Back to Journals » Clinical Ophthalmology » Volume 13
Orbital Compartment Syndrome: An Update With Review Of The Literature
Authors McCallum E, Keren S, Lapira M, Norris JH
Received 2 July 2019
Accepted for publication 3 October 2019
Published 7 November 2019 Volume 2019:13 Pages 2189—2194
DOI https://doi.org/10.2147/OPTH.S180058
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 3
Editor who approved publication: Dr Scott Fraser
Ewan McCallum, Shay Keren, Matthew Lapira, Jonathan H Norris
Oxford Eye Hospital, John Radcliffe Hospital, Oxford, UK
Correspondence: Jonathan H Norris
Oxford Eye Hospital, John Radcliffe Hospital, Oxford, UK
Email [email protected]
Abstract: Orbital compartment syndrome (OCS) is a potentially blinding condition characterized by a rapid increase in intra-orbital pressure. OCS is most commonly seen in the context of intra-orbital hemorrhage secondary to either trauma or surgery. A review of the literature indicates that better visual outcomes are achieved when interventions occur within the first 2 hrs. There are reports of visual recovery after a delay in management and consideration should be given to intervention even when presentation is delayed. Reported interventions include: lateral canthotomy with cantholysis, bony orbital decompression and treatment of the underlying cause.
Keywords: orbit, compartment, management, review
Introduction
Orbital compartment syndrome (OCS) is a sight-threatening emergency that requires urgent intervention to prevent loss of vision.1,2 OCS was first described by Gordon and McCrae in 1950, following traumatic zygoma fracture repair3 and as with other forms of compartment syndrome (e.g., affecting a limb), is associated with significant morbidity owing to rapidly increasing intra-compartment pressure. Subsequent ischemia affecting optic nerve and retinal function can result in irreversible visual loss.4
This paper discusses OCS including its potential causes, presentation, management and prognosis; and reviews the current literature.
Pathogenesis
The orbit is a confined, cone-shaped space, which apart from its anterior aspect is bound on all sides by bony walls. The orbit contains the globe, orbital fat, extraocular muscles, lacrimal gland and neurovascular anatomy.5 Anteriorly, the orbit is limited by the orbital septum and tarsal plates of the upper and lower eyelid. The average adult orbit has a volume of approximately 30mL, with an intraorbital pressure of 3–6mmHg.1,6–9 It is recognized that the orbit has limited compliance related to the limited elasticity of the septum and tarsal plates, beyond which results in an increase in intra-orbital pressure.
The exact mechanism by which OCS causes visual loss has not been fully established. Hargarden et al simulated OCS in primates, by placing a catheter in the retro-bulbar space and inflating it with saline for a minimum of 180 minutes. Following histopathological analysis, the authors concluded that visual loss occurred as a result of damage to the optic nerve from prolonged ischemia.10
Etiology
Any process that results in an increase in mass effect within the confines of the orbit can result in OCS.11 The most common precipitating event of OCS is hemorrhage secondary to trauma which may be within the orbit contents or subperiosteal.12 This is frequently seen in the presence of orbito-facial fractures.4 Ophthalmic surgical trauma is an important iatrogenic cause of orbital hemorrhage. This has been reported in orbital, eyelid and lacrimal surgery.12–14 Peribulbar or retrobulbar injections, commonly performed for anesthesia, can also lead to orbital haemorrhage.15
Non-ophthalmic procedures can also result in orbital hemorrhage. These include: sinus surgery,16,17 craniofacial surgery and neurosurgery.18 The most common ophthalmic complication of functional endoscopic sinus surgery (FESS) is OCS.16,17 Less well reported causes of retrobulbar hemorrhage include: valsalva-related hemorrhage in a patient with sino-nasal carcinoma,19 hemorrhage within an orbital lymphatic malformation20 and from orbital metastases of extra-ocular tumors.21 There has also been a report of extension of an extraorbital hematoma from the forehead into the orbit via the subgaleal space leading to OCS in a patient with factor VII deficiency.22
Accumulation of fluid within the orbit can lead to OCS in certain situations, particularly secondary to prolonged prone (typically spinal) surgery.23,24 It can also occur secondary to facial/periocular chemical burns where the tissue damage precipitates significant fluid egress which is exacerbated by loss of pliability of the eyelids25 and massive fluid resuscitation following extensive thermal burns.26 There is one case report of OCS after traumatic asphyxiation where prolonged hypoxia leads to massive capillary leak syndrome and accumulation of fluid within the orbit.27
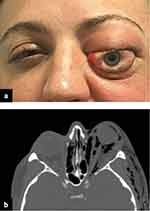
Orbital emphysema is an uncommon cause of OCS.28 It is often seen in the presence of an orbital wall fracture29,30 with a history of trauma followed by coughing, nose blowing or sneezing (Figure 1).31 It can also occur in the absence of a fracture such as following retinal surgery32,33 or injury with compressed gas.34 Fulminant orbital cellulitis, with or without concurrent orbital abscess is a recognized cause of OCS.4
Several rare iatrogenic causes of OCS have been reported in the literature. Inadvertent infiltration of the orbit with saline35 and antimicrobial ointment13 during sinus surgery was observed to immediately induce a tense orbit with proptosis. There are also reports of endovascular treatments leading to OCS. In one case this occurred during the management of a facial vascular malformation with a sclerosant; a small amount the agent unintentionally “leaked” into the orbital space causing OCS.36 In another report, OCS occurred secondary to a superior ophthalmic vein thrombosis which developed on the first day after embolization of a carotid cavernous fistula.37 The therapeutic agent was placed near to the entrance of the vein in the cavernous sinus resulting in extension of the thrombus and obstruction of venous return from the orbit.
There are further case reports of rare extra orbital and systemic conditions complicated by OCS. Bilateral OCS has been reported as an unusual complication of neuropsychiatric systemic lupus erythematosus,38 Richter syndrome39 (the transformation of chronic lymphocytic leukemia into an aggressive lymphoma) and disseminated intravascular coagulation.40 There is one case of a nasal mass rapidly expanded within 3 days of it being biopsied resulting in invasion of the orbit and OCS. Histological analysis later confirmed a diagnosis of eosinophilic angiocentric fibrosis, a rare, usually slowly progressive inflammatory mass.41
The wide spectrum of etiologies highlights the importance that ophthalmologists be alert to the possibility of OCS; not only in more common cases such as traumatic retrobulbar hemorrhage, but also in any patient presenting with reduced vision and proptosis.
Clinical Diagnosis, History And Examination
The diagnosis of OCS is often based on the history and clinical signs without the need for radiological imaging. Possible causative factors such as recent trauma, surgery, sinus disease, oncological disease or systemic inflammatory conditions should be excluded. Risk factors for exacerbation of hemorrhage also should be sought. These include blood dyscrasias or the use of anti-coagulant, anti-platelet or thrombolytic medications.42,43 Other medications that may affect bleeding such as corticosteroids, non-steroidal anti-inflammatories and herbal supplements (ginko, garlic, ginseng and bilboa) may also be relevant.4
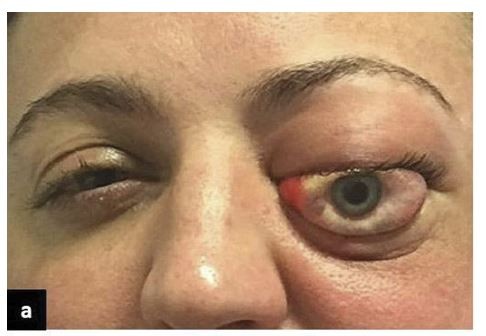
The patient will typically complain of reduced vision, pain and increased prominence of the eye or eyelids, with or without double vision.1,4,44
Examination of the patient should be performed quickly if OCS is suspected, so as not to delay treatment. The patient will usually have marked eyelid swelling or ecchymosis with a combination of proptosis, chemosis and in some cases extensive subconjunctival hemorrhage. The vision is often significantly reduced, with no perception of light (NPL) a common finding along with a relative afferent pupillary defect.1,4 Color vision can be a sensitive marker of disease severity particularly if there is moderately good vision.1,4,6 Digital ocular palpation often demonstrates resistance to retropulsion and a firm globe indicating an elevated IOP. Sensation may also be diminished in the distribution of the supra- and infra-orbital nerves. Examination of eye movements may demonstrate significant or even complete external ophthalmoplegia.
The globe itself should be examined for complications of blunt trauma or rupture dependent on the history. The posterior segment may be normal or exhibit venous congestion, arterial pulsations or retinal artery occlusion.1,4,6
Ancillary Tests
Computed tomography imaging may be helpful in establishing the diagnosis in milder cases where there is uncertainty and vision remains intact. It should not be sought when the diagnosis of OCS is clear, based on the clinical findings, so as not to delay treatment.4 After initial treatment, imaging may help locate a hematoma, emphysema, foreign body or soft tissue expansion (to some degree). This can be useful to guide when considering further decompression of the orbit after initial management.4 Straightening of the optic nerve or “globe tenting” indicates forward displacement of the globe.39 A posterior angle between the globe and sclera lower than 120° indicates severe proptosis and infers a greater risk of permanent loss of vision.4,19 Other measurements that have been postulated as predictors of outcome are globe to apex distance (longer in the affected eye) and stretch angle (the angle between the medial rectus insertion and the optic nerve).11
When a vascular abnormality is thought to be the cause of OCS, magnetic resonance imaging (MR) has an application. MR with an angiography/venography protocol may assist in finding venous or arterial malformations, lymphangiomatosis of the orbit or carotid artery pathologies.20
Management
Surgical Management
If OCS is suspected and there is evidence of optic nerve compromise, then urgent surgical decompression is the mainstay of treatment. A common first-line approach for reducing intra-orbital pressure is a lateral canthotomy and cantholysis (LC/C). This can be done expediently at the bedside under local anesthesia and has been demonstrated to effectively reduce the orbital pressure in cadaveric studies, although there is no consensus on the additional benefit of septolysis.45,46 Bony orbital decompression can be considered as an adjuvant procedure alongside LC/C, or as a secondary procedure if adequate response is not achieved after LC/C. Lee et al47 and Mootha et al48 highlighted the importance, not only of early treatment, but of ensuring that adequate decompression has occurred. Both authors report cases where early LC/C was performed (within 2 hrs) without evidence of relief of tension and with vision remaining NPL despite further surgical decompression (at 7 and 9 hrs respectively). There remains a challenge when LC/C does not adequately decompress the orbit as additional decompression often involves a general anesthetic. This invariably introduces a delay and is associated with a less favorable outcome. The decision to perform a secondary bony orbital decompression is not always straightforward; improvement in visual acuity (VA) may not be immediate after the LC/C despite an improvement in intra-orbital pressure.
Surgery should also target the primary cause of OCS. For example, OCS secondary to air, abscess or hematoma, should be drained and any bleeding source identified and cauterised. This can occur using a variety of surgical approaches, including trans-orbital,20,47,48 trans-sinus1,49,50 or trans-cranial.51–53
The authors preferred approach in suspected OCS is to perform LC/C urgently. If this does not result in a clear improvement in signs and symptoms of OCS we recommend obtaining/reviewing orbital imaging to locate the hematoma or other causative pathology. For a diffuse or medial orbital lesion, a joint approach with ENT is preferred. This allows for endonasal medial wall decompression and/or evacuation of a hematoma. For predominately superior lesions an anterior orbitotomy via an eyelid crease incision is preferred and for inferior lesions a “swinging eyelid” approach offers excellent access.
Medical Therapy
Pharmacological agents can be used as an adjunct to surgery or in very mild cases where vision is preserved. The most commonly used agents are corticosteroids, carbonic anhydrase inhibitors, osmotic agents and aqueous suppressants. All are used with the intention of reducing pressure within the orbital compartment, though their effectiveness in OCS has not been established.4
Timing Of Intervention
OCS is a rare and potentially blinding condition. Rapid diagnosis and initiation of treatment is crucial, with an aim to treat as soon as possible to maximize the chance of visual recovery. Hayreh et al reported retinal ischemia after clamping the optic nerve in rhesus monkeys resulted in irreversible damage after 105 mins but recovery occurred when clamped for less than 97 mins.54
Clinically, most patients treated within 2 hours will achieve a final Snellen visual acuity better than 6/12, though approximately 15% will be worse than 6/12.7,13,18,22,29,35,36,40,49,52,53,55–66 Patients treated after 2 hours have poorer reported outcomes with approximately 25% of patients reaching a final visual acuity of 6/12 or better.1,7,19,24,44,47,48,50,51,53,60,64,65,67–71 For those cases who achieve a favorable visual acuity despite being treated after 2 hrs, more than 50% had a presenting acuity of 6/12 or better. This suggests that in less severe cases, treatment beyond 2 hours is likely to lead to recovery of vision.
There are further reports of visual recovery from hand movements after delayed decompression at 5 days34 and even with no decompression at all.72 It is therefore still worth considering orbital decompression in cases with delayed presentation. It should be noted that whilst Snellen visual acuity is often used as a gross marker of outcome, there may be other forms of visual deficit. Maurer et al reported significant visual field defects and nerve pallor in all six of the patients in their case series, despite good post-intervention acuity.65
Recovery of vision after decompression is not always immediate29,51,55,60,62 with ongoing improvement in VA noted up to 4-week post-intervention.63 IOP can then be used as a surrogate marker of decompression after treatment.73 Most reports in the published literature provide limited follow-up period after treatment for OCS. It is reasonable to deduce that long-term follow-up may show a better final VA and that some of the data regarding milder improvement in VA may be attributable to the short follow-up period. Patients and physicians should be aware of the possibility of delayed improvement in VA.
Conclusion
This review highlights the importance of early recognition and management of OCS in order to maximize visual potential. The diagnosis is often clinical and treatment should be expedited if OCS is suspected.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Li KK, Meara JG, Rubin PA. Orbital compartment syndrome following orthognathic surgery. J Oral Maxillofac Surg. 1995;53(8):964–968. doi:10.1016/0278-2391(95)90294-5
2. Whitford R, Continenza S, Liebman J, Peng J, Powell EK, Tilney PVR. Out-of-hospital lateral canthotomy and cantholysis: a case series and screening tool for identification of orbital compartment syndrome. Air Med J. 2018;37(1):7–11. doi:10.1016/j.amj.2017.11.002
3. Gordon S, Macrea H. Monocular blindness as a complication of the treatment of malar fracture. Plast Reconstr Surg (1946). 1950;6(3):228–232.
4. Lima V, Burt B, Leibovitch I, Prabhakaran V, Goldberg RA, Selva D. Orbital compartment syndrome: the ophthalmic surgical emergency. Surv Ophthalmol. 2009;54(4):441–449. doi:10.1016/j.survophthal.2009.04.005
5. Heinze JB, Hueston JT. Blindness after blepharoplasty: mechanism and early reversal. Plast Reconstr Surg. 1978;61(3):347–354. doi:10.1097/00006534-197803000-00007
6. Perry M. Acute proptosis in Trauma: retrobulbar hemorrhage or orbital compartment syndrome—does it really matter? J Oral Maxillofac Surg. 2008;66(9):1913–1920. doi:10.1016/j.joms.2008.04.012
7. Sun MT, Chan WO, Selva D. Traumatic orbital compartment syndrome: importance of the lateral canthomy and cantholysis. Emerg Med Australas. 2014;26(3):274–278. doi:10.1111/1742-6723.12236
8. Kratky V, Hurwitz JJ, Avram DR. Orbital compartment syndrome. Direct measurement of orbital tissue pressure: 1. Technique. Can J Ophthalmol. 1990;25(6):293–297.
9. Riemann CD, Foster JA, Kosmorsky GS. Direct orbital manometry in patients with thyroid-associated orbitopathy. Ophthalmology. 1999;106(7):1296–1302. doi:10.1016/S0161-6420(99)00712-5
10. Hargaden M, Goldberg SH, Cunningham D, Breton ME, Griffith JW, Lang CM. Optic neuropathy following simulation of orbital hemorrhage in the nonhuman primate. Ophthal Plast Reconstr Surg. 1996;12(4):264–272. doi:10.1097/00002341-199612000-00009
11. Oester AE, Sahu P, Fowler B, Fleming JC. Radiographic predictors of visual outcome in orbital compartment syndrome. Ophthalmic Plast Reconstr Surg. 2012;28(1):7–10. doi:10.1097/IOP.0b013e31822672c4
12. Edmunds MR, Haridas AS, Morris DS, Jamalapuram K. Management of acute retrobulbar haemorrhage: a survey of non-ophthalmic emergency department physicians. Emerg Med J. 2019;36(4):245–247. doi:10.1136/emermed-2018-207937
13. Castro E, Seeley M, Kosmorsky G, Foster JA. Orbital compartment syndrome caused by intraorbital bacitracin ointment after endoscopic sinus surgery. Am J Ophthalmol. 2000;130(3):376–378. doi:10.1016/s0002-9394(00)00557-2
14. Blandford AD, Young JM, Arepalli S, Li A, Hwang CJ, Perry JD. Paracanthal “One-Snip” decompression in a cadaver model of retrobulbar hemorrhage. Ophthal Plast Reconstr Surg. 2018;34(5):428–431. doi:10.1097/IOP.0000000000001032
15. Burkat CN, Lemke BN. Retrobulbar hemorrhage: inferolateral anterior orbitotomy for emergent management. Arch Ophthalmol. 2005;123(9):1260–1262. doi:10.1001/archopht.123.9.1260
16. Neuhaus RW. Orbital complications secondary to endoscopic sinus surgery. Ophthalmology. 1990;97(11):1512–1518. doi:10.1016/s0161-6420(90)32383-7
17. Dunya IM1, Salman SD, Shore JW. Ophthalmic complications of endoscopic ethmoid surgery and their management. - PubMed - NCBI. Am J Otolaryngol. 1996;17(5):322–331. doi:10.1016/s0196-0709(96)90019-8
18. Wladis EJ, Peebles TR, Weinberg DA. Management of acute orbital hemorrhage with obstruction of the ophthalmic artery during attempted coil embolization of a dural arteriovenous fistula of the cavernous sinus. Ophthal Plast Reconstr Surg. 2007;23(1):57–59. doi:10.1097/IOP.0b013e31802c7e5a
19. Yang P, Toomey C, Lin J, Kikkawa DO, Korn BS, Harrison A. Beware of the sneeze. Surv Ophthalmol. 2019. doi:10.1016/j.survophthal.2019.04.001
20. Wyse E, Sorte DE, Mahoney NR, Pearl MS. Orbital compartment syndrome due to acute hemorrhage within an orbital lymphatic malformation: emergency treatment with percutaneous sclerotherapy. J Vasc Interv Radiol. 2016;27(3):453–455. doi:10.1016/j.jvir.2015.11.050
21. Truong H, Counsilman MJ, Gooptu M, Pulte E, Prestipino AJ, Lallas CD. Orbital compartment syndrome as the presenting sign of disseminated intravascular coagulation from metastatic prostatic cancer to the orbit. Urology. 2015;86(2):e7–e8. doi:10.1016/j.urology.2015.05.017
22. Jenkins TL, Zheng CX, Murchison AP, Bilyk JR. Orbital compartment syndrome following post-traumatic subgaleal hematoma. Ophthal Plast Reconstr Surg. 2017;33(2):e33–e36. doi:10.1097/IOP.0000000000000684
23. Leibovitch I, Casson R, Laforest C, Selva D. Ischemic orbital compartment syndrome as a complication of spinal surgery in the prone position. Ophthalmology. 2006;113(1):105–108. doi:10.1016/j.ophtha.2005.09.025
24. Amorim Correa JL, Acioly MA. The enigma of orbital compartment syndrome after lumbar spine surgery in the prone position: case report and literature review. World Neurosurg. 2018;110:309–314. doi:10.1016/j.wneu.2017.11.111
25. Hurst J, Johnson D, Campbell R, Baxter S, Kratky V. Orbital compartment syndrome in a burn patient without aggressive fluid resuscitation. Orbit. 2014;33(5):375–377. doi:10.3109/01676830.2014.881400
26. Sullivan SR, Ahmadi AJ, Singh CN, et al. Elevated orbital pressure: another untoward effect of massive resuscitation after burn injury. J Trauma. 2006;60(1):72–76. doi:10.1097/01.ta.0000197657.25382.b2
27. Prodhan P, Noviski NN, Butler WE, et al. Orbital compartment syndrome mimicking cerebral herniation in a 12-yr-old boy with severe traumatic asphyxia. Pediatr Crit Care Med. 2003;4(3):367–369. doi:10.1097/01.PCC.0000074271.09917.19
28. Roelofs KA, Starks V, Yoon MK. Orbital emphysema. Ophthal Plast Reconstr Surg. 2018;35(1):1. doi:10.1097/IOP.0000000000001216
29. Key SJ, Ryba F, Holmes S, Manisali M. Orbital emphysema – the need for surgical intervention. J Cranio Maxillofacial Surg. 2008;36(8):473–476. doi:10.1016/j.jcms.2008.04.004
30. Tomasetti P, Jacbosen C, Gander T, Zemann W. Emergency decompression of tension retrobulbar emphysema secondary to orbital floor fracture. J Surg Case Rep. 2013;2013(3):rjt011–rjt011. doi:10.1093/jscr/rjt011
31. Dong QN, Ide T, Karino M, Okuma S, Koike T, Kanno T. Retrobulbar orbital emphysema associated with medial orbital wall fracture. J Craniofac Surg. 2019;1. doi:10.1097/SCS.0000000000005390
32. Rodríguez-Cabrera L, Rodríguez-Loaiza JL, Tovilla-Canales JL, Zuazo F. Orbital emphysema as a rare complication of retina surgery. Ophthal Plast Reconstr Surg. 2017;33(6):e141–e142. doi:10.1097/IOP.0000000000000879
33. Iniesta-Sanchez DL, Romero-Caballero F, Aguirre-Alvarado A, Rebollo-Hurtado V, Velez-Montoya R. Management of orbital emphysema secondary to rhegmatogenous retinal detachment repair with hyperbaric oxygen therapy. Am J Ophthalmol Case Rep. 2016;1:26–30. doi:10.1016/j.ajoc.2016.03.002
34. Mellington FE, Bacon AS, Abu-Bakra MAJ, Martinez-Devesa P, Norris JH. Orbital compressed air and petroleum injury mimicking necrotizing fasciitis. J Emerg Med. 2014;47(3):e69–e72. doi:10.1016/j.jemermed.2014.04.030
35. See A, Gan EC. Orbital compartment syndrome during endoscopic drainage of subperiosteal orbital abscess. Am J Otolaryngol. 2015;36(6):828–831. doi:10.1016/j.amjoto.2015.07.017
36. Colletti G, Deganello A, Bardazzi A, et al. Complications after treatment of head and neck venous malformations with sodium tetradecyl sulfate foam. J Craniofac Surg. 2017;28(4):e388–e392. doi:10.1097/SCS.0000000000003723
37. Sia PI, Sia DIT, Scroop R, Selva D. Orbital compartment syndrome following transvenous embolization of carotid-cavernous fistula. Orbit. 2014;33(1):52–54. doi:10.3109/01676830.2013.841717
38. Hamill EB, Weber AC, Patel KR, Yen MT. Bilateral orbital compartment syndrome preceding cerebellar herniation in neuropsychiatric systemic lupus erythematosus. Ophthal Plast Reconstr Surg. 2019;35(3):e55–e57. doi:10.1097/IOP.0000000000001339
39. Stewart CM, McDonald B, Clifford R, Norris JH. Bilateral acute orbital compartment syndrome secondary to Richter syndrome: the “tulip” sign. Clin Experiment Ophthalmol. 2016;44(8):722–724. doi:10.1111/ceo.12759
40. Huang S, Sun MT, Davis G, Fitzgerald J, Selva D, Henderson T. Bilateral orbital compartment syndrome in a patient with disseminated intravascular coagulation. Orbit. 2018;37(5):361–363. doi:10.1080/01676830.2017.1423359
41. Takahashi Y, Takahashi E, Ichinose A, Kakizaki H. Orbital compartment syndrome in eosinophilic angiocentric fibrosis. Ophthal Plast Reconstr Surg. 2015;31(4):e98–e100. doi:10.1097/IOP.0000000000000118
42. Ahmar W, Mason K, Harley N, Hogan C. An unusual complication of thrombolysis–bilateral retro-orbital haematomata. Anaesth Intensive Care. 2005;33(2):271–273. doi:10.1177/0310057X0503300220
43. Chorich LJ, Derick RJ, Chambers RB, et al. Hemorrhagic ocular complications associated with the use of systemic thrombolytic agents. Ophthalmology. 1998;105(3):428–431. doi:10.1016/S0161-6420(98)93023-8
44. Voss JO, Hartwig S, Doll C, Hoffmeister B, Raguse J-D, Adolphs N. The “tight orbit”: incidence and management of the orbital compartment syndrome. J Craniomaxillofac Surg. 2016;44(8):1008–1014. doi:10.1016/j.jcms.2016.05.015
45. Haubner F, Jägle H, Nunes DP, et al. Orbital compartment: effects of emergent canthotomy and cantholysis. Eur Arch Otorhinolaryngol. 2015;272(2):479–483. doi:10.1007/s00405-014-3238-5
46. Oester AE, Fowler BT, Fleming JC. Inferior orbital septum release compared with lateral canthotomy and cantholysis in the management of orbital compartment syndrome. Ophthal Plast Reconstr Surg. 2012;28(1):40–43. doi:10.1097/IOP.0b013e31823646f3
47. Lee KYC, Tow S, Fong K-S. Visual recovery following emergent orbital decompression in traumatic retrobulbar haemorrhage. Ann Acad Med Singapore. 2006;35(11):831–832.
48. Mootha VV, Cowden TP, Sires BS, Dortzbach RK. Subperiosteal orbital hemorrhage from retrobulbar injection resulting in blindness. Arch Ophthalmol. 1997;115(1):123–124. doi:10.1001/archopht.1997.01100150125027
49. Saussez S, Choufani G, Brutus JP, Cordonnier M, Hassid S. Lateral canthotomy: a simple and safe procedure for orbital haemorrhage secondary to endoscopic sinus surgery. Rhinology. 1998;36(1):37–39.
50. Popat H, Doyle PT, Davies SJ. Blindness following retrobulbar haemorrhage—it can be prevented. Br J Oral Maxillofac Surg. 2007;45(2):163–164. doi:10.1016/j.bjoms.2005.06.028
51. Amagasaki K, Tsuji R, Nagaseki Y. Visual recovery following immediate decompression of traumatic retrobulbar hemorrhage via transcranial approach. Neurol Med Chir (Tokyo). 1998;38(4):221–224. doi:10.2176/nmc.38.221
52. Gillum WN, Anderson RL. Reversible visual loss in subperiosteal hematoma of the orbit. Ophthalmic Surg. 1981;12(3):203–209.
53. Korinth MC, Ince A, Banghard W, Huffmann BC, Gilsbach JM. Pterional orbital decompression in orbital hemorrhage and trauma. J Trauma. 2002;53(1):73–78. doi:10.1097/00005373-200207000-00015
54. Hayreh SS, Weingeist TA. Experimental occlusion of the central artery of the retina. IV: retinal tolerance time to acute ischaemia. Br J Ophthalmol. 1980;64(11):818–825. doi:10.1136/bjo.64.11.818
55. Larsen M, Wieslander S. Acute orbital compartment syndrome after lateral blow-out fracture effectively relieved by lateral cantholysis. Acta Ophthalmol Scand. 1999;77(2):232–233. doi:10.1034/j.1600-0420.1999.770225.x
56. Susarla SM, Nam AJ, Dorafshar AH. Orbital compartment syndrome leading to visual loss following orbital floor reconstruction. Craniomaxillofac Trauma Reconstr. 2016;9(2):152–157. doi:10.1055/s-0035-1558456
57. Schwitkis AE, Pollack TL, Torbati SS. Orbital compartment syndrome following mechanical fall. Clin Pract Cases Emerg Med. 2018;2(3):268–269. doi:10.5811/cpcem.2018.4.37810
58. Tran KD, Scawn RL, Whipple KM, Korn BS, Kikkawa DO. Mastication induced retrobulbar hemorrhage. Orbit. 2013;32(6):387–388. doi:10.3109/01676830.2013.815229
59. Sampath R, Shah S, Leatherbarrow B. The management of an optic nerve compromising acute retrobulbar haemorrhage: report of a case. Eye (Lond). 1995;9(Pt 4):533–535. doi:10.1038/eye.1995.123
60. Goodall KL, Brahma A, Bates A, Leatherbarrow B. Lateral canthotomy and inferior cantholysis: an effective method of urgent orbital decompression for sight threatening acute retrobulbar haemorrhage. Injury. 1999;30(7):485–490. doi:10.1016/s0020-1383(99)00137-0
61. Hislop WS, Dutton GN. Retrobulbar haemorrhage: can blindness be prevented? Injury. 1994;25(10):663–665. doi:10.1016/0020-1383(94)90009-4
62. McInnes G, Howes DW. Lateral canthotomy and cantholysis: a simple, vision-saving procedure. CJEM. 2002;4(1):49–52.
63. Carrim ZI, Anderson IWR, Kyle PM. Traumatic orbital compartment syndrome: importance of prompt recognition and management. Eur J Emerg Med. 2007;14(3):174–176. doi:10.1097/MEJ.0b013e3280b17e49
64. Jamal BT, Diecidue RJ, Taub D, Champion A, Bilyk JR. Orbital hemorrhage and compressive optic neuropathy in patients with midfacial fractures receiving low-molecular weight heparin therapy. J Oral Maxillofac Surg. 2009;67(7):1416–1419. doi:10.1016/j.joms.2008.12.044
65. Maurer P, Conrad-Hengerer I, Hollstein S, Mizziani T, Hoffmann E, Hengerer F. Orbital haemorrhage associated with orbital fractures in geriatric patients on antiplatelet or anticoagulant therapy. Int J Oral Maxillofac Surg. 2013;42(12):1510–1514. doi:10.1016/j.ijom.2012.09.024
66. Pamukcu C, Odabaşı M. Acute retrobulbar haemorrhage: an ophthalmologic emergency for the emergency physician. Ulus Travma Acil Cerrahi Derg. 2015;21(4):309–314. doi:10.5505/tjtes.2015.16768
67. Gauden AJ, Hardy T, Mack HG, Danesh-Meyer HV, Kaye AH. Orbital compartment syndrome following aneurysm surgery. J Clin Neurosci. 2012;19(7):1032–1036. doi:10.1016/j.jocn.2012.01.009
68. Pahl FH, de Oliveira MF, Dal Col Lúcio JE, Souza E, Castro EF. Orbital compartment syndrome after frontotemporal craniotomy: case report and review of literature. World Neurosurg. 2018;109:218–221. doi:10.1016/j.wneu.2017.09.167
69. Yu Y-H, Chen W-J, Chen L-H, Chen W-C. Ischemic orbital compartment syndrome after posterior spinal surgery. Spine (Phila PA 1976). 2008;33(16):E569–E572. doi:10.1097/BRS.0b013e31817c55c2
70. Vassallo S, Hartstein M, Howard D, Stetz J. Traumatic retrobulbar hemorrhage: emergent decompression by lateral canthotomy and cantholysis. J Emerg Med. 2002;22(3):251–256. doi:10.1016/s0736-4679(01)00477-2
71. Colletti G, Valassina D, Rabbiosi D, et al. Traumatic and iatrogenic retrobulbar hemorrhage: an 8-patient series. J Oral Maxillofac Surg. 2012;70(8):e464–e468. doi:10.1016/j.joms.2012.05.007
72. Ujam A, Perry M. Emergency management for orbital compartment syndrome-is decompression mandatory? Int J Oral Maxillofac Surg. 2016;45(11):1435–1437. doi:10.1016/j.ijom.2016.08.001
73. Warburton RE, Brookes CCD, Golden BA, Turvey TA. Orbital apex disorders: a case series. Int J Oral Maxillofac Surg. 2016;45(4):497–506. doi:10.1016/j.ijom.2015.10.014
 © 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2019 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.