Back to Journals » Clinical Ophthalmology » Volume 16
Orbital Complications of Acute Invasive Fungal Rhinosinusitis: A New Challenge in the COVID-19 Convalescent Patients
Authors Tadros D , Tomoum MO , Shafik HM
Received 2 October 2022
Accepted for publication 28 November 2022
Published 7 December 2022 Volume 2022:16 Pages 4011—4019
DOI https://doi.org/10.2147/OPTH.S391188
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Dina Tadros,1 Mohamed O Tomoum,2 Heba M Shafik1
1Department of Ophthalmology, Faculty of Medicine, Tanta University, Tanta, Egypt; 2Department of Otorhinolaryngology, Faculty of Medicine, Tanta University, Tanta, Egypt
Correspondence: Dina Tadros, Tanta University Hospital, Department of Ophthalmology, El-Geesh Street, Tanta, El-Gharbia, 31515, Egypt, Tel +201224093354, Email [email protected]
Purpose: Increased incidence of acute invasive fungal rhinosinusitis (AIFR) in the setting of COVID-19 is undeniable. This can be attributed to its effect on innate immunity and extensive use of corticosteroids. The goal of our study was to assess the orbital complications of AIFR and its management in the COVID-19 convalescent patients.
Methods: Our longitudinal prospective study included 45 patients with orbital complications of AIFR in recently recovered COVID-19 patients. We performed otorhinolaryngological, ophthalmological, and neurological examinations to monitor the manifestations of the disease. Computed tomography and contrast enhanced magnetic resonance imaging were performed to detect the extent of infection. Antifungal medications, surgical intervention, and general condition management were all provided to all the patients.
Results: We reported pre-septal cellulitis, orbital cellulitis, and orbital apex syndrome in 18, 13, and 10 patients, respectively. Four patients had cavernous sinus thrombosis. Mucormycosis and Aspergillus species were detected in 80% and 11.11% of our patients, respectively, while the mixed infection was found in 8.88% of our patients. Diabetes mellitus was the most common cause of immunocompromise (95.55% of our patients). Orbital pain and ophthalmoplegia were the most common ocular manifestations, followed by proptosis and relative afferent pupillary defect. All patients underwent surgical intervention, except for one patient who was unfit for surgery. One patient had orbital exenteration. The ophthalmological manifestations were reversible in cases of orbital and pre-septal cellulitis. The overall survival rate was 66.67%.
Conclusion: Early diagnosis and treatment of AIFR can decrease the morbidity and mortality rate of affected patients.
Keywords: acute invasive fungal rhinosinusitis, COVID-19, pre- septal cellulitis, orbital cellulitis, orbital apex syndrome
Introduction
Since the first case of coronavirus disease 2019 (COVID-19) caused by severe acute respiratory syndrome coronavirus 2 (SARS-CoV-2) was discovered in December 2019 in Wuhan, China, there have been discussions concerning the pathophysiology, diagnosis, therapy, and consequences of this novel condition.1 The incidence of acute invasive fungal rhinosinusitis (AIFR) has experienced a dramatic increase in patients recovering from COVID-19. Multiple fungal species have been identified in patients with AIFR, with Mucoraceae and Aspergillus being the two most common pathogens.2 Immunocompromised patients with a reduced neutrophilic response, such as those with uncontrolled diabetes, acquired immunodeficiency syndrome, or hematological malignancies and those who are organ-transplant recipients, are more vulnerable to this potentially fatal sinus infection.3 Patients on long-term steroid medication or mechanical ventilation may also be prone to these opportunistic fungal infections.4 Invasion of the orbital region occurs through bony erosion and/or vascular involvement.5 Clinically, invasive fungal rhino-orbital sinusitis manifests as numbness over the cheek, nasal blockade, crustation, proptosis, facial pain and edema, ptosis, chemosis, ophthalmoplegia, and symptoms of intracranial extension.6,7 Necrosis with black eschar in the nasal cavity and/or the hard palate are characteristic signs of such an infection.8,9 Because of the absence of a circulating antigen-detection test and a pathognomonic radiological sign. Therefore, making the diagnosis depends on clinical suspicion, collection of tissue biopsies for histopathological examination, and fungal cultures.10,11 Our study aimed to assess the orbital complications of AIFRS and its management in the early recovered COVID-19 patient to describe its burden, outcome, and the best management strategies for these patients.
Patients and Methods
This prospective longitudinal study was conducted as a collaboration between the Departments of Ophthalmology and Otorhinolaryngology at Tanta University Hospital (a tertiary referral center) from January 2020 to October 2021. Our study included 45 patients with a recent history of COVID-19 who were diagnosed with AIFR. Our study was approved by the Research Ethics Committee (REC), Tanta University, Egypt (Approval code number: 35889), and it adhered to the principles of the Declaration of Helsinki. Informed written consents were obtained from all patients before they were enrolled into the study. To publish their photographs, we obtained a signed separate consent from the patients whose images were included in our publication.
All patients underwent complete history-taking, otorhinolaryngological and ophthalmological examinations, and a neurological assessment. Laboratory investigations to identify the cause of any immunocompromising conditions, including a complete blood picture with differential to assess the absolute neutrophil count (ANC), and fasting and 2-h post-prandial blood sugar, glycosylated hemoglobin, and renal function tests, were conducted.
Computed tomography (CT) and contrast enhanced magnetic resonance imaging (MRI) were used to detect the extent of the infection and to identify orbital and intracranial affection. When cavernous sinus thrombosis was suspected, magnetic resonance venography (MRV) was performed as well. The diagnosis of AIFR was confirmed by a biopsy taken from the nasal cavity and fungal culture. All included patients had a definite history of COVID-19 diagnosed by polymerase chain reaction (PCR) and CT chest studies.
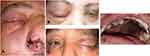
Orbital involvement was diagnosed clinically by eyelid edema, chemosis, proptosis, ophthalmoplegia, and decreased visual acuity, which was confirmed by CT and contrast enhanced MRI. Orbital apex syndrome was identified by simultaneous affection of the optic nerve and cranial nerves; these patients generally presented with a loss of vision, ptosis, relative afferent pupillary defect (RAPD), and complete internal and external ophthalmoplegia because of invasion into the optic canal and the superior orbital fissure. Patients with incomplete data and those lost to follow-up were excluded from the study.
Surgical intervention, antifungal medication, and systemic disease management were all part of our patients’ treatment plan. Antifungal medications, such as intravenous amphotericin B (1–2 mg/kg/daily) and its liposomal form (3–5 mg/kg/daily) followed by a transition to oral medications eg, voriconazole (6 mg/kg for 2 doses and then 4 mg/kg bid), and Posaconazole (400 mg twice daily), were commonly used in conjunction with therapeutic drug monitoring based on the organism found and the renal functions of the affected patients. Duration of therapy should be tailored according to the fungal pathogen, the resolution of the process, and the individual is net state of immunosuppression as guided by clinical, microbiological, or radiological means. In our series, most patients were treated for months (2–14 months). Diabetes was controlled with insulin therapy, and corticosteroids were stopped.
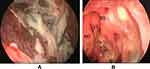
Surgical intervention was not performed until two successive negative swab results for SARS-CoV-2 infection were obtained. All patients were treated using endoscopic or combined endoscopic and open approaches according to the extent of the pathology to remove all the necrotic tissues from the nose and paranasal sinuses. Repeated surgical debridement was required in some cases, and all the debrided specimens were sent for histopathological examination and fungal culture. Anticoagulants were used to treat cerebral sinus thrombosis in collaboration with neurology experts. Patients were considered free from fungal infection after two negative histopathologic endoscopic evaluations. Surviving patients were maintained on endoscopic evaluation during the follow-up period to ensure the infection had been eradicated.
All personnel involved in the patients’ care used full protective equipment, and the hospital’s professional cleaning staff cleaned the surgical environment and all devices based on the American Academy of Ophthalmology’s disinfection recommendations12 after each procedure. Treatment success was defined by the existence of a stable, disease-free patient, while treatment failure was defined by the occurrence of any death due to intracranial extension of the infection. Descriptive data were analyzed using SPSS for Mac OS, version 26.0 (IBM Corporation, Armonk, NY, USA). Continuous variables are presented as mean ± standard deviation and range values.
Results
Our study included 45 patients with a recent history of COVID-19 who had AIFR with orbital manifestations. The diagnosis of AIFR was made at a mean point of 21.63 ± 5.21 days after testing negative via PCR for SARS-CoV-2 infection in all cases except 6 patients who developed AIFR concurrently with ongoing SARS-CoV-2 infection.
Our study included 21 male patients and 24 female patients, with a mean age of 57.17 ± 14.7 years (range, 10–76 years). Table 1 reports the demographic data and associated comorbidities of our patients.
 |
Table 1 Demographic Data and Comorbidities of Our Patients |
Diabetes mellitus was the most common cause of immunocompromise among our patients, affecting 43 of 45 individuals (95.55%) (8 patients were recently diagnosed as diabetic after receiving high corticosteroid doses during treatment for COVID-19). Thirteen patients had renal function impairment, and 2 patients had aplastic anemia.
Mucormycosis species was the most common organism in our patients (36/45, 80%), followed by Aspergillus species (5/45, 11.11%), and 4 patients (8.88%) had mixed fungal infections. The most common rhinological manifestations in our patients were: headache and facial pain (43/45, 95.55%), facial numbness (34/45, 75.55%), and nasal discharge and nasal obstruction (15/45, 33.33%). Palatal necrosis was noticed in 4 patients (8.88%), and 2 patients (4.44%) developed necrosis of the cheek and the lateral nasal wall.
Ophthalmological manifestations were divided according to presentation into pre-septal cellulitis, orbital cellulitis, orbital cellulitis associated with cavernous sinus thrombosis, and orbital apex syndrome. Orbital pain was present in all 45 patients, proptosis was found in 25 patients, ophthalmoplegia was found in 27 patients, and RAPD was found in 14 patients; additionally, fundus examination revealed central retinal artery and vein occlusion in 14 of patients. The classification of ophthalmological manifestation is presented in Table 2.
 |
Table 2 Classifications of Ophthalmological Manifestation of Our Patients |
There were variable radiological findings among our patients. CT scans showed hypoattenuating mucosal thickening and opacification of the affected paranasal sinuses in all our cases, and bone erosion in 34/45 (75.56%) of our patients. Fat stranding outside the sinus perimeter were observed in the intraorbital fat and periantral fat in 39 (86.67%) patients, and 42 (93.33%) patients, respectively. Any of the above findings raised the suspicion of acute invasive fungal rhinosinusitis in high-risk patients.
Contrast enhanced MRI was the modality of choice to evaluate soft tissue extension and invasion beyond the paranasal sinuses including intraorbital and intracranial affection. T1-weighted images showed intermediate low signal of the affected paranasal sinuses, while T2-weighted images revealed intermediate to low signal of the affected paranasal sinuses. Another benefit of contrast enhanced MRI is to detect loss of the mucosal enhancement, which can be a sign of ischemia.
The anterior ethmoid sinuses were the most affected paranasal sinuses by the fungal infection in our study (86.66% of our patients), followed by the maxillary sinuses (75.55% of our patients), while the posterior ethmoid, sphenoid, and frontal sinuses were affected by the fungal infection in 27.60%, 51.1%, and 28.8% of our patients, respectively. Orbital involvement showed evidence of orbital cellulitis in 13 patients (28.88%), dehiscence of lamina papyracea was noticed in 29 patients (64.44%), and orbital apex syndrome was noted in 10 patients (22.22%). Four patients (8.88%) showed radiological evidence of cavernous sinus thrombosis, and one patient had a frontal lobe abscess.
All our patients were admitted to the hospital for early surgical debridement, antifungal therapy, and reversal of the underlying immunodeficiency, all of which were critical components of multidisciplinary treatment.
Except for one patient who was ruled unfit for surgery, all our patients had surgical intervention. An endoscopic-debridement approach was performed in thirty-two patients, while twelve patients underwent a combined (endoscopic and open) approach, and one patient received no surgical intervention at all as he was deemed medically unfit for surgery. Only one patient required orbital exenteration. Despite medical and surgical management, fifteen patients died from bad general condition and complications of intracranial affection.
Debriding of the necrotic sinonasal tissue was performed until bleeding is seen, with repeated surgical debridement as needed. Infected bone may be discolored or just appear thinner and weaker than healthy bone. Extended endoscopic approach was performed to remove all the infected sinus mucosa eg Endoscopic medial maxillectomy, endoscopic Denker’s procedure, Caldwell-Luc operation, transmaxillary approach may be required for pterygopalatine or infratemporal fossa illness, Endoscopic Draf III may be required for frontal sinus affection.
The ophthalmological manifestations were reversible in the case of pre-septal cellulitis and orbital cellulitis, and one patient underwent exenteration of the eye. A representation of our results is shown in Figures 1–3.
Discussion
COVID-19 has a wide variety of clinical presentations, ranging from dry cough and high-grade fever to various multisystem effects, such as shortness of breath, anosmia, ageusia, diarrhea, generalized malaise, and acute cardiac injury. Patients with COVID-19 were discovered to exhibit immunosuppression due to a reduction in CD4+ and CD8+ T-cell counts, in addition to the acute respiratory distress syndromes produced by the disease.13 This leads to the potential for a wide spectrum of bacterial and fungal infections, some of which may co-exist with pre-existing morbidities (eg, diabetes mellitus, lung illness) or emerge as a hospital-acquired infection.14 AIFR was noticed with a high incidence among patients who had recently recovered from COVID-19, especially diabetic patients who had received high doses of corticosteroid therapy. Without early diagnosis and treatment, AIFR may rapidly progress, with reported mortality rates from intra-orbital and intracranial complications of 50–80%.15
AIFR should be recognized early and treated promptly to avoid life-threatening complications. Previous studies have documented a significant increase in the number of patients with AIFR after COVID-19, particularly among immunocompromised patients.16,17 AIFR usually presents with paresthesia over the cheek area; acute onset of facial pain; nasal congestion; and fever with rapid extension to the adjacent paranasal sinus, orbit, and cranial cavity. Orbital involvement can markedly affect the vision, and sinus and cranial extension can lead to proptosis and neurological manifestations.18,19
The most common rhinological manifestations in our study were headache and facial pain (95.55%), facial numbness (75.55%), and nasal discharge and nasal obstruction (33.33%). El-Naaj et al20 previously reported that facial pain and swelling were the most common symptoms of AIFR, while Krusun et al21 found that periorbital cellulitis (75%) and periorbital edema (70%) were the most common manifestations of AIFR. Meanwhile, according to another study by Ketenci et al,22 facial pain, facial swelling, and fever were the most common presentations of the AIFR, and these authors also documented 9 patients (64%) with skin and palatal involvement, while our study identified only four patients (8.88%) with palatal necrosis and two patients (4.44%) with necrosis of the cheek and the lateral nasal wall, respectively.
Diabetes mellitus was the most prevalent comorbidity (95.5%) among our patients, followed by hypertension (82.22%), renal impairment (28.8%), and aplastic anemia (0.44%). Diabetic patients have immune function impairment because of reduced chemotaxis and phagocytosis by macrophages, monocytes, and neutrophils, as well as an environment of hyperglycemia, ketosis, and low oxygen tension, which provides an excellent medium for the fungus to flourish.23 Therefore, uncontrolled diabetes is a major predisposing risk factor for the development and progression of AIFR. A previous non–COVID-19 AIFR study conducted by Krusun et al21 reported that diabetes is a major comorbidity in this patient group, followed by hematological malignancy and renal impairment, similar to the results of the study of Turner et al.24 In the wake of COVID-19, Bayram et al25 documented a diagnosis of diabetes mellitus in 72% of their patients with AIFR. They also claimed that high doses of corticosteroids in COVID-19 patients not only reduce immune system activity but also cause drug-induced hyperglycemia, which worsens the AIFR clinical course. Another study by El-Kholy et al14 revealed that diabetes mellitus and hypertension were present in 27.8% and 16.67% of patients with AIFR, respectively.
Mucormycosis is an aggressive, opportunistic fungal infection that can affect any part of the body, such as the lungs or gastrointestinal tract. The spores of the fungus are inhaled through the mouth and nose but rarely affect a person with an intact immune system. However, in the immunocompromised patient, germination and hyphae formation occur and infections develop mostly in the lungs and the paranasal sinuses. When the fungus invades the paranasal sinus mucosa, it may directly spread to the orbital apex, then gain intracerebral access. The spread of infection to the orbital cavity, orbital apex, and intracranial cavity may progress rapidly, which can threaten the patient’s vision and life.20 Many fungal species, like Rhizomucor, Aspergillus, Rhizopus, and Mucor, have been reported to cause AIFR.26 Our study demonstrated that the mucormycosis species were the most common pathogens in our study, affecting 80% of our patients, followed by Aspergillus species, which affected 11.11% of our patients, while a mixed infection of mucormycosis and Aspergillus species was reported in 8.88% of our patients. These results were concomitant with those of other studies14,21,22 that noted that mucormycosis species were the most common pathogens in patients with AIFR.
In our study, pre-septal cellulitis was found in 40% of our patients, who experienced orbital pain and periocular edema with no limitation of ocular motility. We also recorded orbital cellulitis in 28.8% of our patients, who complained of ocular pain and periocular edema in addition to ptosis, proptosis, and ophthalmoplegia. Previous literature has suggested that proptosis and ophthalmoplegia are the most common ocular manifestations of AIRS.27,28 In our study, ocular pain was found in all cases, followed by proptosis and then ophthalmoplegia, in a manner comparable to the findings of the studies by El-Kholy et al and Bakhshaee et al.14,26
About 22% of our patients had orbital apex syndrome resulting from the affection of the optic nerve and ocular motor nerves in the anatomical region of the orbital apex. Central retinal artery and vein occlusion combined with optic neuropathy were the primary causes of visual loss in our study population due to vascular occlusion causing ischemia. The angioinvasive nature of the identified fungi gives rise to a localized procoagulant state with the formation of a thrombus within the vessel lumen.29 In our study, we reported intracranial involvement in 11.11% of our patients in the form of cavernous sinus thrombosis and frontal lobe abscess associated with orbital cellulitis, which was similar to the result of a previous study conducted by Bayram et al.25 The ophthalmological manifestations identified in our study were ultimately reversible in patients with pre-septal cellulitis and orbital cellulitis because of early surgical intervention.
The radiological findings of the AIFR result from the angioinvasive behavior of the fungal infection with subsequent thrombosis and massive tissue necrosis.30 We utilized CT and contrast enhanced MRI to diagnose the AIFR and to determine the extent of the disease, however the imaging findings were not specific for the diagnosis of such disease especially in the early phases of the disease. Bone erosion and the mucosal thickening of the paranasal sinuses were sometimes very subtle and nonsignificant, as the fungi tend to extend beyond the paranasal sinuses through the blood vessels with intact bony walls. Mild periantral fat stranding was often the first sign of vascular invasion and spread beyond the paranasal sinuses. Contrast enhanced MRI was superior in evaluating the intraorbital and the intracranial extension of the infection, with a lack of enhancement that identifies ischemia zones. We used a biopsy taken from the nasal cavity and fungal culture to confirm the diagnosis of AIFR. A study by Kurubor et al31 confirmed the efficiency of fine-needle aspiration cytology in making an early diagnosis of AIFR.
In our study, the anterior ethmoidal sinuses were the most affected paranasal sinuses with the invasive fungal infection in 86.66% of our patients, followed by the maxillary sinus in 75.55% of our patients, while the frontal sinuses were the least affected paranasal sinuses with the invasive fungal infection in only 28.8% of our patients. These results were similar to those of previously published studies.25,32 Bone erosion is not the only route of the fungal spread between the orbit and nasal cavity, as the fungus may also travel through the natural foramen between the orbit and the nasal cavity or retrograde thrombophlebitis.33 We documented bone erosion of the paranasal sinuses in 75.56% of our patients, while Adulkar et al29 reported bone erosion of the paranasal sinuses in 10 (50%) patients of their study.
Surgery is a keystone for the diagnosis and treatment of AIFR, to arrest the invasion of surrounding structures and to debride the necrotic tissue. The invasive nature of the infection necessitates extensive surgical excision of all affected areas, with a focus on sinus ventilation. Furthermore, biopsies should be obtained during surgery to confirm the diagnosis. Endoscopic techniques should be tried first, but external procedures should be considered if the disease extends into the orbit and along the anterior and medial cranial fossa.
An endoscopic-debridement approach was used to treat 71.11% of our patients, while 26.6% of them underwent a combined endoscopic and open treatment approach, and only 1 patient required orbital exenteration in our study. In the context of AIFR, orbital exenteration remains a point of contention. In their studies of patients with AIFR, Hirabayashi et al34 and Athavale et al35 found no difference in mortality rates between those who had had their orbits exenterated and those who did not. Meanwhile, Terif et al36 argued that orbital exenteration should be considered for individual patients.
In patients with AIFR, previous studies21,22,37 have documented a mortality rate of about 6%–68%. In the pre–COVID-19 era, Turner et al24 and Terif et al36 reported death rates of 49.7% and 78.6% among AIFR patients, respectively. Kasapoglu et al38 stated that AIFR confined to the paranasal sinus has a favorable prognosis; however, orbital or intracranial involvement has been linked to a greater risk of fatality.38 Among patients with AIFR after COVID-19, Bayram et al25 and El-Kholy et al14 noticed mortality rates of about 63.6% and 63.89%, respectively, in their studies. The mortality rate in our study was 33.33%, as most of our cases experienced only limited infection within the nasal and paranasal sinuses with early diagnosis and aggressive debridement and medical intervention.
Conclusion
Early diagnosis of AIFR in COVID-19 patients by clinical suspicion in addition to imaging techniques is essential for achieving better visual outcomes. This, together with rapid initiation of antifungal therapy and prompt early surgical intervention, could affect the prognosis of the ocular manifestations and improve the survival rate of the patient.
Acknowledgments
The authors thank Tanta University hospitals for their support and allowing the collection of patients’ data for this research. Also, thank Charlesworth Author service for helping us in the final editing of the manuscript.
Author Contributions
All authors contributed significantly to the work reported, whether in the conception, study design, execution, acquisition of data, analysis and interpretation. They all participated in drafting, revising, or critically reviewing the article and gave final approval of the version to be published. Also, they have agreed on the journal to which the article has been submitted and agreed to be accountable for all aspects of the work.
Funding
This research did not receive any specific grant from funding agencies in the public, commercial or not-for-profit sectors.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Sharma S, Grover M, Bhargava S, Samdani S, Kataria T. Post coronavirus disease mucormycosis: a deadly addition to the pandemic spectrum. J Laryngol Otol. 2021;135(5):442–447. doi:10.1017/S0022215121000992
2. De Shazo RD, O’Brien M, Chapin K, Soto-Aguilar M, Gardner L, Swain R. A new classification and diagnostic criteria for invasive fungal sinusitis. Arch Otolaryngol Head Neck Surg. 1997;123(11):1181–1188. doi:10.1001/archotol.1997.01900110031005
3. De Shazo RD. Fungal sinusitis. Am J Med Sci. 1998;316(1):39–45. doi:10.1097/00000441-199807000-00006
4. Sarkar S, Gokhale T, Choudhury SS, Kumar Deb A. COVID-19 and orbital mucormycosis. Indian J Ophthalmol. 2021;69(4):1002–1004. doi:10.4103/ijo.IJO_3763_20
5. Levin LA, Avery R, Shore JW, Woog JJ, Baker AS. The spectrum of orbital aspergillosis: a clinicopathological review. Surv Ophthalmol. 1996;41(2):142–154. doi:10.1016/S0039-6257(96)80004-X
6. Scheckenbach K, Cornely O, Hoffmann TK, et al. Emerging therapeutic options in fulminant invasive rhinocerebral mucormycosis. Auris Nasus Larynx. 2010;37(3):322–328. doi:10.1016/j.anl.2009.09.001
7. Vairaktaris E, Moschos MM, Vassiliou S, et al. Orbital cellulitis, orbital subperiosteal and intraorbital abscess. Report of three cases and review of the literature. J Craniomaxillofac Surg. 2009;37(3):132–136. doi:10.1016/j.jcms.2008.10.007
8. Mohindra S, Mohindra S, Gupta R, Bakshi J, Gupta SK. Rhinocerebral mucormycosis: the disease spectrum in 27 patients. Mycoses. 2007;50(4):290–296. doi:10.1111/j.1439-0507.2007.01364.x
9. Munir N, Jones NS. Rhinocerebral mucormycosis with orbital and intracranial extension: a case report and review of optimum management. J Laryngol Otol. 2007;121(2):192–195. doi:10.1017/S0022215106003409
10. Szyfter W, Łaczkowska-Przybylska J. The diagnostic and therapeutical difficulties of acute (fulminant) invasive mycotic rhinosinusitis [in Polish]. Otolaryngol Pol. 2008;62:710–715. doi:10.1016/S0030-6657(08)70345-7
11. DeShazo RD, Chapin K, Swain RE. Fungal sinusitis. N Engl J Med. 1997;337(4):254–259. doi:10.1056/NEJM199707243370407
12. American Academy of Ophthalmology. Important coronavirus updates for ophthalmologists. Available from: https://www.aao.org/headline/alert-important-coronavirus-context#resuming.
13. Yang W, Cao Q, Qin L, et al. Clinical characteristics and imaging manifestations of the 2019 novel coronavirus disease (COVID-19): a multi-center study in Wenzhou city, Zhejiang, China. J Infect. 2020;80(4):388–393. doi:10.1016/j.jinf.2020.02.016
14. El-Kholy NA, El-Fattah AMA, Khafagy YW. Invasive fungal sinusitis in post COVID-19 patients: a new clinical entity. Laryngoscope. 2021;131:2652–2658. doi:10.1002/lary.29632
15. Gillespie MB, O’Malley BW. An algorithmic approach to the diagnosis and management of invasive fungal rhinosinusitis in the immunocompromised patient. Otolaryngol Clin North Am. 2000;33(2):323–334. doi:10.1016/S0030-6665(00)80008-0
16. Werthman-Ehrenreich A. Mucormycosis with orbital compartment syndrome in a patient with COVID-19. Am J Emerg Med. 2020;42:264–265.
17. Mehta S, Pandey A. Rhino-orbital mucormycosis associated with COVID-19. Cureus. 2020;12:10726.
18. Aribandi M, McCoy VA, Bazan C III. Imaging features of invasive and noninvasive fungal sinusitis: a review. Radiographics. 2007;27:1283–1296. doi:10.1148/rg.275065189
19. Momeni AK, Roberts CC, Chew FS. Imaging of chronic and exotic sinonasal disease. AJR Am J Roentgenol. 2007;189:S35–S45. doi:10.2214/AJR.07.7031
20. El-Naaj IA, Leiser Y, Wolff A, Peled M. The surgical management of rhinocerebral mucormycosis. J Craniomaxillofac Surg. 2013;41(4):291–295. doi:10.1016/j.jcms.2012.03.019
21. Kursun E, Turunc T, Demiroglu YZ, Alıskan HE, Arslan AH. Evaluation of 28 cases of mucormycosis. Mycoses. 2015;58(2):82–87. doi:10.1111/myc.12278
22. Ketenci İ, Unlü Y, Kaya H, et al. Rhinocerebral mucormycosis: experience in 14 patients. J Laryngol Otol. 2011;125(8):e3. doi:10.1017/S0022215111000843
23. Gen R, Horasan EŞ, Vaysoğlu Y, Arpaci RB, Ersöz G, Özcan C. Rhino-orbito-cerebral mucormycosis in patients with diabetic ketoacidosis. J Craniofac Surg. 2013;24(2):144–147. doi:10.1097/SCS.0b013e31827c7eb8
24. Turner JH, Soudry E, Nayak JV, Hwang PH. Survival outcomes in acute invasive fungal sinusitis: a systematic review and quantitative synthesis of published evidence. Laryngoscope. 2013;123(5):1112–1118. doi:10.1002/lary.23912
25. Bayram N, Ozsaygılı C, Sav H, et al. Susceptibility of severe COVID-19 patients to rhino-orbital mucormycosis fungal infection in different clinical manifestations. Jpn J Ophthalmol. 2021;65(4):515–525. doi:10.1007/s10384-021-00845-5
26. Bakhshaee M, Bojdi A, Allahyari A, et al. Acute invasive fungal rhinosinusitis: our experience with 18 cases. Eur Arch Otorhinolaryngol. 2016;273(12):4281–4287. doi:10.1007/s00405-016-4109-z
27. Heier JS, Gardner TA, Hawes MJ, McGuire KA, Walton WT, Stock J. Proptosis as the initial presentation of fungal sinusitis in immunocompetent patients. Ophthalmology. 1995;102:713–717. doi:10.1016/S0161-6420(95)30964-5
28. Mody KH, Ali MJ, Vemuganti GK, Nalamada S, Naik MN, Honavar SG. Orbital aspergillosis in immunocompetent patients. Br J Ophthalmol. 2014;98(10):1379–1384. doi:10.1136/bjophthalmol-2013-303763
29. Adulkar NG, Radhakrishnan S, Vidhya N, Kim U. Invasive sino-orbital fungal infections in immunocompetent patients: a clinico-pathological study. Eye. 2019;33(6):988–994. doi:10.1038/s41433-019-0358-6
30. Massry GG, Hornblass A, Harrison W. Itraconazole in the treatment of orbital aspergillosis. Ophthalmology. 1996;103(9):1467–1470. doi:10.1016/S0161-6420(96)30482-X
31. Kuruba SL, Prabhakaran VC, Nagarajappa AH, Biligi DS. Orbital aspergillus infection diagnosed by FNAC. Diagn Cytopathol. 2011;39:523–526. doi:10.1002/dc.21488
32. Bhansali A, Bhadada S, Sharma A, et al. Presentation and outcome of rhino-orbital-cerebral mucormycosis in patient with diabetes. Postgrad Med J. 2004;80:670–674. doi:10.1136/pgmj.2003.016030
33. Patel PJ, Kolawole TM, Malabarey TM, Hulailah A, Hamid F, Chakaki M. CT findings in paranasal sinus aspergillosis. Clin Radiol. 1992;45(5):319–321. doi:10.1016/S0009-9260(05)80083-2
34. Hirabayashi KE, Idowu OO, Kalin-Hajdu E, et al. Invasive fungal sinusitis: risk factors for visual acuity outcomes and mortality. Ophthalmic Plast Reconstr Surg. 2019;35(6):535–542. doi:10.1097/IOP.0000000000001357
35. Athavale DD, Jones R, O’Donnell BA, Forer M, Biggs N. Non-exenteration management of sino-orbital fungal disease. Ophthalmic Plast Reconstr Surg. 2017;33(6):426–429. doi:10.1097/IOP.0000000000000812
36. Trief D, Gray ST, Jakobiec FA, et al. Invasive fungal disease of the sinus and orbit: a comparison between mucormycosis and Aspergillus. Br J Ophthalmol. 2016;100(2):184–188. doi:10.1136/bjophthalmol-2015-306945
37. Kalin-Hajdu E, Hirabayashi KE, Vagefi MR, Kersten RC. Invasive fungal sinusitis: treatment of the orbit. Curr Opin Ophthalmol. 2017;28(5):522–533. doi:10.1097/ICU.0000000000000394
38. Kasapoglu F, Coskun H, Ozmen OA, Akalin H, Ener B. Acute invasive fungal rhinosinusitis: evaluation of 26 patients treated with endonasal or open surgical procedures. Otolaryngol Head Neck Surg. 2010;143(5):614–620. doi:10.1016/j.otohns.2010.08.017
 © 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2022 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.