UC).
Patients and Methods: We retrospectively reviewed the data of patients with metastatic UC who had received TC chemotherapy after the failure of platinum-based chemotherapy and pembrolizumab. The RECIST 1.1 criteria were used to assess the objective response to pembrolizumab and TC chemotherapy at tumor sites.
Results: We analyzed 8 patients (male, n=5; female, n=3; median age, 65 years old). All patients except one had visceral metastasis. The median overall survival for TC was 10.9 months (95% confidence interval, 1.0‑12.7 months), and the objective response rate was 25.0% (partial response [PR]: 2 cases). The metastatic organs were the lymph nodes in 5 cases (number of tumor sites: 8), lung in 4 cases (number of tumor sites: 12), liver in 3 cases (number of tumor sites: 14), bone in 3 cases (number of tumor sites: 12), and primary lesion in 3 cases (number of tumor sites: 3). There were no cases of a complete response or progressive disease in any metastatic organs due to TC chemotherapy. A PR was seen in 2 cases of lymph node metastasis (40.0%), 2 cases of lung metastasis (50.0%), and 2 cases of liver metastasis (66.7%). All 3 cases of bone metastasis showed stable disease, as did all 3 cases of primary lesion. Improvement in the therapeutic effect of TC chemotherapy compared with pembrolizumab was observed in 2 cases (40.0%) of lymph node metastasis, 2 cases (50.0%) of lung metastasis, and 1 case (33.3%) of liver metastasis.
Conclusion: Lymph node, lung, and liver metastases may respond to TC chemotherapy, even if exacerbated with pembrolizumab after platinum-based chemotherapy in metastatic UC.
Keywords: urothelial carcinoma, platinum-based chemotherapy, pembrolizumab, paclitaxel, carboplatin, organ-specific therapeutic effect
Introduction
For many years, the initial therapeutic strategy for metastatic urothelial carcinoma (UC) has been platinum-based combination chemotherapy.1,2 The most commonly used first-line regimens now are gemcitabine plus cisplatin (GC) and/or dose-dense methotrexate, vinblastine, doxorubicin, and cisplatin in cisplatin-eligible patients. However, these treatments result in a median survival of 14–15 months.3,4
Recently, immune checkpoint blockade with antibodies targeting programmed cell death-1 (PD-1) or programmed cell death ligand 1 (PD-L1) antibodies has changed the treatment landscape for many cancers, including UC. At present, only pembrolizumab (a highly selective, humanized monoclonal IgG4κ isotype antibody against PD-1) can be used for platinum-refractory advanced UC in Japan due to the results of the KEYNOTE-045 trial.5 However, the period in which the effect of pembrolizumab treatment was better than that of other chemotherapy treatments was only approximately 3 months, and the overall response rate was only 21.1%; the outcome of advanced UC therefore remains unsatisfactory.
In addition, no standard sequent-line therapy after platinum-based chemotherapy and pembrolizumab has been established. We therefore administer TC chemotherapy as salvage chemotherapy for patients if they desire aggressive treatment. There are still few reports describing the response to chemotherapy after immune checkpoint inhibition in metastatic UC.6–8 Furthermore, to our knowledge, there are no published studies describing the organ-specific therapeutic effect of chemotherapy when administered in the setting of progression on chemotherapy and immune checkpoint inhibitor in metastatic UC.
In the present study, we retrospectively assessed the clinical outcomes of TC chemotherapy in patients who failed platinum-based chemotherapy and pembrolizumab for metastatic UC in order to clarify the organ-specific therapeutic effect of TC chemotherapy.
Patients and Methods
From January 2018 to April 2020, 22 patients received pembrolizumab for metastatic UC at our institution. Of the 13 patients who progressed after pembrolizumab, 8 received TC chemotherapy, and 5 selected best supportive care. In all patients, UC was histopathologically diagnosed, and disease progression after platinum-based chemotherapy and pembrolizumab was radiologically confirmed.9
Pembrolizumab was administered intravenously on day 1 at a dose of 200 mg, and the cycle was basically repeated every 21 days. In the TC regimen, paclitaxel (175 mg/m2) and carboplatin (area under the curve: 5) were administered by intravenous infusion on day 1. The cycle was basically repeated every 21 days. Both treatments were continued until disease progression or unacceptable adverse events occurred. Tumor measurements were generally performed by computed tomography before and after every four to six cycles of pembrolizumab and before and after every one to two cycles of TC chemotherapy, but evaluations were performed as needed when the clinical symptoms worsened.
We selected the organs for which metastases had been confirmed before TC chemotherapy as the target organs for this study. Metastatic organs that were not confirmed in multiple cases were excluded. All metastases that measured ≥5 mm in the long axis (lymph node [LN] metastases ≥15 mm in the short axis) on computed tomography were measured before and during pembrolizumab and TC chemotherapy treatment.10 The tumor burden was defined as the sum of the long axis for all non-LN metastases or the short axis of all LN metastases measured. The overall response was determined based on Response Evaluation Criteria in Solid Tumors, version 1.1 but included all measured lesions.11 For each metastatic organ, the best response was classified as a complete response (CR) (disappearance or reduction to <10 mm in the short axis for an LN metastasis), a partial response (PR) (>30% reduction), stable disease (SD) (neither a CR, PR, nor progressive disease [PD]), or PD (>20% growth).10 In addition, the treatment effect was judged to have been improved by TC therapy in cases where the treatment effect of PD and SD in pembrolizumab was superior to that of PR in TC chemotherapy.
All of the patients provided their written informed consent to participate in this study, and the study protocol was approved by the Ethics Committee of the Kyushu Cancer Center (Fukuoka, Japan) and complied with the 1964 Declaration of Helsinki and its later amendments.
Statistical Analyses
The statistical analyses were carried out using the JMP® Pro, version 14.2.0 software package (SAS Institute, Inc., Cary, NC, USA). The overall response rate (ORR) is defined as the proportion of patients who achieve a partial or complete response to TC chemotherapy. The overall survival (OS) was calculated from the day on which TC chemotherapy was started until the date of the last follow-up examination or death from any cause and was evaluated using the Kaplan–Meier method.
Results
Patient Characteristics
The clinical characteristics of the 8 (male, n=5; female, n=3; median age, 65 years old; range, 57–79 years) patients are listed in Table 1. All patients received platinum-based chemotherapy for UC and selected TC chemotherapy after the failure of pembrolizumab. According to the Eastern Cooperative Oncology Group Performance (ECOG PS), 3 (37.5%), 2 (25.0%), and 3 (37.5%) patients had PS 0, PS 1, and PS ≥2, respectively. Two patients had bladder UC, two had upper urinary tract UC, and four had both types of UC. Regarding the number of treatments attempted before pembrolizumab, 5 (62.5%), 2 (25.0%) and 1 (12.5%) patients had attempted 1, 2, and ≥3 treatments, respectively. The median time from first-line chemotherapy to TC chemotherapy was 13.1 months (range 5.4–46.7). All patients except for one had visceral metastasis.
 |
Table 1 Patients’ Characteristics |
The OS and Organ-Specific Responses to TC Chemotherapy
The median observation period from pembrolizumab and TC chemotherapy was 13.7 and 8.5 months, respectively. The OS of TC chemotherapy is shown in Figure 1. The median OS for TC was 10.9 months (95% confidence interval [CI], 1.0‑12.7 months), and the ORR was 25.0% (PR: 2 cases, SD: 5 cases, PD: 1 case). The objective tumor responses in each metastatic organ are shown in Table 2. Among the 8 patients who received TC chemotherapy after pembrolizumab, the metastatic organs were the LN in 5 cases (number of tumor sites: 8, median size: 20 mm [range 16–65 mm]), lung in 4 cases (number of tumor sites: 12, median size: 12 mm [range 6–27 mm]), liver in 3 cases (number of tumor sites: 14, median size: 24 mm [range 12–84 mm]), bone in 3 cases (number of tumor sites: 12, median size: 19 mm [range 11–40 mm]), and primary lesion in 3 cases (number of tumor sites: 3, median size: 47 mm [range 17–92 mm]).
 |
Table 2 Organ-Specific Responses to TC Chemotherapy |
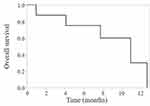 |
Figure 1 Overall survival of TC chemotherapy. |
No CR or PD was observed in any metastatic organs treated by TC chemotherapy. Regarding LN metastasis, 2 cases (40.0%) showed PR and 3 cases (60%) showed SD. Regarding lung metastasis, 2 cases (50.0%) showed PR and 2 cases (50%) showed SD. Regarding liver metastasis, 2 cases (66.7%) showed PR, and 1 case (33.3%) showed SD. Regarding bone metastasis, all three cases showed SD. Regarding primary lesion, all three cases also showed SD.
Organ-Specific Responses to TC Chemotherapy Compared with Pembrolizumab
The treatment effect of pembrolizumab and TC therapy in each metastatic organ confirmed before TC therapy is shown in Table 3. Regarding the treatment effect of pembrolizumab, 1 case (20.0%) showed PR, 2 (40.0%) showed SD, and 2 (40.0%) showed PD among cases of LN metastasis. With regard to lung metastasis, 1 case (25.0%) showed SD, and 3 cases (75.0%) showed PD. With regard to liver metastasis, 1 case (33.3%) showed PR, and 2 cases (66.7%) showed PD. With regard to bone metastasis, all 3 cases showed SD. With regard to primary lesion, all 3 cases showed PD. Improvement in the therapeutic effect of TC chemotherapy compared with pembrolizumab was observed in 2 cases (40.0%) of LN metastasis, 2 cases (50.0%) of lung metastasis, and 1 case (33.3%) of liver metastasis.
 |
Table 3 Organ-Specific Responses to TC Chemotherapy Compared with Pembrolizumab |
Discussion
In the present study, we retrospectively assessed the organ-specific therapeutic effect of TC chemotherapy in patients who failed platinum-based chemotherapy and pembrolizumab for metastatic UC, as no published studies have described the organ-specific therapeutic effect of salvage chemotherapy after progression on platinum-based chemotherapy and immune checkpoint inhibitor. Improvement in the therapeutic effect of TC therapy compared with pembrolizumab was observed in 2 cases (40.0%) of LN metastasis, 2 cases (50.0%) of lung metastasis, and 1 case (33.3%) of liver metastasis in the present study.
Although metastatic UC has been treated with platinum-based chemotherapy for many years, with immunotherapy has recently been performed, the prognosis of metastatic UC remains extremely poor, and metastatic UC is largely incurable.3–5 However, no standard sequent-line therapies after platinum-based chemotherapy and immune checkpoint inhibitors have been established. In recent years, unexpected effects have been reported regarding cases of further treatment after ICI in various carcinomas, with chemotherapy as well as molecular-targeted therapy reported to demonstrate marked efficacy in this setting.12–15 Unexpected responses to cisplatin rechallenge after treatment with immune checkpoint inhibitors have been also reported in patients with metastatic UC refractory to platinum regimens.7
Since metastatic UC has a severe prognosis (patients tend to have a poor PS, and most die after second-line treatment), resulting in insufficient time to assess the therapeutic effect of re-administration of the same regimen, another regimen chemotherapy is typically selected at our institution. In our previous study, we reviewed the efficacy of TC therapy as a second-line regimen for metastatic UC showing resistance to GC chemotherapy as a first‑line chemotherapy regimen.16 The median OS for TC chemotherapy as a second-line regimen was 12.7 months (95% CI, 3.1‑25.4 months), the ORR (CR 6.2%, PR 12.5%) was 18.7%. Although the patient background in the present study differed from that in the previous study, the median OS for TC chemotherapy after platinum-based chemotherapy and pembrolizumab failure was 10.9 months (95% CI, 0.9‑12.7 months), and the ORR was 25.0% in the present study. This indicates that the present findings after platinum-based chemotherapy and pembrolizumab in a third-line or later setting were not inferior to those after platinum-based chemotherapy in a second-line setting.
However, in which metastasized organs therapeutic efficacy of salvage chemotherapy after platinum-based chemotherapy and pembrolizumab in the third-line or later setting is obtained has been unclear. Therefore, we reviewed the organ-specific therapeutic effects of pembrolizumab and TC chemotherapy according to the metastases confirmed after pembrolizumab but before TC chemotherapy. Among the eight evaluated patients, the metastatic organs were LN in five, lung in four, liver in three, bone in three, and primary lesion in three. The therapeutic effects of TC therapy by metastatic organ were not CR or PD in any metastatic organ. A PR was seen in 2 cases of lymph node metastasis (40.0%), 2 cases of lung metastasis (50.0%), and 2 cases of liver metastasis (66.7%). All 3 cases of bone metastasis showed SD, as did all 3 cases of primary lesion. In addition, we also reviewed in which metastatic organs TC chemotherapy showed a better therapeutic effect than pembrolizumab treatment. Improvement in the therapeutic effect of TC chemotherapy compared with pembrolizumab was observed in 2 cases (40.0%) of LN metastasis, 2 cases (50.0%) of lung metastasis, and 1 case (33.3%) of liver metastasis.
Immune checkpoint inhibitors induce antitumor effects by reactivating exhausted T cells, thereby rejuvenating antitumor immunity. Therefore, the differential tumor microenvironments of various organs may influence the therapeutic effect of immune checkpoint inhibitors. Recently, organ-specific tumor responses to immune checkpoint inhibitors were reported in some types of cancers (non-small-cell lung cancer, melanoma, and liver cancer), although no such findings have yet been reported in UC.10,17,18 Those studies found that immune checkpoint inhibitors had different response rates and prognoses depending on the metastatic organ, and liver metastases were also reported to be less responsive to immune checkpoint inhibitors than other metastases. Several prognostic factors have been reported to predict the prognosis of patients with UC. The presence of liver metastases has previously been reported to be a poor prognostic factor.19–21 In addition, in the Phase 3 trial KEYNOTE-045, randomization was stratified according to the presence or absence of liver metastases.5 We also reported liver metastases and time from the previous chemotherapy regimen as independent prognostic factors for patients with advanced UC receiving pembrolizumab after platinum-based chemotherapy in real-world clinical practice.22 The deep and durable responses that occur with immune checkpoint inhibitors are unique to this class of drugs. However, these responses occur only in a minority of patients with solid tumors.23 With regard to the prognosis, the period in which the effect of pembrolizumab treatment was better than that of other chemotherapy treatments was only approximately 3 months, and the ORR was only 21.1% in the phase 3 trial KEYNOTE-045.5 Although the size of other organ metastases (except for the LN, lung, and liver) did not increase during pembrolizumab treatment, if their size (in the LN, lung, and liver) had increased, then switching from pembrolizumab to salvage chemotherapy at an early stage may have been a viable treatment strategy in actual clinical practice. Despite the small number of samples in this study, we feel that these findings have clinical significance.
The biological mechanisms involved in platinum resensitization are still unclear. One hypothesis holds that immunotherapy induces tumor microenvironment modification, resulting in chemosensitivity restoration.14 A previous study systematically evaluated the duration for which the PD-1-blocking antibody pembrolizumab persisted in T cells in non-small-cell lung cancer patients and reported the loss of absolute complete binding to T cells at around 20–25 weeks.24 Another hypothesis therefore holds that pembrolizumab may also confer delayed synergism to subsequent cytotoxic therapy and contribute to the improved treatment efficacy via the overlap between circulating anti-PD1 and cytotoxic agents. In recent years, the therapeutic landscape of urothelial carcinoma has been rapidly evolving. Fibroblast growth factor receptor inhibitors, poly (ADP-ribose) polymerase inhibitors, anti-human epidermal growth factor receptor 2 agents, and antibody-drug conjugates targeting Nectin-4 are emerging as new therapeutic options.25 Notably, maintenance immunotherapy with avelumab following chemotherapy was suggested to improve the survival in advanced urothelial cancer in the Phase III JAVELIN Bladder 100 trial.26 However, chemotherapy following immune checkpoint inhibitors may remain an option for treatment in the future.
We observed a promising response rate to TC chemotherapy challenge in the present study, which is, to our knowledge, the first to describe the metastatic organ-specific therapeutic effects after platinum-based chemotherapy and immune checkpoint inhibitor for metastatic UC. Our study also showed that switching to salvage chemotherapy was a viable treatment strategy when LN, lung, or liver metastases worsened during pembrolizumab treatment. Our study is limited by its retrospective nature and its analysis of a limited number of cases in a single institution. Confirmatory studies with larger populations may be required.
Data Sharing Statement
The datasets used and/or analyzed during the current study are available from the corresponding author on reasonable request.
Ethics Approval and Consent to Participate
The present study was approved by the Institutional Review Board of National Hospital Organization Kyushu Cancer Center (2014-99), and written informed consent was obtained from the patient.
Patient Consent for Publication
The patient provided written informed consent for the publication of any associated data.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for all aspects of the work.
Funding
No funding was received.
Disclosure
The authors declare that they have no conflicts of interest for this study.
References
1. Maase HVD, Sengelov L, Roberts JT, et al. Long-term survival results of a randomized trial comparing gemcitabine plus cisplatin, with methotrexate, vinblastine, doxorubicin, plus cisplatin in patients with bladder cancer. J Clin Oncol. 2005;23(21):4602–4608. doi:10.1200/JCO.2005.07.757
2. National Comprehensive Cancer Network: Guidelines on bladder cancer. May 1, 2020. Available from: https://www.nccn.org/professionals/physician_gls/pdf/bladder.pdf.
3. Kaufman D, Raghavan D, Carducci M, et al. Phase II trial of gemcitabine plus cisplatin in patients with metastatic urothelial cancer. J Clin Oncol. 2000;18(9):1921–1927. doi:10.1200/JCO.2000.18.9.1921
4. Maase HVD, Hansen SW, Roberts JT, et al. Gemcitabine and cisplatin versus methotrexate, vinblastine, doxorubicin, and cisplatin in advanced or metastatic bladder cancer: results of a large, randomized, multinational, multicenter, Phase III Study. J Clin Oncol. 2000;18(17):3068–3077. doi:10.1200/JCO.2000.18.17.3068
5. Bellmunt J, Wit RD, Vaughn DJ, et al. Pembrolizumab as second-line therapy for advanced urothelial carcinoma. N Engl J Med. 2017;376(11):1015–1026. doi:10.1056/NEJMoa1613683
6. Szabados B, Dijk NV, Tang YZ, et al. Response rate to chemotherapy after immune checkpoint inhibition in metastatic urothelial cancer. Eur Urol. 2018;73(2):149–152. doi:10.1016/j.eururo.2017.08.022
7. Gravis G, Billon E, Baldini C, et al. Unexpected response to cisplatin rechallenge after immune checkpoint inhibitors in patients with metastatic urothelial carcinoma refractory to platinum regimen. Eur J Cancer. 2018;104:236–238. doi:10.1016/j.ejca.2018.09.002
8. Beom S-H, Rha SY, Ahn JB, Shin SJ. Response to salvage chemotherapy after progression on immune checkpoint inhibitors in patients with advanced urothelial carcinoma. Available from: https://meetinglibrary.asco.org/record/183994/abstract.
9. Moch H, Cubilla AL, Humphrey PA, Reuter VE, Ulbright TM. The 2016 WHO Classification Of Tumours Of The Urinary System And Male Genital Organs-Part A: renal, penile, and testicular tumours. Eur Urol. 2016;70(1):93–105. doi:10.1016/j.eururo.2016.02.029
10. Silva IPD, Lo S, Quek C, et al. Site-specific response patterns, pseudoprogression, and acquired resistance in patients with melanoma treated with ipilimumab combined with anti-PD-1 therapy. Cancer. 2020;126(1):86–97. doi:10.1002/cncr.32522
11. Eisenhauer EA, Therasse P, Bogaerts J, et al. New response evaluation criteria in solid tumours: revised RECIST guideline (version 1.1). Eur J Cancer. 2009;45(2):228–247. doi:10.1016/j.ejca.2008.10.026
12. Schvartsman G, Peng SA, Bis G, et al. Response rates to single-agent chemotherapy after exposure to immune checkpoint inhibitors in advanced non-small cell lung cancer. Lung Cancer. 2017;112:90–95. doi:10.1016/j.lungcan.2017.07.034
13. Park SE, Lee SH, Ahn JS, et al. Increased response rates to salvage chemotherapy administered after PD-1/PD-L1 inhibitors in patients with non–small cell lung cancer. J Thorac Oncol. 2018;13(1):106–111. doi:10.1016/j.jtho.2017.10.011
14. Saleh K, Daste A, Martin N, et al. Response to salvage chemotherapy after progression on immune checkpoint inhibitors in patients with recurrent and/or metastatic squamous cell carcinoma of the head and neck. Eur J Cancer. 2019;121:123–129. doi:10.1016/j.ejca.2019.08.026
15. Azuma T, Sugihara T, Honda S, et al. Metastatic renal cell carcinoma regains sensitivity to tyrosine kinase inhibitor after nivolumab treatment: a case report. Oncol Lett. 2019;17(4):4011–4015. doi:10.3892/ol.2019.10027
16. Furubayashi N, Negishi T, Yamashita T, et al. The combination of paclitaxel and carboplatin as second-line chemotherapy can be a preferred regimen for patients with urothelial carcinoma after the failure of gemcitabine and cisplatin chemotherapy. Mol Clin Oncol. 2017;7(6):1112–1118. doi:10.3892/mco.2017.1452
17. Schmid S, Diem S, Li Q, et al. Organ-specific response to nivolumab in patients with Non-Small Cell Lung Cancer (NSCLC). Cancer Immunol Immunother. 2018;67(12):1825–1832. doi:10.1007/s00262-018-2239-4
18. Lu L-C, Hsu C, Shao -Y-Y, et al. Differential organ-specific tumor response to immune checkpoint inhibitors in hepatocellular carcinoma. Liver Cancer. 2019;8(6):480–490. doi:10.1159/000501275
19. Bajorin DF, Dodd PM, Mazumdar M, et al. Long-term survival in metastatic transitional-cell carcinoma and prognostic factors predicting outcome of therapy. J Clin Oncol. 1999;17(10):3173–3181. doi:10.1200/JCO.1999.17.10.3173
20. Bellmunt J, Choueiri TK, Fougeray R, et al. Prognostic factors in patients with advanced transitional cell carcinoma of the urothelial tract experiencing treatment failure with platinum-containing regimens. J Clin Oncol. 2010;28(11):1850–1855. doi:10.1200/JCO.2009.25.4599
21. Sonpavde G, Pond GR, Fougeray R, et al. Time from prior chemotherapy enhances prognostic risk grouping in the second-line setting of advanced urothelial carcinoma: a retrospective analysis of pooled, prospective phase 2 trials. Eur Urol. 2013;63(4):717–723. doi:10.1016/j.eururo.2012.11.042
22. Furubayashi N, Kuroiwa K, Tokuda N, et al. Treating Japanese patients with pembrolizumab for platinum-refractory advanced urothelial carcinoma in real-world clinical practice. J Clin Med Res. 2020;12(5):300–306. doi:10.14740/jocmr4162
23. Yarchoan M, Hopkins A, Jaffee EM. Tumor mutational burden and response rate to PD-1 Inhibition. N Engl J Med. 2017;377(25):2500–2501. doi:10.1056/NEJMc1713444
24. Osa A, Uenami T, Naito Y, et al. Monitoring antibody binding to T cells in a pembrolizumab-treated patient with lung adenocarcinoma on hemodialysis. Thorac Cancer. 2019;10(11):2183–2187. doi:10.1111/1759-7714.13197
25. Mollica V, Rizzo A, Montironi R, et al. Current strategies and novel therapeutic approaches for metastatic urothelial carcinoma. Cancers (Basel). 2020;12(6):1449. doi:10.3390/cancers12061449
26. Avelumab outduels supportive care for urothelial cancer. Cancer Discov. 2020;10(7):OF4. doi: 10.1158/2159-8290.CD-NB2020-049
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
