Back to Journals » Psoriasis: Targets and Therapy » Volume 10
Optimal Management of Plaque Psoriasis in Adolescents: Current Perspectives
Authors Mahé E
Received 28 September 2020
Accepted for publication 29 October 2020
Published 27 November 2020 Volume 2020:10 Pages 45—56
DOI https://doi.org/10.2147/PTT.S222729
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Prof. Dr. Uwe Wollina
Emmanuel Mahé
Service De Dermatologie Et Médecine Vasculaire, Hôpital Victor Dupouy, Argenteuil 95100, France
Correspondence: Emmanuel Mahé Tel +33 134 232 628
Fax +33 134 232 261
Email [email protected]
Abstract: The skin is at the interface between the body and its environment and is therefore at the center of adolescent concerns during this period of identity formation and increased awareness of body image issues, and stigmatization. Managing an adolescent with psoriasis involves managing the illness and the individual during their transition from being an older child to a young adult. In addition to ensuring that the patient adheres to treatments and is engaged with the therapeutic strategy, dermatologists may also need to manage issues linked to unspoken suffering or conflicts between the adolescent and their parents, who are often present during consultations. The impact of psoriasis on the social interactions, school life and sexuality of the patients, together with the influence of the internet and social networks, also have to be taken into account. In this review, we summarize the epidemiologic, clinical, and therapeutic data available on psoriasis in adolescents, and propose specific management strategies, adapted to the 21st century, for patients in this age group.
Keywords: psoriasis, adolescent, treatment, biotherapies, acitretin, methotrexate, proactive treatment
Introduction
Adolescence is a period of identity formation and increased awareness of physical appearance. At the interface between the human body and its environment, the skin is at the center of justified or unjustified body image concerns, and real or perceived stigmatization during this period. The management of adolescents can be demanding for physicians, as these issues may be compounded by a lack of interest or engagement from the patients.
Managing an adolescent with psoriasis involves managing the illness, as well as managing the individual as they transition from being an older child to a young adult. Dermatologists need to ensure adherence to treatments and the therapeutic strategy, and may also need to manage issues linked to unspoken suffering or conflict between the adolescent and their parents, who are often present during the consultation. Indeed, it may be important to reserve time to consult with the adolescent alone. Verbal communication is sometimes difficult: every dermatologist has been confronted with the “monosyllabic, closed-off adolescent” who provides responses limited to “yes”, “no”, “maybe”. Understanding the suffering and hopes of these patients is a major challenge for dermatologists. The impact of the disease on social interactions, sexuality, and school work, as well as the role of the internet and social networks, have to be taken into account in patients of this age.
New perspectives in the field of psoriasis are often associated with therapeutic developments. In this review, we provide a summary of the epidemiologic, clinical, and therapeutic data available on psoriasis in adolescence. More importantly, we propose specific management strategies adapted for adolescent patients, and also discuss the management of adolescents in the 21st century environment.
Epidemiology
Prevalence
Psoriasis is frequent in adolescence. In Europe, the prevalence of psoriasis in adolescents ranges from 1.2% to 2.0%, with a roughly linear increase during adolescence.1,2 Prevalence is lower in the United States, Asia, and Africa, ranging from 0.02% to 0.1%.3–7 A Californian study showed that incidence doubled between 1970 and 1999, with rates of 29.6 per 100,000 in 1970–1974 increasing to 62.7 per 100,000 in 1995–1999.5
Gender and Prevalence
Most studies show that the prevalence of psoriasis in adolescents is higher in girls than in boys.7–11 As the sex ratio among adults with psoriasis is balanced, this suggests that psoriasis starts earlier in girls. Behavior, genetic background, or earlier puberty could all explain this difference. There are also slight differences in the clinical aspects of psoriasis according to sex: girls may have a higher prevalence of scalp involvement, whereas boys appear to have a higher prevalence of nail involvement.11–13
Comorbidities
Obesity
Being overweight or obese, or having central obesity, has been associated with psoriasis in adolescents. The same trend has been observed in adults.2,7,14–18 and is independent of the clinical type of psoriasis. A family history of being overweight or obese is the major risk factor for obesity in children with psoriasis.14 Management of obesity in adolescents with psoriasis is important, both for their general health and for the management of their skin disease.
- The consequences of obesity in adolescence continuing into adulthood are serious, not only because the obesity persists in the majority of cases but also because some of the complications that manifest in adulthood start in adolescence.19
- The degree of obesity is associated with the severity of psoriasis.15,16 In adults, weight reduction has been shown to improve psoriasis, psoriatic arthritis and treatment responses. It is likely that weight loss could have the same impact for obese or overweight adolescent patients with psoriasis.20
Other Metabolic and Cardiovascular comorbidities
As reported in adults, some publications suggest that there is a link between psoriasis in childhood and adolescence, and dyslipidemia, diabetes, and high blood pressure.2,15,17,21 There are discussed evidences for these associations, and thus systematic screening for these comorbidities has not been proposed.16 However, screening must be discussed when there is a family history of comorbidity (familial dyslipidemia) or when a comorbidity is identified following clinical investigation (diabetes, dyslipidemia, or blood pressure).22
Dermatologists should also consider assessing comorbidities in adolescents with psoriasis when proposing a treatment that can have an impact on these comorbidities: corticosteroids can induce hyperlipidemia, hyperglycemia, and weight gain; acitretin may lead to hyperlipidemia; cyclosporine can cause hypertension; and tumor necrosis factor (TNF)-alpha inhibitors can result in weight gain.23
Clinical Aspects
Skin Psoriasis
The clinical picture of psoriasis in childhood resembles that of the adult disease. Plaque psoriasis (Figure 1) is the most frequent clinical type of psoriasis occurring in adolescents. The inverse, palmoplantar, and guttate forms, which are frequent in infants and children, are less common in adolescents.8–12 Although all clinical types of psoriasis can be observed in adolescents, the pustular, erythrodermic, and linear forms are rare.23
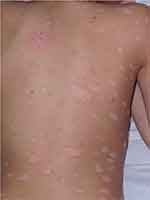 |
Figure 1 Plaque psoriasis in a 13-year-old boy. |
Psoriasis plaques in young children are often atypical (small, pink, poorly demarcated lesions with mild scaling) and may resemble the lesions in atopic dermatitis. In adolescents, the plaques usually display typical psoriasis characteristics (monomorphic, sharply demarcated, red plaques covered with grey lamellar scales). The plaques may be localized and only appear in body areas typically affected by psoriasis (such as the elbows, knees, scalp, and umbilicus), but can also be generalized.23
The scalp is often the only location affected by psoriasis in adolescents, leading to misdiagnosis as simple dandruff or tinea capitis.24 The hairline (Figure 2) and occipital scalp are often the first sites to develop the typical plaque form. Some severe forms can have a pityriasis amiantacea presentation, with thick, fixed, silver scales. In mild forms, scalp psoriasis resembles seborrheic dermatitis. Scalp psoriasis has been associated with an increase in the severity of psoriasis and with the Koebner phenomenon, suggesting a possible role for trauma or scratching in the aggravation of scalp psoriasis.25 In adults, scalp psoriasis is associated with psoriatic arthritis but there are no data confirming such an association in children or adolescents.
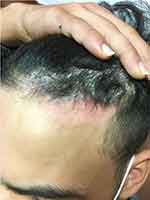 |
Figure 2 Scalp psoriasis in a 16-year-old boy. This adolescent was suspected of having tinea capitis. |
Nail Psoriasis
Nails are affected in more than one-third of adolescents with psoriasis, mainly with pitting of the fingernails, and onycholysis and pachyonychia of the toenails. Nail involvement has been associated with an increase in the overall severity of psoriasis and psoriatic arthritis.26 However, although there is a suspected link between nail psoriasis and psoriatic arthritis in adults, the predictive value of nail involvement in adolescence for developing psoriatic arthritis in adulthood remains uncertain.27
Psoriatic Arthritis
Psoriatic arthritis affects between 5% and 10% of adolescents with psoriasis.7–12,14,15,24 Only limited data are available about the distribution of the clinical types of arthritis – peripheral arthritis, spondyloarthropathy, or enthesitis – in this population. As articular pain has a negative impact on the quality of life (QoL), all children and adolescents with psoriasis should be screened for arthritis.28 A four-question evaluation of clinical history has been proposed to screen for arthritis in children with psoriasis (Box 1). Assessment of adolescents using the Psoriasis Epidemiology Screening Tool (PEST) has also been proposed, together with the referral of patients to a rheumatology department if any signs or symptoms of arthritis are reported.29,30
 |
Box 1 Four-Question Evaluation of Clinical History to Screen for Arthritis in Children with Psoriasis |
Health Quality of Life and Psoriasis in Adolescence
Altered QoL is a major concern for adolescents with psoriasis: the disease can have an impact on all aspects of the social life of these patients, including school, sports, social interactions, and sexuality.31 Studies using classical scoring tools, such as the Dermatology Life Quality Index (DLQI) or the children’s version of this tool (CDLQI) adapted to patients aged from 4–5 years to 16 years, have yielded interesting results, showing that QoL scores correlate with disease severity and that the scores improve with successful treatment of the disease.31–33 However, the validity of these scores in adolescent patients with psoriasis is debatable: the tools are not specific for psoriasis and there is confusion over whether to use the DLQI or the CDLQI for patients aged between 15 and 17 years. Indeed, comparative studies of the CDLQI and the DLQI in patients aged 16 and 17 years have revealed differences between the total scores obtained from the two instruments. A QoL assessment tool dedicated to adolescents with psoriasis, the Adolescent Psoriasis Quality of Life instrument, has recently been developed. The preliminary form of this tool showed promising psychometric properties.33
Evolution of the Psoriasis
Triggering Factors
Psoriasis flares can be provoked by nonspecific triggers. In children, flares have been noted to occur in association with stress, mild trauma (Koebner phenomenon), and infection (streptococcal or viral). No such associations have been documented for adolescents with psoriasis, with the possible exception to the implication of scratching in the maintenance of scalp psoriasis.25 Systemic drugs (beta-blockers, lithium, antimalarials, etc.) and HIV infection, are less frequently implicated in the onset of psoriasis in adolescents than they are in adults.
Outcomes in Adolescents with Psoriasis
Only limited and discordant information is available about outcomes for adolescents with psoriasis. Indeed, previous studies have mainly evaluated outcomes for “child-onset” psoriasis rather than collecting specific data on adolescents with the disease. Nevertheless, recent large-scale studies have provided reassuring data about disease outcomes in adolescents. They suggest that adolescent onset of psoriasis does not influence the prevalence of cardiovascular and metabolic comorbidities, or that of psoriatic arthritis, and does not seem to influence disease severity in adulthood.9,34,35
Social Life and Psoriasis
School and University
Psoriasis in adolescence has been associated with cognitive impairment, the development of addictions (tobacco, alcohol, and drug abuse), and psychiatric symptoms such as attention deficit disorder or attention deficit hyperactivity disorder.36–39 These data led to the hypothesis that early psoriasis onset affects the educational development of young patients. In a recent study, our group found that there were no significant associations between early onset of psoriasis and educational level, working activity or living conditions, or with alcohol consumption in adulthood (personal data, submitted for publication). However, vigilance is required concerning the immediate impact of psoriasis on absenteeism, psychological distress, and schooling.
Extracurricular Activities and Psoriasis
Adolescence is a key period for maintaining or engaging individuals in sporting activities. Participating in sports is widely considered as beneficial for maintaining health and psychological wellbeing during adolescence. However, the impact of skin disease on sporting activity is evaluated in only one question in the DLQI and CDLQI. There can be many reasons why adolescents with psoriasis stop practicing sports, including issues surrounding the psoriasis being visible (for example, when swimming or practicing fighting sports), exacerbation of itchy or painful psoriatic skin by sweat and contact, and psoriatic arthritis limiting sporting activity. Problems may also be associated with psoriasis treatments: oily topical treatments or side effects may be incompatible with practicing sports. Indeed, methotrexate can induce asthenia, and acitretin can induce myalgia or alterations of night vision. A recent case has been reported where an adolescent practicing aviation refused treatment acitretin because of the risk of alteration of night vision (personal case).40 The treatments needed to control psoriasis therefore need to be adapted to facilitate sporting activity among adolescent patients, particularly as physical activity seems to have a direct impact on psoriasis, independently of its effect on obesity. The results of a recent study involving adolescents and young adults (mean age: 19 years) indicated that regular physical activity lowers the risk of psoriasis and has a beneficial effect on the natural course of the disease.41
Sex Life, Psoriasis, and Treatments
Sex life, fertility, and contraception are common clinical concerns among adults with psoriasis. However, it can be difficult to discuss these concerns with adolescents because of a reluctance to raise these issues on the part of the adolescent or the dermatologist, or because discussions are hindered by the presence of parents at consultations. Adolescents will most likely be uncomfortable initiating a discussion on these topics and may expect physicians to bring up the subject first. Although these discussions may be initiated by pediatricians or general practitioners, this role often needs to be assumed by dermatologists who can raise these issues in the context of psoriasis and its treatments.
Sexuality
Psoriasis affects the genital areas in up to 40% of adult patients, impacting QoL and sexual health.42 There is currently no information about the true frequency of genital involvement in adolescents, with any available data likely to lead to an undervaluation as the CDLQI, unlike the adult version, does not cover sexuality issues. Thus, one way to indirectly assess adolescent sexuality issues might be to offer patients an early adolescent QoL assessment using the DLQI instead of the CDLQI.32
Fertility
The adverse effects of psoriasis and its treatment on future fertility is a major concern for adolescent patients, and, perhaps even more so, for their parents. Although fertility issues are a cause of additional concern during therapeutic management, there is no evidence that fertility is negatively affected by psoriasis.43 Acitretin has teratogenic potential in females but does not affect fertility. After stopping treatment for 2 to 3 years, the teratogenicity can be considered to be nil. Methotrexate has been associated with impaired fertility in adulthood in males and females. However, low doses and short-term treatments have been shown to have no impact on fertility.44 Cyclosporine and biologics have no known impact on fertility. Thus, data on the effect of psoriasis and its treatments on the future fertility of adolescent patients are reassuring.
Contraception
Contraception has no impact on the course of psoriasis. However, contraception is necessary for female patients using acitretin and is strongly recommended for both males and females using methotrexate and some biologics. These issues should be discussed before initiating treatment with any systemic therapy, particularly in adolescent girls, as the need for contraception may impact the choice of treatment. The choice of a contraception will depend on the preferences of the adolescent and their parents, as well as on ethical considerations and country-specific regulations (for example, in France, parental authorization is not needed before initiating contraception in adolescent girls).
Adolescent Psoriasis, the Internet and Social Networks
The internet provides information on virtually every possible subject, including all aspects of psoriasis. However, the nature and quality of this information is not controlled.45 Adolescents have access to this universal data but, unlike their parents and physicians, they may not be able to judge the quality of the information or impose constraints. It is therefore important to discuss these issues with adolescents and possibly guide them to quality sites, such as institutional websites (for example, in France: “Dermato-Info”, https://dermato-info.fr/fr/les-maladies-de-la-peau/le-psoriasis; “Resopso”: https://www.resopso.fr/2018/02/05/psoriasis-chez-lenfant/) or websites created by patient associations (for example, in France: “France Psoriasis”: https://francepsoriasis.org/lassociation/documentation/fiches-conseil/le-psoriasis-de-lenfant/).
Social networks now play a prominent role in the life of adolescents. They can be used as an educational resource, and as a source of psychological and social support. They are tools for sharing and expressing difficulties, anxieties, and life experiences, as well as judgements and criticism. Generally, dermatologists have no access to this information. However, the data collected from these networks are likely to have a strong impact on the decisions made by adolescents regarding their disease and treatments.45,46 Thus, we must try to identify the false or inappropriate information found by adolescents on social networks to counterbalance with appropriate information given during consultations. Dermatologists should direct adolescent patients towards associative networks, such as associations of patients that have chat groups for teenage patients (for example, in France, https://francepsoriasis.org/).
Adolescents have a tendency to flee from physicians, who represent a form of authority in connivance with their parents. The use of teleconsultations has recently become widespread in some countries during the COVID-19 crisis47 and could provide a way to narrow the communication gap between physicians and their adolescent patients by finally adapting to the young person’s mode of communication. Teleconsultations impose fewer constraints and may facilitate direct communication with the adolescents via a more modern approach. However, although the technical resources for teleconsultations may be available, the tools used to monitor chronic inflammatory dermatoses like psoriasis (severity scores, self-assessment, etc.) need to be improved.
General Management
Education is always the first step in the management of young patients and should be adapted for adolescents and their parents. Dermatologists should aim to reserve time during consultations for discussions with the adolescent alone, without the parents being present. This dedicated consulting time can be used to discuss difficulties with psoriasis or treatments, or problems with parents and the underlying causes of these issues. Adolescents should also be given key information about their disease. They should be provided with an explanation of the disease pathophysiology and information about outcomes, and informed of the fact that psoriasis is a disease that can be controlled but not cured. They should also be told that psoriasis is not contagious and that no alimentary, sport or vaccine restrictions are necessary, and should also be informed of the aims of the treatments. Although initially very time-consuming, this level of patient education is invaluable for long-term management.24
In general, all adolescents with psoriasis will need treatment for all lesions. Assessing the need for treatment and convincing patients to adhere to therapeutic regimens are important issues for all patients, and can be particularly difficult for adolescents. There is no “perfect” argument for convincing patients of the need to initiate and adhere to treatments: treating the disease early does not modify its long-term outcome; the treatments available only manage the symptoms and do not cure the disease; the disease is not lethal; psoriasis requires long-term treatment; and all the treatments have side effects. Several consultations are often required before the adolescent will be willing to accept the most appropriate treatment, either topical or systemic.
Treatment options include local treatments, phototherapy and systemic treatments (conventional systemic therapy and biologics). In most cases, these treatments are sufficient to control the psoriasis. Subsequent management of adolescents should focus on maintaining therapeutic efficacy and preventing relapses, whilst reducing any treatment-related toxicity to improve patient QoL. This can be considered as “proactive” treatment.24,48
One of the major limiting factors in the management of young patients with psoriasis is that some treatments are not licensed for use in adolescents. In France, combined betamethasone-calcipotriol therapy and methotrexate are not licensed for use in adolescents, and cyclosporine is not licensed for use in patients aged under 16 years. However, in a recent study, we found that up to 100% of adolescents with severe psoriasis had received unlicensed treatments.49 There are now enough data from registers, cohorts and clinical trials to assess the real-world benefits and tolerance of adult treatments in adolescents.50–55 In France, treatments can be prescribed for “off-label” use under the following circumstances: 1) there is no alternative licensed drug; 2) the treatment is recognized as effective and safe by the scientific community and literature; 3) the indication is “essential” with regard to the patient’s condition, is necessary for the patient, and is consistent with current scientific knowledge; 4) the prescriber has informed the patient of the absence of official authorization for the prescription, the absence of therapeutic alternatives, the expected benefits, and the risks or constraints of the drug, as well as the conditions of health insurance coverage; and 5) justification for the prescription is recorded in the patient’s medical file.48 Recent national guidelines have been published for the management of psoriasis in children and adolescents in Germany, Italy and the United States.56–58 These guidelines should help improve the care of all young patients with psoriasis worldwide. Overall, nearly all of the therapeutic arsenal used to treat adults with psoriasis can be used in adolescents.
Specific Physical Conditions of Adolescence and Psoriasis Treatments
Some skin diseases linked to adolescence have to be taken into account before choosing the most appropriate treatment for psoriasis.
- Acne can be induced or exacerbated by the application of topical steroids to the face or scalp (Figure 3), or by the use of cyclosporine. Thus, extra care should be taken when managing adolescents with both acne and psoriasis.
- Striae distensae develop frequently in adolescence. Several risk factors have been identified such as obesity, and rapid variations in weight and height. Another major factor in the development of striae distensae is the chronic use of topical corticosteroids on the same area of skin (Figure 4). This type of use may occur during the treatment of chronic inflammatory skin diseases such as psoriasis. The role of the dermatologist is to reduce this risk in psoriatic patients by prescribing either nonsteroidal treatments or systemic therapies.
- Androgenic alopecia occurs in around 15% of adolescent boys. It is strongly associated with a family history of androgenic alopecia and can severely impair QoL. As acitretin and methotrexate can also induce transient alopecia, these treatments should be avoided in these patients.59
- Hair growth is one of the main physical changes occurring in adolescence. Cyclosporine can induce facial hair growth, which may be problematic in adolescent girls with dark hair.60
 |
Figure 3 Clobetasol propionate shampoo-induced acne in a 14-year-old girl with scalp psoriasis. |
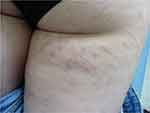 |
Figure 4 Severe striae distensae in a 16-year-old girl with psoriasis. The patient received treatment with topical steroids for a long time. |
Topical Treatments
There are no major concerns specifically linked to the use of topical treatments in adolescents, except those concerning topical steroids described in Specific Physical Conditions of Adolescence and Psoriasis Treatments. Emollients are an essential part of the long-term care of patients with psoriasis. As in atopic dermatitis, emollient treatment of the areas usually affected by psoriasis reduces the frequency of relapse. In addition, by reducing pruritus, emollients limit the risk of scratching and the Koebner phenomenon on dry skin.61 The choice of emollient should be made with the adolescent, after discussions to determine the most appropriate product (with or without urea, not too oily, with a pleasant fragrance, etc.).
Topical steroids are the reference treatment for psoriasis, most commonly prescribed as a combination of calcipotriol (50 μg/g) and betamethasone dipropionate (0.5 mg/g).49,51 These treatments are recommended for skin-limited disease. The potency of the steroids prescribed must be adapted according to disease severity and location. To be effective in plaque psoriasis, topical steroids need to be used over the long term, both to treat symptomatic skin and as preventative treatments. Thus, the duration of the prescription should not be restricted and the treatment should be maintained “until clearing” of the lesions. Several options can be proposed to reduce the frequency and severity of disease relapses after clearing: reducing the use of topical corticosteroids very gradually, using the steroid as a weekend therapy, and ensuring very early treatment of flare-ups (Table 2).48
 |
Table 1 Management of Adolescents with Psoriasis Using Methotrexate, Acitretin, or Cyclosporine |
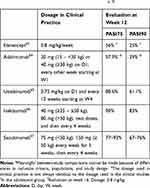 |
Table 2 Efficacy of Biologics Licensed for Use in Adolescents with Psoriasis: Data from Randomized Studiesa, b |
Topical calcineurin inhibitors (tacrolimus or pimecrolimus) can be beneficial for pediatric psoriasis, particularly for sites where there is a risk of atrophy, such as the face. After the lesions have cleared, weekend therapy can be proposed. Dithranol, applied under careful parental supervision, can be used as a short-term therapy. Good results are usually obtained in 70% to 80% of cases.
Phototherapies
Psoralen–UV-A (PUVA) therapy, UV-B therapy, and local therapy can be proposed for adolescents. There are two major limitations to the use of these therapies in this age group. First, the main limitation is the increased risk of skin cancer and the potential cumulative carcinogenic risk when we offer other potential carcinogenic drugs later such as cyclosporine and some biologics. For example, cyclosporine is associated with increased risks of nonmelanoma skin cancer and photocarcinogenic potential during long-term treatment, particularly in patients who have received high cumulative doses of PUVA (no evidence for UVB therapy).62 Second, these treatments are frequently incompatible with the daily life of adolescent patients, either because of a lack of treatment availability close to home or school or because they interrupt the schooling, family life or sporting activities of the adolescent.
Conventional Systemic and Biologic Treatments
During the past few years, the number of treatments available for the treatment of psoriasis has increased in some countries. Available treatments may now include conventional systemic treatments, acitretin, methotrexate, cyclosporine, and fumaric acid. Etanercept, adalimumab (two TNF-alpha inhibitors), and ustekinumab (an anti-interleukin-12/23 monoclonal antibody) have now been licensed for use in adolescents with psoriasis for a few years. In 2020, secukinumab, and ixekizumab were licensed in Europe. The choice of treatment depends on several patient-related factors (age, gender, type of psoriasis, comorbidities, etc.), on product availability and their eligibility for reimbursement, on country-specific recommendations or treatment habits, and on the personal experience of the dermatologist. Recent guidelines have been published in Italy, Germany, and the united states for the use of systemic treatments in patients with onset of psoriasis in childhood and adolescence.56–58 Systemic treatments are summarized in Table 1 and data on the efficacy of biologics in adolescents, obtained from industry-sponsored randomized studies, are summarized in Table 2. Real-life data evaluating other factors, such as comparative drug survival, are now becoming available and will be of use during discussions concerning treatment choices.50,53
Proactive Treatments
Proactive patient management means anticipating disease progression to limit its severity and the occurrence of new flare-ups. This approach must be distinguished from “active or reactive” management given at the time of the active episode, and also from “prophylactic” management, conducted before onset of the disease. Proactive management plays a key role in the successful treatment of adolescents as it appears to increase adherence to therapeutic strategies, increase patient wellbeing, reduce the burden of the disease and its treatment, and decrease the cumulative toxicity of the treatments. We have recently published a proposed set of proactive measures that can be used in the management of adolescents (detailed in Table 3).48
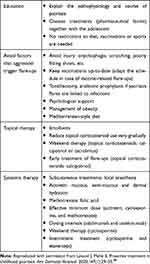 |
Table 3 Proactive Management Options That Can Be Proposed After Flare-Ups are Under Control, or Used Together with Active Flare-Up Treatments |
Conclusion
The role of dermatologists in the management of adolescents with psoriasis is not limited to proposing more recent and effective treatments. These patients require multidimensional and long-term treatment strategies that take into account the particularities associated with this difficult period of personal development and have been adapted to life in the 21st century.
Disclosures
E. Mahé has paid activities as a consultant, advisor, or speaker for Abbvie, Amgen, Janssen Cilag, Celgene, Leo Pharma, Lilly, and Novartis, and reports no other potential conflicts of interest for this work.
References
1. Gelfand JM, Weinstein R, Porter SB, Neimann AL, Berlin JA, Margolis DJ. Prevalence and treatment of psoriasis in the United Kingdom: a population-based study. Arch Dermatol. 2005;141(12):1537–1541. doi:10.1001/archderm.141.12.1537
2. Augustin M, Glaeske G, Radtke MA, Christophers E, Reich K, Schäfer I. Epidemiology and comorbidity of psoriasis in children. Br J Dermatol. 2010;162(3):633–636. doi:10.1111/j.1365-2133.2009.09593.x
3. Yamamah GA, Emam HM, Abdelhamid MF, et al. Epidemiologic study of dermatologic disorders among children in South Sinai, Egypt. Int J Dermatol. 2012;51(10):1180–1185. doi:10.1111/j.1365-4632.2012.05475.x
4. Chang YT, Chen TJ, Liu PC, et al. Epidemiological study of psoriasis in the national health insurance database in Taiwan. Acta Derm Venereol. 2009;89(3):262–266. doi:10.2340/00015555-0642
5. Tollefson MM, Crowson CS, McEvoy MT, Maradit Kremers H. Incidence of psoriasis in children: a population-based study. J Am Acad Dermatol. 2010;62(6):979–987.
6. Wu JJ, Black MH, Smith N, Porter AH, Jacobsen SJ, Koebnick C. Low prevalence of psoriasis among children and adolescents in a large multiethnic cohort in southern California. J Am Acad Dermatol. 2011;65(5):957–964. doi:10.1016/j.jaad.2010.09.005
7. Burden-Teh E, Thomas KS, Ratib S, Grindlay D, Adaji E, Murphy R. The epidemiology of childhood psoriasis: a scoping review. Br J Dermatol. 2016;174(6):1242–1257. doi:10.1111/bjd.14507
8. Raychaudhuri SP, Gross J. A comparative study of pediatric onset psoriasis with adult onset psoriasis. Pediatr Dermatol. 2000;17(3):174–178. doi:10.1046/j.1525-1470.2000.01746.x
9. Ferrándiz C, Pujol RM, García-Patos V, Bordas X, Smandía JA. Psoriasis of early and late onset: a clinical and epidemiologic study from Spain. J Am Acad Dermatol. 2002;46(6):867–873. doi:10.1067/mjd.2002.120470
10. Bonigen J, Phan A, Hadj-Rabia S, et al. Impact de l’âge et du sexe sur les aspects cliniques et épidémiologiques du psoriasis de l’enfant. Données d’une étude transversale, multicentrique française. Ann Dermatol Venereol. 2016;143(5):354–363. doi:10.1016/j.annder.2016.02.006
11. Mercy K, Kwasny M, Cordoro KM, et al. Clinical manifestations of pediatric psoriasis: results of a multicenter study in the United States. Pediatr Dermatol. 2013;30(4):424–428. doi:10.1111/pde.12072
12. Morris A, Rogers M, Fischer G, Williams K. Childhood psoriasis: a clinical review of 1262 cases. Pediatr Dermatol. 2001;18(3):188–198. doi:10.1046/j.1525-1470.2001.018003188.x
13. Al-Mutairi N, Manchanda Y, Nour-Eldin O. Nail changes in childhood psoriasis: a study from Kuwait. Pediatr Dermatol. 2007;24(1):7–10. doi:10.1111/j.1525-1470.2007.00324.x
14. Mahé E, Beauchet A, Bodemer C, et al. Psoriasis and obesity in French children: a case-control, multicentre study. Br J Dermatol. 2015;172(6):1593–1600. doi:10.1111/bjd.13507
15. Paller AS, Mercy K, Kwasny MJ, et al. Association of pediatric psoriasis severity with excess and central adiposity: an international cross-sectional study. JAMA Dermatol. 2013;149(2):166–176. doi:10.1001/jamadermatol.2013.1078
16. Badaoui A, Tounian P, Mahé E. Psoriasis and metabolic and cardiovascular comorbidities in children: A systematic review. Arch Pediatr. 2019;26(2):86–94. doi:10.1016/j.arcped.2018.12.005
17. Phan K, Lee G, Fischer G. Pediatric psoriasis and association with cardiovascular and metabolic comorbidities: systematic review and meta-analysis. Pediatr Dermatol. 2020;37(4):661–669. doi:10.1111/pde.14208
18. Guidolin L, Borin M, Fontana E, Caroppo F, Piaserico S, Fortina AB. Central obesity in children with psoriasis. Acta Derm Venereol. 2018;98(2):282–283. doi:10.2340/00015555-2816
19. Tounian P. Conséquences à l’âge adulte de l’obésité de l’enfant. Arch Pediatr. 2007;14(6):718–720. doi:10.1016/j.arcped.2007.02.043
20. Ko SH, Chi CC, Yeh ML, Wang SH, Tsai YS, Hsu MY. Lifestyle changes for treating psoriasis. Cochrane Database Syst Rev. 2019;7(7):CD011972.
21. Caroppo F, Ventura L, Belloni Fortina A. High blood pressure in normal-weight children with psoriasis. Acta Derm Venereol. 2019;99(3):329–330. doi:10.2340/00015555-3076
22. Osier E, Wang AS, Tollefson MM, et al. Pediatric psoriasis comorbidity screening guidelines. JAMA Dermatol. 2017;153(7):698–704. doi:10.1001/jamadermatol.2017.0499
23. Mahé E. Childhood psoriasis. Eur J Dermatol. 2016;26(6):537–548. doi:10.1684/ejd.2016.2932
24. Mahé E, Maccari F, Ruer-Mulard M, et al. Psoriasis de l’enfant vu en milieu libéral: les aspects cliniques et épidémiologiques diffèrent des données habituellement publiées. Ann Dermatol Venereol. 2019;146(5):354–362. doi:10.1016/j.annder.2019.01.024
25. Bronckers IMGJ, Maatkamp M, Kievit W, van de Kerkhof PCM, de Jong EMGJ, Seyger MMB. The association of scalp psoriasis with overall psoriasis severity and koebnerization in children: cross-sectional findings from the Dutch child-capture registry. Br J Dermatol. 2019;181(5):1099–1101. doi:10.1111/bjd.18168
26. Pourchot D, Bodemer C, Phan A, et al. Nail psoriasis: a systematic evaluation in 313 children with psoriasis. Pediatr Dermatol. 2017;34(1):58–63. doi:10.1111/pde.13028
27. Eder L, Haddad A, Rosen CF, et al. The incidence and risk factors for psoriatic arthritis in patients with psoriasis: a prospective cohort study. Arthritis Rheumatol. 2016;68(4):915–923. doi:10.1002/art.39494
28. Meneghetti TC, Padilha TMH, Azevedo VF, Cat MNL, Sarolli BMS, de Carvalho VO. The musculoskeletal impairment negatively impacts the quality of life of children and adolescents with psoriasis. Adv Rheumatol. 2020;60(1):33. doi:10.1186/s42358-020-00136-6
29. Burden-Teh E, Thomas KS, Rangaraj S, Cranwell J, Murphy R. Early recognition and detection of juvenile psoriatic arthritis: a call for a standardized approach to screening. Clin Exp Dermatol. 2017;42(2):153–160. doi:10.1111/ced.13010
30. Coates LC, Aslam T, Al Balushi F, et al. Comparison of three screening tools to detect psoriatic arthritis in patients with psoriasis (CONTEST study). Br J Dermatol. 2013;168(4):802–807. doi:10.1111/bjd.12190
31. Randa H, Todberg T, Skov L, Larsen LS, Zachariae R. Health-related quality of life in children and adolescents with psoriasis: a systematic review and meta-analysis. Acta Derm Venereol. 2017;97(5):555–563. doi:10.2340/00015555-2600
32. Van Geel MJ, Maatkamp M, Oostveen AM, et al. Comparison of the dermatology life quality index and the children’s dermatology life quality index in assessment of quality of life in patients with psoriasis aged 16–17 years. Br Dermatol. 2016;174(1):152–157. doi:10.1111/bjd.14163
33. Randa H, Khoury LR, Grønborg TK, Lomholt JJ, Skov L, Zachariae R. Development and preliminary validation of the adolescent psoriasis quality of life instrument: a disease-specific measure of quality of life in adolescents with psoriasis. Br J Dermatol. 2020;183(1):96–104. doi:10.1111/bjd.18719
34. de Jager ME, de Jong EM, Meeuwis KA, van de Kerkhof PC, Seyger MM. No evidence found that childhood onset of psoriasis influences disease severity, future body mass index or type of treatments used. J Eur Acad Dermatol Venereol. 2010;24(11):1333–1339. doi:10.1111/j.1468-3083.2010.03645.x
35. Mahé E, Maccari F, Beauchet A, et al. Childhood onset psoriasis: association with future cardiovascular and metabolic comorbidities. Br J Dermatol. 2013;169(4):889–895. doi:10.1111/bjd.12441
36. Ramsay B, O’Reagan M. A survey of the social and psychological effects of psoriasis. Br J Dermatol. 1988;118(2):195–201. doi:10.1111/j.1365-2133.1988.tb01774.x
37. Bilgic A, Ö B, Akış HK, Eskioğlu F, Kılıç EZ. Psychiatric symptoms and health-related quality of life in children and adolescents with psoriasis. Pediatr Dermatol. 2010;27(6):614–617. doi:10.1111/j.1525-1470.2010.01195.x
38. Carter-Pokras OD, Bugbee BA, Gold RS, et al. Utilizing student health and academic data: a county-level demonstration project. Health Promot Pract. 2019. doi:10.1177/1524839919862796
39. Zink A, Herrmann M, Fischer T, et al. Addiction: an underestimated problem in psoriasis health care. J Eur Acad Dermatol Venereol. 2017;31(8):1308–1315. doi:10.1111/jdv.14204
40. Chroni E, Monastirli A, Tsambaos D. Neuromuscular adverse effects associated with systemic retinoid dermatotherapy: monitoring and treatment algorithm for clinicians. Drug Saf. 2010;33(1):25–34. doi:10.2165/11319020-000000000-00000
41. Balato N, Megna M, Palmisano F, et al. Psoriasis and sport: a new ally? J Eur Acad Dermatol Venereol. 2015;29(3):515–520. doi:10.1111/jdv.12607
42. Larsabal M, Ly S, Sbidian E, et al. GENIPSO: a French prospective study assessing instantaneous prevalence, clinical features and impact on quality of life of genital psoriasis among patients consulting for psoriasis. Br J Dermatol. 2019;180(3):647–656. doi:10.1111/bjd.17147
43. Lambe M, Bergstrom AV, Johansson ALV, Weibull CE. Reproductive patterns and maternal and pregnancy outcomes in women with psoriasis-A population-based study. J Am Acad Dermatol. 2020;82(5):1109–1116. doi:10.1016/j.jaad.2019.05.099
44. Cacciapuoti S, Scala E, Megna M, et al. Impact of current anti-psoriatic systemic treatments on male and female fertility: what the endocrinologist needs to know. Minerva Endocrinol. 2020. doi:10.23736/S0391-1977.20.03236-8
45. Schuster B, Ziehfreund S, Biedermann T, Zink A. Psoriasis 2.0: facebook as a source of disease-related information for patients with psoriasis. J Dtsch Dermatol Ges. 2020;18(6):571–581.
46. Amir M, Sampson BP, Endly D, et al. Social networking sites: emerging and essential tools for communication in dermatology. JAMA Dermatol. 2014;150(1):56–60. doi:10.1001/jamadermatol.2013.6340
47. Perkins S, Cohen JM, Nelson CA, Bunick CG. Teledermatology in the era of COVID-19: experience of an academic department of dermatology. J Am Acad Dermatol. 2020;83(1):e43–e44. doi:10.1016/j.jaad.2020.04.048
48. Lavaud J, Mahé E. Proactive treatment in childhood psoriasis. Ann Dermatol Venereol. 2020;147(1):29–35. doi:10.1016/j.annder.2019.07.005
49. Mahé E, Corgibet F, Maccari F, et al. Prescriptions hors AMM (autorisation de mise sur le marché) dans le psoriasis de l’enfant. Ann Dermatol Venereol. 2020;147(6–7):429–438. doi:10.1016/j.annder.2020.01.021
50. Phan C, Beauchet A, Burztejn AC, et al. Biological treatments for paediatric psoriasis: a retrospective observational study on biological drug survival in daily practice in childhood psoriasis. J Eur Acad Dermatol Venereol. 2019;33(10):1984–1992. doi:10.1111/jdv.15579
51. Seyger M, Abramovits W, Liljedahl M, Hoejen MN, Teng J. Safety and efficacy of fixed-dose combination calcipotriol (50 μg/g) and betamethasone dipropionate (0.5 mg/g) cutaneous foam in adolescent patients (aged 12 to <7 years) with plaque psoriasis: results of a Phase II, open-label trial. J Eur Acad Dermatol Venereol. 2020. doi:10.1111/jdv.16233
52. Charbit L, Mahé E, Phan A, et al. Systemic treatments in childhood psoriasis: a French multicentre study on 154 children. Br J Dermatol. 2016;174(5):1118–1121. doi:10.1111/bjd.14326
53. Bronckers IMGJ, Paller AS, West DP, et al. A comparison of psoriasis severity in pediatric patients treated with methotrexate vs biologic agents. JAMA Dermatol. 2020;156(4):384–392. doi:10.1001/jamadermatol.2019.4835
54. Bronckers IMGJ, Seyger MMB, West DP, et al. Safety of systemic agents for the treatment of pediatric psoriasis. JAMA Dermatol. 2017;153(11):1147–1157. doi:10.1001/jamadermatol.2017.3029
55. De Jager ME, de Jong EM, van de Kerkhof PC, Seyger MM. Efficacy and safety of treatments for childhood psoriasis: a systematic literature review. J Am Acad Dermatol. 2010;62(6):1013–1030. doi:10.1016/j.jaad.2009.06.048
56. Fortina AB, Bardazzi F, Berti S, et al. Treatment of severe psoriasis in children: recommendations of an Italian expert group. Eur J Pediatr. 2017;176(10):1339–1354. doi:10.1007/s00431-017-2985-x
57. Menter A, Cordoro KM, Davis DMR, et al. Joint American Academy of dermatology-national psoriasis foundation guidelines of care for the management and treatment of psoriasis in pediatric patients. J Am Acad Dermatol. 2020;82(1):161–201. doi:10.1016/j.jaad.2019.08.049
58. Eisert L, Augustin M, Bach S, et al. S2k guidelines for the treatment of psoriasis in children and adolescents - Short version part 2. J Dtsch Dermatol Ges. 2019;17(9):959–973.
59. McClure SL, Valentine J, Gordon KB. Comparative tolerability of systemic treatments for plaque-type psoriasis. Drug Saf. 2002;25(13):913–927. doi:10.2165/00002018-200225130-00003
60. Matos RS, Torres T. Facial hair growth in a patient with psoriasis. Aust Fam Physician. 2016;45(9):641–642.
61. Man MQ, Ye L, Hu L, Jeong S, Elias PM, Lv C. Improvements in epidermal function prevent relapse of psoriasis: a self-controlled study. Clin Exp Dermatol. 2019;44(6):654–657. doi:10.1111/ced.13888
62. Balak DMW, Gerdes S, Parodi A, Salgado-Boquete L. Long-term safety of oral systemic therapies for psoriasis: a comprehensive review of the literature. Dermatol Ther (Heidelb). 2020;10(4):589–613. doi:10.1007/s13555-020-00409-4
63. Paller AS, Siegfried EC, Langley RG, et al. Etanercept treatment for children and adolescents with plaque psoriasis. N Engl J Med. 2008;358(3):241–251. doi:10.1056/NEJMoa066886
64. Papp K, Thaçi D, Marcoux D, et al. Efficacy and safety of adalimumab every other week versus methotrexate once weekly in children and adolescents with severe chronic plaque psoriasis: a randomised, double-blind, Phase 3 trial. Lancet. 2017;390(10089):40–49. doi:10.1016/S0140-6736(17)31189-3
65. Landells I, Marano C, Hsu MC, et al. Ustekinumab in adolescent patients age 12 to 17 years with moderate-to-severe plaque psoriasis: results of the randomized phase 3 CADMUS study. J Am Acad Dermatol. 2015;73(4):594–603. doi:10.1016/j.jaad.2015.07.002
66. Paller AS, Seyger MMB, Alejandro Magariños G, et al. Efficacy and safety of ixekizumab in a Phase III, randomized, double-blind, placebo-controlled study in paediatric patients with moderate-to-severe plaque psoriasis (IXORA-PEDS). Br J Dermatol. 2020;183(2):231–241. doi:10.1111/bjd.19147
67. Summary of product characteristics. Available from: https://www.ema.europa.eu/en/documents/product-information/cosentyx-epar-product-information_en.pdf.
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
