Back to Journals » Drug Design, Development and Therapy » Volume 16
Orexin-A Reverse Bone Mass Loss Induced by Chronic Intermittent Hypoxia Through OX1R-Nrf2/HIF-1α Pathway
Authors Gu H, Ru Y , Wang W, Cai G, Gu L, Ye J , Zhang WB, Wang L
Received 20 February 2022
Accepted for publication 24 May 2022
Published 5 July 2022 Volume 2022:16 Pages 2145—2160
DOI https://doi.org/10.2147/DDDT.S363286
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Tin Wui Wong
Hong Gu,1,* Yiwen Ru,1,* Wei Wang,1,2 Guanhui Cai,1 Lanxin Gu,1 Junjie Ye,1 Wei-Bing Zhang,3,4 Lin Wang1,2
1Jiangsu Key Laboratory of Oral Diseases, Nanjing Medical University, Nanjing, People′s Republic of China; 2Department of Orthodontics, Affiliated Hospital of Stomatology, Nanjing Medical University, Nanjing, People′s Republic of China; 3Department of Stomatology, Dushu Lake Hospital Affiliated to Soochow University, Suzhou, People′s Republic of China; 4Department of Stomatology, Medical Center of Soochow University, Suzhou, People′s Republic of China
*These authors contributed equally to this work
Correspondence: Wei-Bing Zhang, Department of Stomatology, Dushu Lake Hospital Affiliated to Soochow University, 9 Chongwen Road, Suzhou, 215000, People′s Republic of China, Tel +86-512-67505200, Email [email protected] Lin Wang, Department of Orthodontics, Affiliated Hospital of Stomatology, Nanjing Medical University, 136 Hanzhong Road, Nanjing, 210029, People′s Republic of China, Tel +86-025-69593060, Email [email protected]
Background: Recent studies suggest that there is a potential connection between obstructive sleep apnea (OSA) and osteoporosis through dysregulation of bone metabolism. Orexin-A, a neuroprotective peptide secreted by the hypothalamus, is at a lower level in the plasma of OSA patients, which regulates appetite, energy expenditure and sleep-wake states. However, the protective effect of orexin-A on bone metabolism in OSA is unclear.
Purpose: To investigate whether the activation of OX1R by orexin-A can reverse bone mass loss induced by chronic intermittent hypoxia (CIH).
Methods: Mice were randomly divided into the normoxia group and CIH group. Within the CIH or normoxia groups, treatment groups were given a subcutaneous injection of either orexin-A or saline vehicle once every day for 4 weeks and then femurs were removed for micro-CT scans. Histology and immunohistochemical staining were performed to observe and calculate the changes in femurs as a result of hypoxia. Cell immunofluorescence and immunohistochemical staining were used to detect the expression of orexin receptors in MC3T3-E1 cells or in bones. CCK-8 assay, ALP assay kit and alizarin red staining were used to detect the viability, alkaline phosphatase (ALP) activity, and capacity of mineralization, respectively. The effect of orexin-A on osteogenic differentiation of MC3T3-E1 cells was evaluated using qRT-PCR, Western blot and cell staining.
Results: CIH led to a decrease in the amount and density of trabecular bone, downregulated OCN expression while increasing osteoclast numbers in femurs and inhibited the expression of RUNX2, OSX, OPN and Nrf2 in MC3T3-E1 cells. Orexin-A treatment alleviated these CIH-induced effects by combining to OX1R. The level of HIF-1α was elevated both in CIH and orexin-A treatment groups.
Conclusion: CIH environment inhibits osteogenesis and orexin-A can reverse bone mass loss induced by CIH through OX1R-Nrf2/HIF-1α pathway.
Keywords: obstructive sleep apnea, dysregulation, bone metabolism, osteogenesis
Introduction
Obstructive sleep apnea (OSA), a sleeping disorder linked to many other diseases, is characterized by intermittent and recurrent upper airway obstruction during sleep.1 Patients with OSA have a higher probability of suffering from cardiovascular diseases, neurocognitive dysfunction, metabolic disturbances, and the condition can also result in sudden death.2 Chronic intermittent hypoxia (CIH), a typical pathophysiological hallmark of obstructive sleep apnea, is a pathogenic factor because CIH-induced hypoxia/reoxygenation can produce lots of reactive oxygen species.3 Chronic intermittent hypoxia has been proved to cause oxidative stress, increase ROS levels and induce HIF-1α expression.4 HIF-1α transcriptional activity can be powerfully promoted by hypoxia, which may in turn activate inflammatory cytokines.5 In addition, chronic intermittent hypoxia can elevate sympathetic activity.6 Previous research on OSA mainly focused on respiratory, cardiovascular and neurodegenerative diseases, but bone metabolism of OSA has rarely been studied. Recent studies suggest that there is a potential connection between OSA and osteoporosis through dysregulation of bone metabolism.7,8 It is reported that chronic intermittent hypoxia can increase bone destruction and disrupt the dynamic balance between osteoblasts and osteoclasts,9 which may result from enhanced oxidative stress, sympathetic nervous activity, inflammatory reaction and HIF-1α expression and then probably lead to lower bone mass, worse skeletal microstructure, increased bone fragility and secondary osteoporosis.10 However, the intrinsic mechanism of the bone mass loss induced by chronic intermittent hypoxia remains unrevealed. Therefore, research on the mechanism of bone metabolism in chronic intermittent hypoxia conditions may provide an effective approach for OSA-related metabolic bone diseases.
Orexin, also known as hypocretin, is a peptide hormone released by the hypothalamus and found in the small intestine, liver, and many other organs in the form of orexin-A and orexin-B and the two types can identify and bind to both OX1R and OX2R. Upon orexin binding to OX1R or OX2R, orphan G protein-coupled receptors, it regulates sleep-wake states, feeding behaviors and energy homeostasis.11,12 Furthermore, it is reported that orexin-A can accelerate osteoblast differentiation and matrix mineralization in the murine osteoblastic cell-line MC3T3-E1 cells in vitro13 and orexin knockout mice display a lower-bone-mass phenotype.14 Previous studies have suggested that orexin-A expression affects the occurrence of airway diseases, such as OSA, chronic obstructive pulmonary disease, and narcolepsy.15 So we hypothesize that orexin-A may impact osteogenesis in OSA patients. However, there is no comprehensive estimate of the protective effect of orexin-A on bone metabolism in CIH.
CIH is similar to the process of ischemia-reperfusion, and the body will produce a series of oxygen free radicals when exposed to repeated hypoxia-reoxygenation.16 Oxygen free radicals involved in CIH will induce activation of many transcriptional factors including HIF-1α, Nrf217 and so on. Hypoxia-inducible factor (HIF)-1 consists of two subunits named HIF-1α and HIF-1β, which are heavily upregulated in response to hypoxia.18 The transcription of genes coding for proteins can be activated by HIF-1 and play a role in angiogenesis, glycometabolism and cell survival.19,20 Hypoxia can promote osteogenesis through inducing angiogenesis by modulating glycolysis metabolism and promoting the formation of blood vessels. The growth of blood vessels in bone is often coupled with osteogenesis.21 Nuclear factor erythroid 2-related factor 2 (Nrf2) is considered as a vital regulator in the response to cellular response against hypoxia and is sequestered by Kelch-like ECH-associated protein 1 (Keap1) in the cytoplasm in normal resting state. When activated in hypoxia condition, Nrf2 separates from Keap1 and transfers into the nucleus.22 Nrf2 is expressed in bone cells and involved in maintaining bone homeostasis.23 The activation of Nrf2 in osteoblasts will significantly reduce apoptosis and promote osteoclast differentiation.24 Thus, we speculate that Nrf2/HIF-1α is involved in the process by which orexin-A regulates bone homeostasis and bone metabolism.
In the present study, we discovered that orexin-A could reverse bone mass loss induced by CIH and accelerate osteoblast differentiation through OX1R-Nrf2/HIF-1α pathway by using a CIH model in MC3T3-E1 cells and in C57BL/6 mice.
Materials and Methods
Cell Culture and Drug Treatment
MC3T3-E1 cells were obtained from ATCC. Cells were cultured in α-MEM (Hyclone, USA) supplemented with 10%FBS (ScienCell, USA) and 1% penicillin/streptomycin (Sigma, USA). When the cells were cultured until approximately 70% of dish was covered by cells, the culture medium was changed into osteogenic media containing 10%FBS, 1% penicillin/streptomycin, 10mM β-glycerophosphate, and 100 µg /mL ascorbic acid (Sigma-Aldrich, USA). Orexin-A peptide and OX1R specific inhibitor SB334867 (MCE, China) were stored in α-MEM. 3T3 cells were stimulated with OM in the presence or absence of orexin-A (5µM) and SB334867 (10 nM)13 for 14 days.
CIH-Exposed Cell Culture Model
Synthesis of other references, the normoxia cells were cultured at 37°C in 5% CO2, while the CIH group cells were carried out under a recurrent hypoxia environment in which O2 levels were alternated between 21% for 25 min and 1% for 35 min.25 The cells were exposed to CIH 6 times a day.
Animals Model
Animal: 8-week-old male C57BL/6 mice (from Nanjing Medical University Experimental Animal Center, Jiangsu, China) were kept under a constant temperature of 24 ±1°C. After adapting to the feeding diet for a week. At first, mice were divided into normoxia and CIH groups and then took out femurs for micro-CT scans. Afterwards, the mice were randomly divided into four groups: Normoxia mice treated with vehicle (0.9% NaCl), normoxia mice treated with orexin-A (57 ug/kg/day), CIH mice treated with vehicle (0.9% NaCl), CIH mice treated with orexin-A (57 ug/kg/day).26 During CIH exposure, mice were transferred from breeding cages to cabins (S1008, Yuyan, China). The cabins were equipped with gas and an O2 sensor in order to adjust the concentration of O2. The analyzer continuously measured and controlled oxygen concentration by a computerized system. Gas-control delivery equipment adjusted the flow of nitrogen and oxygen. During chronic intermittent hypoxia, oxygen saturation in the cabin fluctuated from 21% to 5% and back in the two-minute cycle. The mice were exposed to CIH during daytime from 9:00 am to 17:00 pm for 4 weeks.27 The normoxia group was put in the same cabin, while they were exposed to the room. This study was approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (Approval number 2106015) and conformed to the guidelines of the National Institute of Health (NIH) policies in the Guide for the Care and Use of Laboratory Animals (NIH Publications No.80-23, revised 1996).
CCK8 Assay
MC3T3-E1 cells were cultured in 96-well plates containing complete α-MEM at the density of 1000 cells/well. The cells were divided into Control group and CIH group. Each group was cultured for 0, 1, 2, 3, 5, and 7 days. Time-dependent cell viability was measured using the Cell Counting Kit-8 (CCK-8) assay (Dojindo, Japan). Each well was incubated with 10 µL of CCK8 reagent for two hours at 37°C. Optical density was measured at 450 nm to count cell viability as a percentage on a microplate reader (Spectramax, USA).
RNA Extraction and Real-Time Quantitative PCR (RT-qPCR)
RNA was isolated from MC3T3-E1 cells using Trizol reagent (Invitrogen, USA) and then we measured the purity and concentration of RNA on microplate spectrophotometer (Spectramax, USA). After the concentration was measured, cDNA was synthesized in the volume of 10 µL using PrimeScript RT reagent kit (Takara, Japan). The levels of relative transcription were measured by quantitative real-time polymerase chain reaction in ABI PRISM QuantStudio 7 Flex (Thermo Fisher, USA) according to manufacturer’s protocol. The messenger RNA (mRNA) expression levels of OX1R, OX2R, RUNX2, OPN, OSX, HIF-1α, Nrf2 were normalized with β-actin. The primers used are described in Table 1.
 |
Table 1 Primer Sequences Used for RT-PCR |
Western Blot Analysis
MC3T3-E1 cells were collected with 0.25% trypsin and then lysed using RIPA lysis buffer (Beyotime, China) for total protein. The nuclear protein was fracted with NE-PER® Nuclear and Cytoplasmic Extraction Reagents (Thermo Fisher Scientific, USA). The concentration of the protein was determined by BCA assay (Beyotime, China), total cell lysates were separated by 10% sodium dodecyl sulfate-polyacrylamide gel electrophoresis (SDS-PAGE), and then transferred to a polyvinylidene fluoride membrane. After blocking with 5% skimmed milk in Tris-buffered saline containing 0.05% Tween 20 (TBST), the membranes were incubated overnight with the following primary antibodies: anti-Runx2 (12556, CST), anti-OSX (ab22552, Abcam), anti-OPN (ab8448, Abcam), anti-Lamin B1 (ab229025, Abcam), anti-HIF-1α (20960-1-AP, Proteintech), anti-Nrf2 (ab62352, Abcam), anti-OX1R (1837-1-AP, Proteintech), anti-OX2R (PA5-77567, ThermoFisher) and anti-β-actin (23660-1-AP, Proteintech). After washing the membranes with TBST, we chose an appropriate secondary antibody to incubate the blots for one hour at room temperature. The bolts were visualized using Millipore Immobilon ECL (WBKLS0100) in Tanon 5200 chemiluminescence imaging system.
Cell Immunofluorescence
Upon the density of the cell reach to about 70%, we washed them three times with PBS and then fixed the cells with paraformaldehyde for 30 minutes. We chose the goat serum (BOSTER, AR009) to block the samples at room temperature for 30 minutes. After that, we chose the primary antibody against OX1R and OX2R for incubation at 4°C overnight. On the second day, the samples were washed 3 times in PBS. They are incubated with Cy3-labeled secondary antibody (SA00009-2, Proteintech) for one hour at 37°C. Finally, the samples were then counterstained with DAPI (VECTASHIELD, H-1500) for 30 seconds. The images were captured with an immunofluorescence microscope (Olympus, Japan).
Alkaline Phosphatase Staining and ALP Activity Assay
CIH group and normoxia group cells were washed by PBS, and then we fixed the cells with paraformaldehyde for 30 minutes at room temperature. Then, we stained the samples with BCIP/NBT Alkaline Phosphatase Color Development Kit (Beyotime, China) according to manufacturer’s protocol. The images were captured with a scanner (GE Image Scanner III). At the same time, the staining was visualized by a microscope. Ten random fields were chosen to quantify the alp enzyme using Image-J software. The cellular ALP activity was detected using Alkaline Phosphatase Assay Kit (Jiancheng, China). MC3T3-E1 cells were collected with 0.25% trypsin and then lysed in 1% triton on ice for 30 minutes. Then, we mixed 50 µL matrix liquid and 50 µL buffer solution to incubate at 37°C for 15 minutes in 96-well-plates out of light. After that, 150 µL spectrophotometric chromogenic reagent was added into the 96-well plates. Alp activity was measured using a microtiter plate spectrophotometer (Spectrama) at 520 nm.
Osteogenic Differentiation Alizarin Red Staining
MC3T3-E1 cells were cultured in plates with or without the presence of orexin-A and SB334867 for 14 days. After the 14-day osteogenic induction, cells were rinsed three times by PBS and fixed in paraformaldehyde for 30 minutes at room temperature. Then, we washed the samples three times and stained them with Alizarin Red S (Leagene, China) at room temperature. The images were captured with a scanner (GE Image Scanner III). At the same time, the staining was visualized by a microscope. Ten random areas were chosen to quantify the alp enzyme using Image-J software.
Micro-CT Scans
All the mice were sacrificed, and femurs were extracted and then fixed in paraformaldehyde overnight. The femurs of each group were taken out for micro-CT scan (Skyscan 1176, Bruker, Germany) with a voltage of 70 kVp and a current of 83 µA with a resolution of 15.6 μm after four weeks of feeding as previously described.28 The samples were enclosed in tightly fitting plastic wrap to prevent movement and dehydration during scanning. We defined the region of interest (ROI) as the distal end of the femur. Three-dimensional images were reconstructed for analysis (CTAn, Skyscan) to display the structure of trabecular bone based on five consecutive images of ROI. The most relevant parameters including trabecular thickness (Tb.Th), trabecular separation (Tb.Sp), bone volume per tissue volume (BV/TV%) and trabecular number (Tb.N) were measured.
Histology and Immunohistochemical Staining
After being fixed in paraformaldehyde overnight, the samples were decalcified in 10% EDTA and embedded in paraffin. The femurs were cut into 4-µm-thick sections. The slices were stained with hematoxylin and eosin (H&E). The trabecular structure and osteocyte lacunae were observed to evaluate the changes in bones.
In addition, tartrate-resistant acid phosphatase (TRAP) staining with TRAP kit (387A-1KTF, Sigma-Aldrich, Germany) was conducted to observe osteoclast distribution in femurs.
Immunohistochemical assays of femurs were first deparaffinized and heat-repaired for antigen retrieval. Tissue sections were incubated in 3% H2O2 for 30 minutes in order to remove endogenous peroxidase activity. After being washed three times, they were blocked in goat serum for 30 minutes at room temperature. The primary antibodies OX-1R, OX-2R and OCN were added and incubated at 4°C overnight. On the second day, the samples were washed 3 times in PBS. Then, the sections were incubated with goat anti-rabbit secondary antibody at 37°C for 30 minutes. The sections were incubated with diaminobenzidine to color the antigens. Hematoxylin was added to counterstain the nuclei. Finally, images were analyzed using Image-J software to quantify the intensities of each group.
Statistical Analysis
GraphPad Prism 8.4.0 (GraphPad Software, LLC) and SPSS 26.0 were used to analyze the statistics. The data were recorded as mean ± SD. Statistical significance was assessed with Student’s t-test to analyze the effect of normoxia and CIH in mice and cells, and the main influence of CIH exposure and orexin-A treatment was analyzed by 2 × 2 factorial ANOVA. Multiple comparisons were performed between groups using post hoc by Student-Newman-Keuls test. P < 0.05 was considered to be statistically significant. All data shown were repeated at least three times.
Results
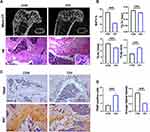
CIH Inhibits Bone Formation and Increases Bone Resorption in C57BL/6 Mice
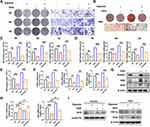
Following 4-weeks’ exposure to either CIH or normoxia conditions, histological and micro-CT scan images of femurs from the CIH and normoxia group were performed. Micro-CT results showed that the trabecular bone mass density was lower, and the trabecular microarchitecture was compromised in the CIH group as compared with the normoxia group. H&E staining demonstrated that the CIH group had a reduced number of femoral trabeculae, a lower bone structure density and a disordered arrangement of bone marrow cells. Bone trabecular structure was sparse and distributed uneven (Figure 1A). Quantitative analysis further demonstrated that Tb.Th (0.15 ± 0.003 mm vs 0.17 ± 0.03 mm, 11% absolute decrease), BV/TV% (11.51 ± 0.933% vs 25.75 ± 2.12%, 50% absolute decrease), and Tb.N (5.108 ± 0.46 mm−1 vs 6.43 ± 0.34 mm−1, 20% absolute decrease) were lower in the CIH group. Contrary to the other results, Tb.Sp (1.00 ± 0.07 mm vs 0.60 ± 0.025 mm, 60% absolute increase) was higher when mice were exposed to the CIH environment (Figure 1B). Tartrate-resistant acid phosphatase is the main marker of osteoclasts, and TRAP staining can show the shape and number of osteoclasts. TRAP staining indicated a significant increase in the number of TRAP-positive cells induced by CIH and positive cells are mainly located on the surface of bone tissue (Figure 1C and D, 168% absolute increase). The expression of OCN measured by IHC was inhibited in CIH group (Figure 1C and D, 56% absolute decrease). These results indicated that femoral morphology was changed and indicated the development of conditions for osteoporosis development in CIH mice. Therefore, we set out to explore effective measures to restore the balance of bone metabolism under CIH conditions.
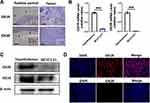
OX1R is Expressed in Femurs and MC3T3-E1 Cells
Two orexin receptor subtypes, OX1R and OX2R, exist in mammals. Upon orexin binding to OX1R or OX2R, orexin can regulate sleep-wake states, feeding behaviors and energy homeostasis. Orexin-A shows the same affinity to OX1R and OX2R. To determine the expression of the specific receptors for orexin-A in the femurs, we performed IHC staining for OX1R and OX2R. As shown in Figure 2, OX1R was expressed in the cortical substance of bone, while OX2R was expressed minimally (Figure 2A).
We evaluated whether OX1R or OX2R were expressed in MC3T3-E1 cells by using hypothalamus as a positive control. RT-PCR (Figure 2B), Western blot (Figure 2C), and immunofluorescence staining (Figure 2D) demonstrated that OX1R but not OX2R was expressed in the cells at both the mRNA and protein level. All these results showed that orexin-A might regulate bone metabolism through OX1R but not OX2R.
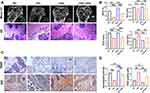
Exogenous Administration of Orexin-A Improves Bone Formation in CIH Mice
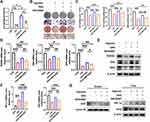
To verify the effect of orexin-A on bone metabolism, we treated mice in the normoxia and CIH groups with orexin-A or saline vehicle once every day for 4 weeks. The micro-CT scan images showed that the trabecular bone microstructure in the CIH group recovered greatly after injection with orexin-A once every day for 4 weeks compared to the mice who received saline vehicle daily, but the bone microstructure was not restored to that of the normoxia group (Figure 3A). In addition, we observed by histological methods that substantial trabecular bone filled the medullary cavity of femurs in the normoxia and CIH + orexin-A groups, while a very small amount of trabecular bone and a large amount of fractured trabecular were observed in the CIH + 0.9%NaCl group. H&E staining showed that the CIH group had a reduced amount of trabecular bone, while large amounts of trabecular bone were observed in the normoxia group, particularly in the normoxia + orexin-A group (Figure 3A). The quantitative comparison of Tb.Th, BV/TV%, Tb.N and Tb.Sp indicated that injection of orexin-A showed a positive impact on bone formation. In short, the normoxia + orexin-A group showed the best bone microscopic parameters (BV/TV: 27.52 ± 1.43%, Tb.N: 4.58 ± 0.12 mm−1, Tb.Th:0.17 ± 0.001 mm, Tb.Sp: 0.47 ± 0.01 mm), normoxia + 0.9%NaCl group came second (BV/TV: 20.80 ± 0.77%, Tb.N: 4.62 ± 0.12 mm−1, Tb.Th:0.16 ± 0.002 mm, Tb.Sp: 0.57 ± 0.02 mm), CIH+ orexin-A came third (BV/TV: 15.77 ± 1.24%, Tb.N: 3.32 ± 0.11 mm−1, Tb.Th: 0.15 ± 0.003 mm, Tb.Sp: 0.63 ±0.03 mm) and the parameters in the CIH + 0.9%NaCl group (BV/TV: 6.59 ± 0.66%, Tb.N: 3.06 ± 0.04 mm−1, Tb.Th:0.14 ± 0.001 mm, Tb.Sp: 0.77 ± 0.03 mm) showed the most compromised features (Figure 3B). The number of TRAP-positive cells was significantly decreased when treated with orexin-A both in the CIH (7.6 ± 1.1 vs 11.2 ± 1.3, absolute 32% decrease) and normoxia (2.8 ± 0.83 vs 4.6 ± 0.89, absolute 40% decrease) group (Figure 3C and D). The orexin-A-treated group showed upregulated OCN expression (Figure 3C and D) in normoxia (0.53 ± 0.01 vs 0.46 ± 0.01, absolute 13% increase) and CIH (0.41 ± 0.01 vs 0.19 ± 0.01, absolute 115% increase) group. Additionally, Micro-CT, H&E staining, TRAP staining and IHC staining were performed on femurs and more comprehensively confirmed that orexin-A can reverse loss of bone mass induced by chronic intermittent hypoxia. Orexin-A treatment alleviated these CIH-induced effects by improving microstructure, promoting the expression of OCN and decreasing the number of osteoclasts. These results indicated that orexin-A could reverse bone mass loss induced by chronic intermittent hypoxia in vivo.
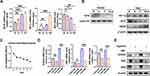
Chronic Intermittent Hypoxia Inhibits MC3T3-E1 Cell Activity and Osteogenesis
Previous studies have reported the effect of sustained hypoxia or orexin-A on murine osteoblastic cell-line MC3T3-E1 cells. To investigate the effects of CIH on the cell viability of MC3T3-E1 cells, the CCK8 assay was conducted. Results of CCK8 assay indicated that the viability of MC3T3-E1 cells was significantly inhibited after repeated exposure to the hypoxia and reoxygenation (Figure 4C). Compared to day 0, cell viability on day 1 decreased 10.79%, decreased 11.08% on day 2, decreased 15.8% on day 3, decreased 17.66% on day 5 and decreased 22.77% on day 7. The expression of HIF-1α and OX1R increased over time, while Nrf2 in both the nucleus and cytoplasm was decreased with these markers showing a time-dependent expression by RT-PCR (Figure 4A) and Western blot (Figure 4B). Therefore, we chose the day 14 timepoint of CIH for subsequent experiments. Moreover, the mRNA expression and the protein expression levels of osteogenesis markers such as OSX, RUNX2, and OPN were significantly decreased in CIH groups in both complete medium and osteogenic medium (Figure 4D and E).
Orexin-A Improves Bone Formation Through the OX1R-Nrf2/HIF-1α Pathway
Since the exogenous administration of orexin-A reduced the impact of CIH damage in vivo, we speculated that orexin-A would play a protective role in regulating osteogenesis in 3T3 cells. ALP is a typical biological marker of the early stage of osteoblast differentiation.29 Orexin-A treatment significantly increased ALP activity and the ALP positive area compared with the vehicle in both the normoxia and CIH groups on day 3, 5 and 7. In addition, we found that orexin-A increased the activity and positive area in a time-dependent manner (Figure 5A, C and D). Alizarin Red Assay showed that orexin-A promoted mineralization of MC3T3-E1 cells (Figure 5B and E). The expression of osteogenic markers increased in both complete medium and osteogenic medium with the presence of orexin-A on day 14 (Figure 5F and G). The expression of HIF-1α in MC3T3-E1 cells increased after the administration of orexin-A. In addition, Nrf2 increased both in the nucleus and in the cytoplasm (Figure 5H and I). The above results suggested that Nrf2 and HIF-1α involved in the process of orexin-mediated osteogenesis under CIH condition.
Orexin-A Combined with OX1R Alleviates the Injury Induced by CIH and Promotes Osteogenesis Through OX1R-Nrf2/HIF-1α Pathway
To verify that orexin-A reverses decreased bone mass through binding OX1R, we used the CCK8 assay to measure cell viability (Figure 6A). When exposed to CIH for 14 days, cell viability decreased to 68.51%. Due to the presence of orexin-A in the culture medium for 14 days in CIH environment, cell viability increased 33.82% compared to CIH group. Cell viability decreased 16.48% after co-treatment of orexin-A and SB334867 for 14 days in CIH environment compared to CIH+orexin-A group. The results indicated that orexin-A treatment alleviated the injury induced by CIH. What is more, after co-treatment with the OX1 receptor antagonist SB334867, the cell viability declined in vitro model, suggesting that inhibition of OX1R binding weakened the protective effect of orexin-A. In conclusion, orexin-A alleviates the injury induced by CIH and is dependent on signaling through OX1R.
Previous studies confirmed the positive effect of orexin-A on the formation of bones.14,30 In this study, we demonstrated the positive effect of orexin-A on osteogenesis. To further delineate the protective mechanism of orexin-A, we treated cells with the OX1R antagonist SB334867 to investigate whether the effect of orexin-A on osteogenesis is the result of signaling through OX1R. The co-treatment of orexin-A and SB334867 decreased the activity and positive area of ALP in MC3T3-E1 cells compared with orexin-A group on day 7 and co-treatment significantly abolished the orexin-A-induced increase in the mineralization of MC3T3-E1 cells on day 14 (Figure 6B and C).
As shown in Figure 6, co-intervention significantly decreased the mRNA expression (Figure 6D) and the protein expression levels (Figure 6E) of the osteogenesis markers RUNX2, OPN, OSX compared to the orexin-A treated group. However, the expressions of osteogenic markers in the co-intervention group were higher than in the CIH group without treatment of orexin-A or SB334867. At the same time, the mRNA expression and the protein expression levels of HIF-1α were highest in the CIH+orexin-A group, then the CIH group, with the expression lowest in the normoxia group. The nuclear and cytoplasmic expression levels of Nrf2 were lowest in the CIH group and highest in the normoxia group. Treatment with orexin-A can improve the expression amount of Nrf2, while co-treatment with SB334867 decreased the expression (Figure 6F and G).
These results revealed that orexin-A regulated osteogenesis through OX1R-mediated HIF-1α/Nrf2 pathway in CIH.
Discussion
Many people in the world suffer from OSA and the rate is increasing.31,32 Chronic intermittent hypoxia was first reported as a model for OSA in 1992.33,34 Animals exposed to cycles of intermittent hypoxia for several days were found to develop a long-term hypertensive response, similar to OSA patients.
In this study, the results of micro-CT scans and histology staining showed that exposure to CIH for 4 weeks induced bone loss in mice. When MC3T3-E1 cells were exposed to CIH for 14 days, the expression of osteogenesis markers decreased as well.
It has been suggested that chronic intermittent hypoxia, secondary inflammation, endothelial dysfunction, oxidative stress, sleep disturbances, leptin resistance, and sympathetic excitation induced by OSA all interfere with normal bone metabolism.7,35 Firstly, the damage to chronic intermittent hypoxia is much more than sustained hypoxia. Hypoxia is often accompanied by acidosis because vascular perfusion decreases and glycolytic metabolism increases. The skeleton is composed of cells, fibers and matrix. The matrix contains a large amount of solid inorganic salts and alkaline minerals. Cells in bone are so extremely sensitive to PH that alkaline mineral can eventually neutralize the metabolism of H+ if the acid-base balance is not maintained in a narrow range.36 Secondly, chronic intermittent hypoxia can interrupt angiogenesis and cause endothelial dysfunction, which decreases bone perfusion and bone mass.37 Thirdly, patients with OSA are accompanied by sleep fragmentation. The immune system will be altered, and inflammatory pathways will be amplified if sleep is disturbed.38 Osteoblasts and osteoclasts also exhibit circadian rhythms that are adversely affected by the disruption of circadian rhythms.39 Fourthly, OSA patients often accompany with changes in hormone levels, including melatonin,40 leptin41 and so on, which can influence bone metabolism. The balance of bone tissues is maintained by bone resorption by osteoclasts and bone formation by osteoblasts. When bone resorption exceeds formation, it can lead to bone diseases, particularly osteoporosis. Osteoporosis, a systemic disease characterized by a decline in bone mass density and microstructure destruction, extremely increases the risk of bone fracture.42 Treatment of osteoporosis brings a heavy burden on the economy, both to the affected families and society, so the demand for anti-osteoporosis drugs is increasing. Now drugs for osteoporosis used on the market come with side effects, high prices and other disadvantages. Therefore, effective measures to restore the balance of bone metabolism in OSA patients are of clinical significance.
Although a few studies report that chronic intermittent hypoxia in OSA brings a negative effect on bone formation, some other studies find that chronic intermittent hypoxia does no harm to the bone and it can even enhance bone fracture healing.43,44 It is critical to define key characteristics of CIH protocol, including the exposure periods per day, the duration of hypoxic episodes and hypoxia levels. Severe/chronic CIH protocols tend to be pathogenic, while beneficial effects are more likely to result from modest/acute CIH exposures.45 When exposed to chronic intermittent for a short time or at a slight/moderate hypoxia level, CIH promotes bone formation through up-regulating HIF-1α and activating angiogenesis via HIF-1α/VEGF pathway.46 Chronic intermittent hypoxia in our study was much more severe and the increasing level of HIF-1α might activate proinflammatory cytokines in turn5 which could inhibit osteogenic differentiation.47
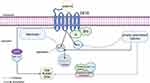
Multiple studies report that OSA patients have lower levels of orexin-A in the plasma.48–51 Additionally, previous studies show that orexin-A neurons are injured when exposed to the CIH environment.52 When orexin-A neurons are injured, the level of orexin-A decreases. It is reported that orexin-A plays an important role in reoxygenation after hypoxia53 and improves the cognitive impairment induced by chronic intermittent hypoxia.52 Thus, we hypothesize that lower bone mass due to CIH exposure can be the result of lower levels of orexin-A in plasma. Here, we demonstrated a significant contribution of orexin-A to bone formation. We identified the expression of OX1R but did not detect the presence of OX2R in femurs and 3T3 cells. After administration of orexin-A, bone formation in the normoxia and CIH groups significantly increased. Moreover, the OX1R activation induced by orexin-A binding promoted the expression of Nrf2 and HIF-1α (Figure 7).
Some studies focused on the role of orexin-A in osteogenesis are consistent with our results on the positive effect of orexin-A on osteogenesis.13,14
The function of orexin-A on osteogenesis was attributed to many aspects, and the increasing expression of Nrf2 and HIF-1α might be partly responsible. Nrf2 is a major regulator of antioxidants and bone acquisition54 and induces the expression of cytoprotective genes to fight against oxidative stress and chemical insults. It can regulate cell autophagy and prevent osteocyte death.55 Nrf2 deficiency leads to an increase in bone resorption and reduces bone formation.56 In addition, it affects apoptosis of osteoblasts and osteoclasts and decreases apoptosis.57 For these reasons, Nrf2 is considered as a critical antiosteoporotic factor.58 In our findings, when exposed to CIH environment, the proliferation of osteoblasts was inhibited in time-dependent manner and the expression of Nrf2 was downregulated gradually in vitro which revealed that oxidative stress was increased. Therefore, the activation of Nrf2 could contribute to alleviate oxidative stress and promote osteogenesis. HIF-1α is one of the master regulators of the hypoxia response and plays an important role in bone modeling, remodeling, and homeostasis through HIF-1α/VEGF pathway.46 The level of HIF-1α is low under normoxia because of the ubiquitylation and degradation of proteasome by VHL. When exposed to hypoxia environment, HIF-1α escapes from degradation and transfers to the nucleus.59 Recent reports demonstrate that the orexin-A-mediated up-regulation of HIF-1α is due to the activation of PI3K/Akt/mTOR pathway.60,61
When 3T3 cells were exposed to the chronic intermittent hypoxia, the level of HIF-1α increased in a time-dependent manner. however, the level of osteogenesis markers decreased gradually. In addition, the level of HIF-1α increased in orexin-A treated groups and promoted osteogenesis. It seemed that the results conflicted with each other. We speculated that nerve damage and bone injury caused by long-term CIH acted much more than the protective effect in bone formation caused by the increasing level of HIF-1α. When exposed to chronic intermittent hypoxia, the increased level of HIF-1α could activate proinflammatory cytokines in turn5 which could inhibit osteogenic differentiation,47 while exogenous orexin-A alleviated inflammatory response, enhanced angiogenesis and promoted osteogenesis through HIF-1α/VEGF pathway.
Our research links orexin-A, chronic intermittent hypoxia and bone formation for the first time and reveals the positive effect of orexin-A on bone formation, especially in chronic intermittent hypoxia conditions, which provides a new therapeutic method for OSA patients with bone metabolic diseases.
Despite our best efforts, our study has some limitations. First, OSA is a complex chronic disease and the CIH model cannot completely recapitulate the nuances of human pathogenesis. Secondly, CIH induces many pathophysiological processes, but due to the complexity of the underlying mechanism, lower bone mass in CIH cannot be solely attributed to the function of protection or the effect of injury. Thirdly, the role of orexin-A on osteogenesis can be attributed to pathways other than Nrf2/HIF-1α pathway, but the precise mechanisms remain unknown and further studies are necessary to clarify the exact molecular mechanisms by which orexin-A promotes osteogenesis. Here, we provide a new insight into the potential use of orexin-A as an available agent for the treatment of osteoporosis.
Conclusion
In summary, our results demonstrated that chronic intermittent hypoxia induced bone loss both in vitro and in vivo through the establishment of CIH models. Orexin-A played an important role in regulating osteogenesis through Nrf2/HIF-1α in combination of OX1R.
Abbreviations
OX1R, orexin receptor 1; OX2R, orexin receptor 2; RUNX2, runt-related transcription factor 2; OPN, osteopontin; OSX, osterix; HIF-1α, hypoxia-inducible factor-1 alpha; Nrf2, nuclear factor erythroid-derived 2-like 2; OSA, Obstructive sleep apnea; CIH, chronic intermittent hypoxia; H&E, Hematoxylin–eosin staining; TRAP, tartrate-resistant acid phosphatase staining; IHC, immunohistochemical staining; ALP, alkaline phosphatase; OCN, osteocalcin; Keap1, Kelch-like ECH-associated protein 1; ARS, alizarin red staining; Tb.Th, trabecular thickness; Tb.Sp, trabecular separation; BV/TV%, bone volume per tissue volume; Tb.N, trabecular number.
Data Sharing Statement
All data generated or analyzed during this study are included in this published article and its additional information files.
Ethics Approval and Consent to Participate
This study was approved by the Institutional Animal Care and Use Committee of Nanjing Medical University (Approval number 2106015) and conformed to the guidelines of the National Institute of Health (NIH) policies in the Guide for the Care and Use of Laboratory Animals (NIH Publications No.80-23, revised 1996).
Acknowledgments
The authors want to thank Nanjing Medical University Experiment Animal Center for supplying the animals, and Jiangsu Key Laboratory of oral diseases for the platform support.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for aspects of work.
Funding
This research was funded by the National Natural Science Foundation of China (81901067, 81870797) and Priority Academic Program Development of Jiangsu Higher Education Institutions (PAPD 2018–87).
Disclosure
The authors declare that they have no competing interests.
References
1. Lu Y, Liu Y, Li Y. Comparison of natural estrogens and synthetic derivative on genioglossus function and estrogen receptors expression in rats with chronic intermittent hypoxia. J Steroid Biochem Mol Biol. 2014;140:71–79.
2. Morselli LL, Guyon A, Spiegel K. Sleep and metabolic function. Pflugers Arch. 2012;463(1):139–160. doi:10.1007/s00424-011-1053-z
3. Lal C, Strange C, Bachman D. Neurocognitive impairment in obstructive sleep apnea. Chest. 2012;141(6):1601–1610. doi:10.1378/chest.11-2214
4. Kalogeris T, Baines CP, Krenz M, et al. Ischemia/Reperfusion. Compr Physiol. 2016;7(1):113–170. doi:10.1002/cphy.c160006
5. Wang W, Gu H, Li W, et al. SRC-3 knockout attenuates myocardial injury induced by chronic intermittent hypoxia in mice. Oxid Med Cell Longev. 2021;2021:6372430.
6. Tamisier R, Pépin JL, Rémy J, et al. 14 nights of intermittent hypoxia elevate daytime blood pressure and sympathetic activity in healthy humans. Eur Respir J. 2011;37(1):119–128. doi:10.1183/09031936.00204209
7. Swanson CM, Shea SA, Stone KL, et al. Obstructive sleep apnea and metabolic bone disease: insights into the relationship between bone and sleep. J Bone Miner Res. 2015;30(2):199–211. doi:10.1002/jbmr.2446
8. Hamada S, Ikezoe K, Hirai T, et al. Evaluation of bone mineral density by computed tomography in patients with obstructive sleep apnea. J Clin Sleep Med. 2016;12(1):25–34. doi:10.5664/jcsm.5386
9. Zhuang Y, Yan Y, Yang X, et al. Osteoporosis in a rat model co-exposed to cigarette smoke and intermittent hypoxia. Int J Chron Obstruct Pulmon Dis. 2020;15:2817–2825.
10. Boyle WJ, Simonet WS, Lacey DL. Osteoclast differentiation and activation. Nature. 2003;423(6937):337–342. doi:10.1038/nature01658
11. Sakurai T, Amemiya A, Ishii M, et al. Orexins and orexin receptors: a family of hypothalamic neuropeptides and G protein-coupled receptors that regulate feeding behavior. Cell. 1998;92(5):1 page following 696. doi:10.1016/S0092-8674(00)80949-6
12. Nixon JP, Mavanji V, Butterick TA, et al. Sleep disorders, obesity, and aging: the role of orexin. Ageing Res Rev. 2015;20:63–73.
13. Han X, Zhou J, Peng W. Orexins facilitates osteogenic differentiation of MC3T3-E1 cells. IUBMB Life. 2018;70(7):633–641. doi:10.1002/iub.1757
14. Wei W, Motoike T, Krzeszinski JY, et al. Orexin regulates bone remodeling via a dominant positive central action and a subordinate negative peripheral action. Cell Metab. 2014;19(6):927–940. doi:10.1016/j.cmet.2014.03.016
15. Saper CB, Scammell TE, Lu J. Hypothalamic regulation of sleep and circadian rhythms. Nature. 2005;437(7063):1257–1263. doi:10.1038/nature04284
16. Li L, Ren F, Qi C, et al. Intermittent hypoxia promotes melanoma lung metastasis via oxidative stress and inflammation responses in a mouse model of obstructive sleep apnea. Respir Res. 2018;19(1):28. doi:10.1186/s12931-018-0727-x
17. Kang HS, Kwon HY, Kim IK, et al. Intermittent hypoxia exacerbates tumor progression in a mouse model of lung cancer. Sci Rep. 2020;10(1):1854. doi:10.1038/s41598-020-58906-7
18. Brent MB. A review of the skeletal effects of exposure to high altitude and potential mechanisms for hypobaric hypoxia-induced bone loss. Bone. 2022;154:116258.
19. Drager J, Harvey EJ, Barralet J. Hypoxia signalling manipulation for bone regeneration. Expert Rev Mol Med. 2015;17:e6.
20. Semenza GL. Targeting HIF-1 for cancer therapy. Nat Rev Cancer. 2003;3(10):721–732. doi:10.1038/nrc1187
21. Kusumbe AP, Ramasamy SK, Adams RH. Coupling of angiogenesis and osteogenesis by a specific vessel subtype in bone. Nature. 2014;507(7492):323–328. doi:10.1038/nature13145
22. Tonelli C, Chio IIC, Tuveson DA. Transcriptional regulation by Nrf2. Antioxid Redox Signal. 2018;29(17):1727–1745. doi:10.1089/ars.2017.7342
23. Sánchez-de-Diego C, Pedrazza L, Pimenta-Lopes C, et al. NRF2 function in osteocytes is required for bone homeostasis and drives osteocytic gene expression. Redox Biol. 2021;40(17):101845. doi:10.1016/j.redox.2020.101845
24. Sun YX, Li L, Corry KA, et al. Deletion of Nrf2 reduces skeletal mechanical properties and decreases load-driven bone formation. Bone. 2015;74(1):1–9. doi:10.1016/j.bone.2014.12.066
25. Lin G, Huang J, Chen Q, et al. miR-146a-5p mediates intermittent hypoxia-induced injury in H9c2 cells by targeting XIAP. Oxid Med Cell Longev. 2019;2019(6581217):1–11. doi:10.1155/2019/6581217
26. Shin SK, Song SE, Oh JU, et al. Orexin A-induced inhibition of leptin expression and secretion in adipocytes reducing plasma leptin levels and hypothalamic leptin resistance. Pflugers Arch. 2019;471(11–12):1407–1418. doi:10.1007/s00424-019-02318-8
27. Lee EJ, Heo W, Kim JY, et al. Alteration of inflammatory mediators in the upper and lower airways under chronic intermittent hypoxia: preliminary animal study. Mediators Inflamm. 2017;2017(4327237):1–7. doi:10.1155/2017/4327237
28. Li W, Zhao J, Sun W, et al. Osteocytes promote osteoclastogenesis via autophagy-mediated RANKL secretion under mechanical compressive force. Arch Biochem Biophys. 2020;694(108594):108594. doi:10.1016/j.abb.2020.108594
29. Yang JX, Xie P, Li YS, et al. Osteoclast-derived miR-23a-5p-containing exosomes inhibit osteogenic differentiation by regulating Runx2. Cell Signal. 2020;70:109504. doi:10.1016/j.cellsig.2019.109504
30. Adeghate E, Lotfy M, D’Souza C, et al. Hypocretin/orexin modulates body weight and the metabolism of glucose and insulin. Diabetes Metab Res Rev. 2020;36(3):e3229. doi:10.1002/dmrr.3229
31. Bradley TD, Floras JS. Obstructive sleep apnoea and its cardiovascular consequences. Lancet. 2009;373(9657):82–93. doi:10.1016/S0140-6736(08)61622-0
32. Ismail K, Roberts K, Manning P, et al. OSA and pulmonary hypertension: time for a new look. Chest. 2015;147(3):847–861. doi:10.1378/chest.14-0614
33. Vilovic M, Dogas Z, Ticinovic Kurir T, et al. Bone metabolism parameters and inactive matrix Gla protein in patients with obstructive sleep apnea. Sleep. 2020;43(3). doi:10.1093/sleep/zsz243.
34. Fletcher EC, Lesske J, Qian W, et al. Repetitive, episodic hypoxia causes diurnal elevation of blood pressure in rats. Hypertension. 1992;19(6 Pt 1):555–561. doi:10.1161/01.HYP.19.6.555
35. Guo H, Zhang Y, Han T, et al. Chronic intermittent hypoxia aggravates skeletal muscle aging by down-regulating Klc1/grx1 expression via Wnt/β-catenin pathway. Arch Gerontol Geriatr. 2021;96(3):104460. doi:10.1016/j.archger.2021.104460
36. Arnett TR. Acidosis, hypoxia and bone. Arch Biochem Biophys. 2010;503(1):103–109. doi:10.1016/j.abb.2010.07.021
37. Yellowley CE, Genetos DC. Hypoxia signaling in the skeleton: implications for bone health. Curr Osteoporos Rep. 2019;17(1):26–35. doi:10.1007/s11914-019-00500-6
38. McAlpine CS, Kiss MG, Rattik S, et al. Sleep modulates haematopoiesis and protects against atherosclerosis. Nature. 2019;566(7744):383–387. doi:10.1038/s41586-019-0948-2
39. Hirai T, Tanaka K, Togari A. α1-adrenergic receptor signaling in osteoblasts regulates clock genes and bone morphogenetic protein 4 expression through up-regulation of the transcriptional factor nuclear factor IL-3 (Nfil3)/E4 promoter-binding protein 4 (E4BP4). J Biol Chem. 2014;289(24):17174–17183. doi:10.1074/jbc.M113.546135
40. Tysoe O. Melatonin prevents diabetes mellitus-induced bone loss. Nat Rev Endocrinol. 2021;17(12):707. doi:10.1038/s41574-021-00581-3
41. Yadav VK, Oury F, Suda N, et al. A serotonin-dependent mechanism explains the leptin regulation of bone mass, appetite, and energy expenditure. Cell. 2009;138(5):976–989. doi:10.1016/j.cell.2009.06.051
42. Compston JE, McClung MR, Leslie WD. Osteoporosis. Lancet. 2019;393(10169):364–376. doi:10.1016/S0140-6736(18)32112-3
43. Zhang L, Jin L, Guo J, et al. Chronic intermittent hypobaric hypoxia enhances bone fracture healing. Front Endocrinol. 2020;11:582670.
44. Bromer FD, Brent MB, Pedersen M, et al. The effect of normobaric intermittent hypoxia therapy on bone in normal and disuse osteopenic mice. High Alt Med Biol. 2021;22(2):225–234. doi:10.1089/ham.2020.0164
45. Navarrete-Opazo A, Mitchell GS. Therapeutic potential of intermittent hypoxia: a matter of dose. Am J Physiol Regul Integr Comp Physiol. 2014;307(10):R1181–97. doi:10.1152/ajpregu.00208.2014
46. Zhu J, Tang Y, Wu Q, et al. HIF-1α facilitates osteocyte-mediated osteoclastogenesis by activating JAK2/STAT3 pathway in vitro. J Cell Physiol. 2019;234(11):21182–21192. doi:10.1002/jcp.28721
47. Lacey DC, Simmons PJ, Graves SE, et al. Proinflammatory cytokines inhibit osteogenic differentiation from stem cells: implications for bone repair during inflammation. Osteoarthritis Cartilage. 2009;17(6):735–742. doi:10.1016/j.joca.2008.11.011
48. Sánchez-de-la-Torre M, Barceló A, Piérola J, et al. Plasma levels of neuropeptides and metabolic hormones, and sleepiness in obstructive sleep apnea. Respir Med. 2011;105(12):1954–1960. doi:10.1016/j.rmed.2011.08.014
49. Aksu K, Firat Güven S, Aksu F, et al. Obstructive sleep apnoea, cigarette smoking and plasma orexin-A in a sleep clinic cohort. J Int Med Res. 2009;37(2):331–340. doi:10.1177/147323000903700207
50. Busquets X, Barbé F, Barceló A, et al. Decreased plasma levels of orexin-A in sleep apnea. Respiration. 2004;71(6):575–579. doi:10.1159/000081757
51. Sakurai S, Nishijima T, Takahashi S, et al. Low plasma orexin-A levels were improved by continuous positive airway pressure treatment in patients with severe obstructive sleep apnea-hypopnea syndrome. Chest. 2005;127(3):731–737. doi:10.1378/chest.127.3.731
52. Zhu J, Tang S, Zhao D, et al. Orexin A improves the cognitive impairment induced by chronic intermittent hypoxia in mice. Brain Res Bull. 2021;173(203):203–210. doi:10.1016/j.brainresbull.2021.05.022
53. Xu D, Kong T, Zhang S, et al. Orexin-A protects against cerebral ischemia-reperfusion injury by inhibiting excessive autophagy through OX1R-mediated MAPK/ERK/mTOR pathway. Cell Signal. 2021;79(109839):109839. doi:10.1016/j.cellsig.2020.109839
54. Park CK, Lee Y, Kim KH, et al. Nrf2 is a novel regulator of bone acquisition. Bone. 2014;63(36):36–46. doi:10.1016/j.bone.2014.01.025
55. Fonseca H, Moreira-Gonçalves D, Esteves JL, et al. Voluntary exercise has long-term in vivo protective effects on osteocyte viability and bone strength following ovariectomy. Calcif Tissue Int. 2011;88(6):443–454. doi:10.1007/s00223-011-9476-2
56. Sun YX, Xu AH, Yang Y, et al. Role of Nrf2 in bone metabolism. J Biomed Sci. 2015;22(101):1–7.
57. Pellegrini GG, Morales CC, Wallace TC, et al. Avenanthramides prevent osteoblast and osteocyte apoptosis and induce osteoclast apoptosis in vitro in an Nrf2-independent manner. Nutrients. 2016;8(7):423. doi:10.3390/nu8070423
58. Chen X, Zhu X, Wei A, et al. Nrf2 epigenetic derepression induced by running exercise protects against osteoporosis. Bone Res. 2021;9(1):15. doi:10.1038/s41413-020-00128-8
59. Meijer TW, Kaanders JH, Span PN, et al. Targeting hypoxia, HIF-1, and tumor glucose metabolism to improve radiotherapy efficacy. Clin Cancer Res. 2012;18(20):5585–5594. doi:10.1158/1078-0432.CCR-12-0858
60. Wan X, Liu Y, Zhao Y, et al. Orexin A affects HepG2 human hepatocellular carcinoma cells glucose metabolism via HIF-1α-dependent and -independent mechanism. PLoS One. 2017;12(9):e0184213. doi:10.1371/journal.pone.0184213
61. Sikder D, Kodadek T. The neurohormone orexin stimulates hypoxia-inducible factor-1 activity. Genes Dev. 2007;21(22):2995–3005. doi:10.1101/gad.1584307
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.