Back to Journals » OncoTargets and Therapy » Volume 13
Optimal Management of First-Line Advanced Renal Cell Carcinoma: Focus on Pembrolizumab
Authors Singh A, Singh I, Singh N, Puzanov I
Received 4 December 2019
Accepted for publication 3 April 2020
Published 13 May 2020 Volume 2020:13 Pages 4021—4034
DOI https://doi.org/10.2147/OTT.S215173
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Jianmin Xu
Abhay Singh, 1,* Inderpreet Singh, 2,* Namrata Singh, 3 Igor Puzanov 1
1Department of Medicine, Roswell Park Comprehensive Cancer Center, Buffalo, NY, USA; 2Department of Medicine, University of Colorado Anschutz Medical Campus, Aurora, CO, USA; 3Department of Medicine, Punjab Institute of Medical Sciences, Punjab, India
*These authors contributed equally to this work
Correspondence: Igor Puzanov
Department of Medicine, Roswell Park Comprehensive Cancer Center, Elm and Carlton Street, Buffalo, NY 14263, USA
Tel +1 716 845 4101
Fax +1 716 845 3423
Email [email protected]
Abstract: Renal cell carcinoma (RCC) is among the 10 most common cancers in the USA. One-third of the patients diagnosed with this cancer present with locally advanced or metastatic disease. In the past, advanced disease conferred poor survival outcomes; however, the treatment paradigm for RCC has been revolutionized twice since 2005. The initial wave of revolution came with the emergence of vascular endothelial growth factor (VEGF) inhibitors and a second wave arose more recently with the emergence and unprecedented success of checkpoint inhibitors in RCC. A third wave combining these two strategies is well underway and likely represents the new paradigm to improve survival outcomes for afflicted patients. In this review, we discuss the current treatment landscape for patients with advanced RCC, focusing on approved VEGF and checkpoint inhibitors in the first-line setting as well as highlighting landmark combination clinical trials.
Keywords: renal cell carcinoma, metastatic, VEGF inhibitors, checkpoint inhibitors, axitinib, pembrolizumab
Introduction
Renal cell carcinoma (RCC) arises from the renal tubular epithelium. It is clinically divided into two histological subtypes: clear cell (cc) RCC and non-clear cell (ncc) RCC.1 ccRCC is the most common subtype of RCC, so named because the dissolution of high lipid contents during histological preparation leaves a clear residual cytoplasm.2 The majority of deaths from kidney cancer are attributed to ccRCC, due to the predominance of this subtype in the metastatic disease. RCC can be classified into several other subtypes, namely, medullary, chromophobe, papillary, and collecting duct, and an increasingly expanding list of additional subtypes makes up the nccRCC group.1 Various subtypes of RCC are shown in Table 1.
 |
Table 1 WHO Classification of Major Tumor Subtypes of Renal Cell Cancer, Clinical Presentation and Molecular Alterations |
Computed tomography has an established role in tumor staging to define local invasion, lymph-node involvement, or metastatic disease.2 Although 65% of renal cancers at detection are confined to the primary site and the disease has an excellent 5-year survival of 92.5%, a significant proportion of patients with RCC have advanced disease at presentation, accounting for 16% of patients with metastatic disease and 17% of patients with regionally spread disease.3 Distant metastatic disease accounts for the worst prognosis, with a 5-year survival rate of approximately 10%.4 The survival curve is changing with the advent of newer therapies.
Metastatic renal cell carcinoma (mRCC) is managed by surgical therapy such as cytoreductive nephrectomy (CN).5–7 In recent years, recognition of new targets for systemic therapies, such as vascular endothelial growth factor (VEGF) and mammalian target of rapamycin (mTOR) pathway inhibitors, has emerged as new developments in the treatment of mRCC.8 Immunotherapy with interleukin-2 (IL-2) had shown convincing results, including durable complete remission in 7–8% of patients;9 however, it was associated with serious toxicity such as capillary leak syndrome and fatal end-organ failure.10 The emergence of VEGF inhibitors and new immunotherapy in the form of checkpoint inhibitors since 2005 has been revolutionary for the treatment of RCC.11
Epidemiology
RCC is among 10 most commonly diagnosed cancers in the USA for both sexes, being responsible for more than 14,000 estimated deaths in 2019.3 RCC is more predominant in males than females (2:1 ratio) and has a median age at presentation of around 60 years.2 The presentation of RCC as an incidental finding has increased gradually over the years, in part owing to improved imaging modalities. Established risk factors for RCC include cigarette smoking,12 obesity,13 and hypertension.14 However, most patients do not have any identifiable risk factors and pathological mechanisms for identifiable risk factors are not fully understood.2 Moreover, 2–3% cases of RCC have also been linked with autosomal dominant syndromes such as von Hippel–Lindau (VHL) syndrome.15 In VHL syndrome, the tumor suppressor gene has been identified on chromosome 3 (3p25–26).16 A defect in one allele of the VHL gene is inherited in patients with VHL syndrome and a defect in the other allele is acquired in the affected organ. An acquired defect in both VHL alleles is seen in most patients with sporadic/non-inherited ccRCC (Table 1), resulting in dysfunction of the VHL protein.2 Alterations in the VHL tumor suppressor gene on chromosome 3 can be seen in 90% of cases of ccRCC.17
Risk Stratification
Two prognostic models are widely accepted and used in risk stratification/prognostication of mRCC: the Memorial Sloan Kettering Cancer Center (MSKCC) model18,19 and the International mRCC Database Consortium (IMDC) model.20,21 The MSKCC criteria were used more during the cytokine therapy era; however, with the advent of newer VEGF therapies in recent years, the IMDC criteria have gained more acceptance and use in contemporary clinical trials.20,22–24 In both models, patients are stratified according to survival outcomes into poor-, intermediate-, and favorable-risk groups. The clinical criteria and comparison of both models can be seen in Table 2, along with their interpretation.
 |
Table 2 Prognostic Risk Criteria |
Management of Locally Advanced RCC
Locally advanced RCC encompasses inferior vena cava involvement, extension to adjacent organs, retroperitoneal lymph-node involvement, and local recurrence after radical surgery.25 Radical extirpative surgery is strongly indicated for locally advanced RCC. Radical nephrectomy is the management of choice for locally advanced tumors, along with occasional en bloc resection of adjacent organs, venous thrombectomy, and regional vasculature isolation and temporary occlusion.25 Such an aggressive approach can cure up to 40–60% of patients.26 Despite extensive surgical resection in patients with locally advanced RCC, they have a significant risk of disease recurrence and progression. These adverse clinical outcomes have typically been associated with the presence of advanced pathological state, extracapsular extension, venous involvement, or nodal metastases.27,28 The development of new multimodality strategies (especially combination with immunotherapy, neoadjuvantly or adjuvantly) to be used in combination with surgical resection is an essential step towards further improving the outcome of these patients.
Management of Metastatic Renal Cell Carcinoma
Cytoreductive Nephrectomy
While surgical resection is the standard for localized or organ-confined disease, historically, its role was less defined in the management of metastatic disease. This changed after prospective trials in the early 2000s showed a benefit of CN prior to systemic therapy. Surgical therapy thus became an important part of the management of patients with mRCC. Two landmark prospective trials reported in 2001 established the role of CN in the setting of mRCC. In the EORTC-30947 trial, radical nephrectomy plus interferon-α (IFN-α)-based immunotherapy was compared with IFN-α alone in mRCC. The EORTC cohort showed a 10-month overall survival (OS) benefit in the CN group (17 vs 7 months).6 Another randomized comparison of nephrectomy followed by IFN-α2b versus IFN-α2b alone in patients with advanced RCC (SWOG 8949) demonstrated a 3-month OS benefit in the CN group (11.1 vs 8.1 months).7 A subsequent combined analysis of 331 patients from two pooled cohorts revealed a 6-month OS benefit of CN (13.6 vs 7.8 months).5 These were the first trials to prospectively demonstrate a survival advantage for CN followed by systemic therapy versus systemic therapy alone. However, more recently the role of CN has been challenged, especially in the era of targeted therapies. A large phase III randomized trial evaluated the efficacy of sunitinib alone or after CN in metastatic disease29 and demonstrated the non-inferiority of sunitinib in comparison to CN followed by sunitinib, but in patients classified as having intermediate- or poor-risk disease. Given such competing challenges and equivocal results, the selection of patients plays a critical role when considering CN in mRCC, and in properly chosen patients CN still represents an essential component of care. This especially holds true for patients with low-burden or slow-growing metastatic disease post-nephrectomy, where prolonged surveillance periods can be utilized until overt progression occurs, at which stage systemic therapy can be appropriately employed.30
Systemic Therapy
Improved understanding of the pathogenesis of mRCC has revolutionized the treatment of mRCC twice since 2005 . First was the increased understanding of the vital role of angiogenesis contributing to the development of VEGF inhibitors; and then, the success of immune checkpoint inhibitors (ICIs) underscoring the immunogenicity of renal cancer cells. Figure 1 depicts the approved systemic therapies for advanced or metastatic RCC according to year of approval.
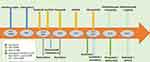 |
Figure 1 Systemic therapies for advanced or metastatic renal cell carcinoma according to year of approval. |
Proangiogenic Factors
Alteration in the VHL tumor suppressor gene on chromosome 3 can be seen in 90% of cases of ccRCC.17 Loss of function of the VHL gene can result in the accumulation of hypoxia inducible factor (HIF). Increased HIF accumulation leads to the production of proangiogenic factors, namely VEGF, platelet-derived growth factor (PDGF), and fibroblast growth factor (FGF).31 Inhibition of these pathways via targeted therapies such as tyrosine kinase inhibitors (TKIs) (sunitinib, axitinib, pazopanib) is depicted in Figure 2 and discussed in detail in the following text. HIF also induces activation of MET and AXL (also proangiogenic factors), which support growth, survival, invasion, and metastasis.32 They also have a vital role in treatment resistance to VEGF inhibitors resulting from upregulation of the alternative angiogenesis pathway.33 Cabozantinib is an oral TKI that targets vascular endothelial growth factor receptors (VEGFRs) in addition to MET and AXL (Figure 2), and therefore results in the simultaneous suppression of metastasis, angiogenesis, and tumor growth.34
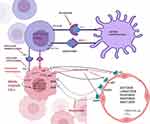 |
Figure 2 Pathways of tumor growth in renal cell carcinoma and systemic targeted therapies of these pathways. |
Currently Approved First-Line Agents and Combinations
Single-Agent Angiogenesis Inhibitors
Sunitinib, in a phase III trial against IFN-α, demonstrated significant improvement in median progression-free survival (PFS) (11 vs 5 months), with a hazard ratio (HR) of 0.42 (95% CI 0.32–0.54) and an objective response rate (ORR) of 31% versus 6% in favor of sunitinib.35 Pazopanib, in a phase III placebo-controlled trial, improved PFS in treatment-naïve or cytokine-treated good- and intermediate-risk patients (HR 0.46; 95% CI 0.34–0.62). In the subset of treatment-naïve patients, the median PFS was 11 versus 2.8 months.36,37 When pazopanib and sunitinib were compared against each other in a non-inferiority phase III COMPARZ trial, pazopanib was found to be non-inferior to sunitinib, with similar PFS (HR 1.05; 95% CI 0.9–1.22) and OS (HR 0.91; 95% CI 0.76–1.08).38 However, the safety and quality-of-life profiles favored pazopanib in 11 of 14 health-related quality-of-life domains, particularly those related to fatigue or palmar–plantar erythrodysesthesia. At the same time, sunitinib was more commonly associated with thrombocytopenia but less frequently with transaminitis compared to pazopanib.38 Given comparable efficacy and safety profiles, both sunitinib and pazopanib are indicated as agents for first-line therapy in favorable-risk disease.
Cabozantinib, an inhibitor of VEGFR, MET, and AXL, was compared to sunitinib in a phase II CABOSUN trial involving IMDC intermediate- and poor-risk patients.39 Compared with sunitinib, cabozantinib had favorable outcomes for both PFS (8.2 vs 5.6 months; HR 0.66; 95% CI 0.46–0.95) and ORR (33% vs 12%). Following this development, cabozantinib was approved as a first-line option for mRCC on December 19, 2017. Common toxicities of cabozantinib include fatigue, hypertension, diarrhea, abnormal liver function tests, anorexia, and palmar–plantar erythrodysesthesia syndrome, similarly to other VEGFR TKIs.39
Axitinib, an oral TKI and a potent VEGFR inhibitor, demonstrated clinical benefit as a second-line agent for patients with advanced RCC. In a phase III randomized trial, patients with treatment failure from a first-line therapy with sunitinib/bevacizumab with INF-α or temsirolimus were randomly assigned to receive either axitinib or sorafenib. Although median OS was similar in both treatment groups, PFS of 6.7 months versus 4.7 months (HR 0.67; 95% CI 0.54–0.81) and ORR (19% vs 11%) in an intention-to-treat (ITT) population favored axitinib over sorafenib.40,41 These results established axitinib as a second-line agent for treatment of mRCC. In the first-line setting, axitinib alone has a limited role and is useful under certain circumstances (category 2B recommendation by the National Comprehensive Cancer Network [NCCN]).93
Mammalian Target of Rapamycin (mTOR) Signaling Pathway
The constitutively activated mTOR signaling pathway plays a significant role in the tumorigenesis and growth of RCC. The mTOR pathway can be activated by cancer cells via different mechanisms, including loss of p53, mutations in upstream components of PI3K (Figure 2), and paracrine growth factor production, or via mTOR complexes such as TSC1/2, PTEN, Lkb1, and Nf1.42 mTOR inhibitors, also known as rapalogs (analogs of rapamycin), inhibit the phosphorylation of mTOR, resulting in altered translation of messenger RNA that codes for the proteins involved in cell survival, cell proliferation, and angiogenesis.42
Temsirolimus, an mTOR inhibitor, was compared with IFN-α in a phase III Global Advanced Renal Cell Carcinoma (Global ARCC) three-arm trial involving patients with previously untreated, poor-prognosis mRCC, divided into treatment groups with temsirolimus, IFN-α, and a combination of temsirolimus and IFN-α. The temsirolimus arm demonstrated superior OS versus IFN-α (HR 0.73; 95% CI 0.58–0.92), although the addition of temsirolimus to IFN-α in the combination group did not show any improved survival versus IFN-α alone.43 Temsirolimus is indicated for use in intermediate- and especially poor-risk patients in the first-line setting under select circumstances (category 1 recommendation by the NCCN).93
Other approved therapies include selective monoclonal antibodies, such as bevacizumab, directed against VEGF, which also inhibit angiogenesis and therefore impede tumor growth.44
However, after favorable outcomes of combinatorial immunotherapy (ipilimumab plus nivolumab) trials and now the success of immunotherapy combinations with VEGF inhibitors (discussed in the following sections), the role of several of the aforementioned drugs as single agents is less distinct and useful only under certain circumstances, for example in cases of absolute contraindication to the use of upfront immunotherapy.
Immunotherapy
The concept of immunosurveillance and acquisition of somatic mutations over an individual’s lifetime has been foundational to understanding tumorigenesis and, therefore, the exploration of mechanisms to potentiate mutant clone clearance via reinvigoration of immune surveillance has been a cornerstone of cancer drug development in recent years.45–47
The finding of IFN-γ's role in host protection from transplanted tumors was highlighted in the mid-1980s.48 More recently, focus has shifted on to the role of Tcells in both cellular and humoral immunological effects on tumor growth. Short peptides of 8–10 and 13–20 amino acids bind to class I and class II major histocompatibility complex (MHC) molecules, respectively. T lymphocytes recognize these peptide MHC complexes via T-cell receptors forming immunological synapses (Figure 2); the quality of the synapse determines whether a tumor-associated antigen (TAA) will elicit a T-cell response. The humoral arm of the immune system exerts antitumor effects through antibody-dependent cellular cytotoxicity (ADCC). ADCC-derived tumor cell lysis is mainly dependent on the interaction between Fcγ receptors on immune effector cells, such as macrophages, neutrophils, and natural killer (NK) cells, and the Fc region of an antibody bound to a tumor cell.49 Over the years, many different immunotherapeutic modalities have been studied to induce specific tumor responses, including those for mRCC.50
Cytokine Therapy
There exists an abundance of evidence to suggest that RCC is associated with the innate host-mediated immune response.51 In the early development of immunotherapy for RCC, IFN-α and IL-2 showed promising antitumor activity and remained the only viable options in mRCC for almost two decades.52–54
IL-2 stimulates the cytotoxic activity of T lymphocytes against tumor cells. It was first used in mRCC in the mid-1980s. The US Food and Drug Administration (FDA) approved high-dose intravenous IL-2 therapy for advanced RCC in 1992. It was associated with an ORR of 15% and a durable complete response (CR) of 5% in patients with mRRC.9 A more recent trial for patients with mRCC utilizing high-dose IL-2 resulted in an ORR of 25%, including 7.5% CRs, and a median response duration of 20.6 months.55 However, high-dose IL-2 was associated with serious toxicities such as capillary leak syndrome, hypotension, and fatal end-organ failure, thereby necessitating the judicious use of high-dose IL-2 in carefully selected patients with good performance status and preserved organ function.10 With the dawn and approval of several better tolerated and efficacious treatments in mRCC in recent years, the utility of high-dose IL-2 has declined steadily.56 High-dose IL-2 is now indicated for only a very select group of young patients and mandates careful selection of patients with excellent performance status and normal organ function.
Another cytokine-based therapy with IFN-α showed minimal antitumor activity in patients with advanced RCC, with overall response in only 10% of patients.57 Over the years, cytokines have been tested in combination with other chemotherapeutic agents to improve the response rates, with a combination of bevacizumab with IFN-α obtaining FDA approval in the first-line setting after the demonstration of significant improvement in PFS and a trend towards improved OS, although this was not statistically significant.58
The development of newer ICIs has revolutionized the role of immunotherapy in the management of mRCC, among other malignancies. In normal physiology, the programmed cell death protein-1 (PD-1)/programmed death ligand-1 (PD-L1) and cytotoxic T-lymphocyte-associated antigen-4 (CTLA-4) checkpoints attenuate T-cell activation and are vital in maintaining the immunological balance between self-defense and self-tolerance (Figure 2).59 Expression of checkpoint proteins such as PD-L1 by tumor cells can promote their immune tolerance, hence the blockers of PD-1/PD-L1 and CTLA-4 can strengthen the antitumor response of exhausted CD8 T cells.60 Several ICI combinations are now approved as first-line agents for advanced clear cell renal cell carcinoma (accRCC), as discussed in the following subsections. Major phase III trials of first-line Immunotherapy-based combination therapies are shown in Table 3.
 |
Table 3 Phase III Trials of First-Line Immunotherapy-Based Combination Therapies |
Ipilimumab Plus Nivolumab
CheckMate 214 was the first phase III randomized trial to demonstrate the clinical activity of ICI combination therapy in IMDC intermediate- or poor-risk patients.61 This study compared the combination of ipilimumab (an anti-CTLA-4 inhibitor) plus nivolumab (an anti-PD-1 inhibitor) to sunitinib. The ORR was 42% versus 27% (9% vs 1% CR) favoring combination compared to sunitinib. The median OS was still not reached at 30 months62 for the combination and it appeared that increasing PFS benefit was emerging in the ITT population as well as in the intermediate/poor-risk population.62 Sunitinib showed superior ORR (29% vs 52%) and PFS (15.3 vs 25.1 months; HR 2.18; 99.1% CI 1.29–3.68) in favorable-risk patients versus the combination therapy and this superiority continued to persist, although the gap was closing at 30-month follow-up, with ORR 39% versus 50% and PFS 13.9 versus 19.9 months (HR 1.23; 95% CI 0.90–1.69). In the most recent 42-month follow-up,63 OS HRs remained favorable for patients treated with combination in both the ITT (HR 0.72; 95% CI 0.61–0.86) and intermediate/poor-risk population (HR 0.66; 95% CI 0.55–0.80). The ORR for combination has continued to be higher in the ipilimumab/nivolumab cohort (ORR 42%, including 10% CRs). The PFS HR has been reported as 0.76, with 35% of patients in combination ipilimumab/nivolumab without progression, compared to 13% with sunitinib. Among favorable-risk patients, ORR continues to be higher with sunitinib at 42-month follow-up (54% vs 29%); however, more patients have achieved CR with combination (13% vs 6%). The responses appear more durable with combination than with sunitinib across all IMDC risk groups, and the PFS probabilities are stabilizing with combination and declining with sunitinib in the favorable-risk patients. This 42-month follow-up is the longest follow-up for any immunotherapy combination phase III trial in first-line treatment of advanced RCC, making it an attractive first-line option.63
Despite the questions about appropriate patient selection, in the setting of different outcomes in different IMDC risk groups, this trial challenged the treatment paradigm of first-line use of VEGF TKIs in RCC by showing the superiority of ipilimumab plus nivolumab over sunitinib.61 The toxicity profile of the combination therapy was found to be significantly different from that of sunitinib, with more immune-related adverse effects such as hepatitis, colitis, rash, hypophysitis, adrenal insufficiency, nephritis, pneumonitis, and diabetes seen with the combination. Moreover, 35% of patients in the combination arm also required high-dose glucocorticoid (40 mg of prednisone per day or equivalent) therapy owing to toxicities.61 In addition, it is now emerging that this combination holds promise in RCCs with sarcomatoid differentiation (sRCC), a highly aggressive form of RCC with poor prognosis. sRCCs are considered rather inflamed tumors and express PD1/PD-L1 at rates higher than ccRCC.64 In a 2019 post-hoc analysis of CheckMate 214, ipilimumab plus nivolumab combination demonstrated promising efficacy (ORR 56.7% vs 19.2% in the sunitinib arm). Prolonged survival was also demonstrated (median OS 31.2 months vs 13.6 months, HR 0.55; 95% CI 0.33–0.90; p<0.0155).65 Based on these encouraging results, the consensus among experts is to consider the use of nivolumab plus ipilimumab in sRCCs.
Avelumab Plus Axitinib
Another anti-PD-L1 antibody, avelumab, has been compared with the standard of care, sunitinib. In a phase III trial involving treatment-naïve patients with advanced RCC, combination therapy of avelumab plus axitinib versus sunitinib demonstrated significantly longer PFS with avelumab plus axitinib than with sunitinib. Median PFS of 13.8 months was seen with avelumab plus axitinib, compared with 7.2 months with sunitinib (HR for disease progression or death 0.61; 95% CI 0.47–0.79) in patients with PD-L1-positive tumors. In the overall study population, median PFS of 13.8 months with avelumab plus axitinib was observed, compared with 8.4 months with sunitinib (HR 0.69; 95% CI 0.56–0.84). Patients with PD-L1-positive tumors had an ORR of 55.2% with avelumab plus axitinib and 25.5% with sunitinib.66 Following these results, the FDA approved avelumab in combination with axitinib for first-line treatment of patients with advanced RCC on May 14, 2019.
Atezolizumab Plus Bevacizumab
Atezolizumab is another PD-L1 inhibitor, which has been compared in combination with bevacizumab to sunitinib. A phase II study was conducted of atezolizumab with or without bevacizumab versus sunitinib for mRCC in treatment-naïve patients. Atezolizumab plus bevacizumab resulted in encouraging antitumor activity in the PD-L1-positive tumors, showing PFS HRs for combination versus sunitinib of 0.64 (95% CI 0.38–1.08) and 1.03 (95% CI 0.63–1.67) for atezolizumab alone versus sunitinib, prompting the study initiation of combination therapy of atezolizumab with bevacizumab in the large phase III trial,67 IMmotion151. This trial compared atezolizumab plus bevacizumab combination with sunitinib, enrolling 915 treatment-naïve patients with advanced RCC.68 In the PD-L1-positive population, the median PFS was 11.2 months in the atezolizumab plus bevacizumab group versus 7.7 months in the sunitinib group (HR 0.74; 95% CI 0.57–0.96). In the ITT population, the median OS HR was 0.93 (95% CI 0.76–1.14), which was not significant in the interim analysis. The ORR was 43% for atezolizumab plus bevacizumab group compared to 35% in the sunitinib group. The duration of response was not reached for atezolizumab plus bevacizumab, compared to 12.9 months for sunitinib in PD-L1-positive patients.69 Altogether, 24 (5%) in the atezolizumab plus bevacizumab group and 37 (8%) in the sunitinib group had treatment-related all-grade adverse events, which led to discontinuation of the treatment regimen.68 Atezolizumab and bevacizumab combination is not FDA approved as a first-line therapy for accRCC. In terms of this combination’s role in sRCC, subgroup analysis showed that patients who had sarcomatoid histology had median PFS of 8.3 versus 5.3 months with atezolizumab plus bevacizumab versus sunitinib, and median OS was not reached for the combination versus 15.0 months in the sunitinib arm. The combination also demonstrated a higher ORR of 49% versus 14% for sunitinib. As discussed previously, it was noted that PD-L1-positive disease was more common in sarcomatoid versus non-sarcomatoid tumors.70
Important Position of Pembrolizumab in Combination with Axitinib in the First-Line Setting
Pembrolizumab is another anti-PD-1 monoclonal antibody that has a significant molecular similarity to nivolumab. In recent studies pembrolizumab has shown encouraging antitumor activity as monotherapy74 as well as in combination with axitinib71 in patients with previously untreated accRCC.
In a phase Ib dose-finding and dose-expansion trial of axitinib in combination with pembrolizumab in treatment-naïve patients with accRCC, 11 patients were enrolled in the dose-finding and 41 patients in the dose-expansion phase. Of these patients, 73% achieved an objective response, including four CRs (8%) and 34 partial responses (65%). More than 90% of patients showed tumor shrinkage with >20-month median PFS.71 Previously, two first-line trials of axitinib monotherapy had shown median PFS of 10–15 months.72,73 Early-phase pembrolizumab combination trials in advanced RCC are outlined in Table 4.
 |
Table 4 Early-Phase Pembrolizumab Combinations in Advanced/Metastatic Renal Cell Carcinoma |
Pembrolizumab as a single agent has also demonstrated convincing results. In a phase II pembrolizumab monotherapy trial, pembrolizumab showed an ORR of 38% across all IMDC risk groups and even higher ORRs of 42% and 50% in patients in IMDC intermediate/poor-risk patients and patients with PD-L1-positive tumors, respectively.74
More recently, antitumor efficacy with a combination of pembrolizumab and axitinib was further tested in a large phase III trial (KEYNOTE-426) of treatment-naïve patients with accRCC. In this trial, 861 patients were randomly assigned to receive pembrolizumab plus axitinib versus sunitinib. The primary endpoints were OS and PFS in the ITT population. After a median follow-up of 12.8 months, pembrolizumab plus axitinib resulted in significantly longer OS and PFS (15.1 vs 11.1 months) in comparison to sunitinib. Pembrolizumab and axitinib combination also seems to have a higher objective response than other combination therapies. The ORR (59.3%) of pembrolizumab and axitinib is higher than in other combination trials, which found an ORR of 51.4% in the avelumab plus axitinib group66 and 39% ORR in the nivolumab and ipilimumab group.61 Cross-trial comparisons can often be misleading and the higher ORR noted in KEYNOTE-426 may have been driven by higher percentages of favorable-risk patients in KEYNOTE-426 in comparison to other groups. Nonetheless, pembrolizumab plus axitinib proved its efficacy in the ITT population across all IMDC-defined risk categories and these findings resulted in FDA approval of this combination on April 19, 2019. The benefit across all risk categories was not seen in the ipilimumab and nivolumab group and, therefore, the approval was limited to only IMDC intermediate- and poor-risk categories. NCCN guidelines recommend pembrolizumab and axitinib combination as first-line therapy in IMDC good-, intermediate-, and poor-risk patients (Table 5). Given its applicability across all IMDC risk strata, pembrolizumab plus axitinib combination now represents a new standard as front-line treatment for metastatic ccRCC.
 |
Table 5 NCCN Treatment Guidelines for First-Line Therapy for Stage IV Disease (Clear Cell Histology) |
In the intermediate- and poor-risk categories, the challenge remains to decide between nivolumab plus ipilimumab and pembrolizumab plus axitinib. In addition, in patients with sarcomatoid RCC (similar to ipilimumab plus nivolumab combination), pembrolizumab plus axitinib showed favorable results versus sunitinib in IMDC intermediate/poor-risk patients with sarcomatoid features. The exploratory analysis demonstrated improved PFS (HR 0.54; 95% CI 0.29–1.00; median not reached vs 8.4 months), OS (HR 0.58; 95% CI 0.21–1.59; 12-month rate 83.4% vs 79.5%), and CR of 11.8% (95% CI 4.4–23.9) versus 0% (95% CI 0.0–6.6) in pembrolizumab plus axitinib versus sunitinib.75 Given comparable efficacy profiles in both combinations (ipilimumab plus nivolumab and pembrolizumab plus axitinib), the clinical decision in choosing one combination over the other in IMDC intermediate/poor-risk patients will take into account patient preference of anticipated toxicities, contraindication to each therapy, and previous history of autoimmune conditions. Ipilimumab plus nivolumab combination has the longest follow-up for any immunotherapy combination phase III trial in first-line treatment of advanced RCC, making it an attractive treatment option. In KEYNOTE-426, the incidence of grade 3 or 4 elevations in liver enzyme levels with pembrolizumab and axitinib was much higher than previously observed profiles for monotherapy with either of these agents.76 Therefore, careful treatment assignment will be prudent in patients, based on the side-effect profiles of each regimen.
Despite all the successes, it remains important to acknowledge that above-mentioned immunotherapy trials, despite enrolling patients with intermediate/poor risk, have generally selected for patients with a good performance status (ECOG 0–1). The trial patients, therefore, are less representative of the general population, and how these immunotherapy combinations improve outcomes in the real-world setting remains to be determined.
Prospective Agents and Combination Trials in Progress
Several combinations of immunotherapeutic agents as well as VEGF TKIs are in the pipeline. PDIGREE (NCT03793166),77 an adaptive phase III trial of nivolumab and ipilimumab with VEGF TKI cabozantinib in metastatic untreated RCC, aims to determine which patients benefit most from the combination of immunotherapy and VEGF inhibitors, and the optimal sequence of these therapies. In this trial, patients start treatment with induction nivolumab plus ipilimumab. Then, based on 3-month radiographic assessment, patients with CR receive maintenance nivolumab every 4 weeks, those with progressive disease (PD) switch to cabozantinib daily, and patients with non-CR/non-PD are randomized to nivolumab every 4 weeks versus cabozantinib daily plus nivolumab every 4 weeks, with OS as the primary endpoint. Another phase III study (COSMIC-313, NCT03937219)78 aims to study the same drugs, testing the efficacy of “triplet” cabozantinib in combination with nivolumab and ipilimumab versus “doublet” nivolumab and ipilimumab (plus placebo), with PFS being the primary endpoint. CheckMate 9ER (NCT03141177) is another RCT comparing cabozantinib plus nivolumab versus sunitinib in the first-line setting in patients with previously untreated advanced or metastatic RCC. The trial includes around 630 participants and results are expected to be released sometime in 2020.79 CLEAR 307, a phase III, open-label study (NCT02811861) with three treatment arms, is using a different TKI (lenvatinib) and comparing its efficacy and safety in combination with everolimus or pembrolizumab versus sunitinib alone in first-line treatment of subjects with advanced RCC. This study will include around 1067 patients and results are expected sometime in 2021.80 In addition to these phase III trials, another ongoing trial is the Titan RCC (NCT02917772). Titan RCC is a phase II single-arm clinical trial of a tailored immunotherapy approach with nivolumab in subjects with metastatic or advanced RCC. This study aims to optimize therapy based on responses and to boost suboptimal responses using immunotherapy “boosts” in patients with stable and progressive disease. This trial includes around 200 participants and is expected to complete by 2021.81
Conclusions
Since the turn of the twenty-first century, tremendous progress has been made in the management and treatment of advanced RCC. Better understanding of molecular profiles has led to the development of targeted therapies and improved understanding of antitumor immunity has changed the clinical landscape of the disease. The paradigm has shifted twice in the past decade and it continues to shift towards improving patient outcomes with the use of combination regimens. The immune checkpoint–VEGF inhibitor combinatorial regimens (axitinib plus pembrolizumab or avelumab) are now approved and form part of the ever-expanding treatment armamentarium. However, this poses a new challenge of optimal sequencing of therapy, since the efficacy of second-line options after the newer first-line therapies is unknown. This is an area of active exploration (PDIGREE study; discussed in the previous section) and evidence is eagerly awaited to inform clinical practice. In addition, from anecdotal physician experience with ICIs and available data on long-term durable responses with high-dose IL-2,82 very long disease-free survival and possible cures for advanced RCC are on the horizon. Maintaining these long disease-free survival periods will be highly desirable and preferably off-therapy. Therefore, newer combination regimens aiming for prolonged disease-free survival should evaluate strategies for drug-free periods. One such effort is currently underway (OMNIVORE Study: NCT03203473), which aims to optimize treatments based on response, and the results of this and other endeavors are eagerly awaited. In addition, patients with RCC of sarcomatoid differentiation historically have had a worse prognosis, with poor response to VEGF therapy alone. However, it now appears that these patients have promising options with immune-based therapies. This is being explored with all of the combinations; however, the response to nivolumab plus ipilimumab is notably impressive. Lastly, given the expanding treatment armamentarium, there exists a huge unmet need to discover predictive and prognostic biomarkers to better risk-stratify patients to guide appropriate clinical decision making, with the goal of maximizing efficacy and minimizing toxicity.
Disclosure
The authors report no conflicts of interest in this work.
References
1. Renshaw AA. Subclassification of renal cell neoplasms: an update for the practising pathologist. Histopathology. 2002;41(4):283–300. doi:10.1046/j.1365-2559.2002.01420.x
2. Rini BI, Campbell SC, Escudier B. Renal cell carcinoma. The Lancet. 2009;373(9669):1119–1132. doi:10.1016/S0140-6736(09)60229-4
3. National Cancer Institute. Surveillance, Epidemiology, and End Results Program. Cancer stat facts: kidney and renal pelvis cancer. Available from: https://seer.cancer.gov/statfacts/html/kidrp.html. Accessed October 27, 2019.
4. Li P, Wong YN, Armstrong K, et al. Survival among patients with advanced renal cell carcinoma in the pretargeted versus targeted therapy eras. Cancer Med. 2016;5(2):169–181. doi:10.1002/cam4.574
5. Flanigan RC, Mickisch G, Sylvester R, Tangen C, Van Poppel H, Crawford ED. Cytoreductive nephrectomy in patients with metastatic renal cancer: a combined analysis. J Urol. 2004;171(3):1071–1076. doi:10.1097/01.ju.0000110610.61545.ae
6. Mickisch GH, Garin A, van Poppel H, de Prijck L, Sylvester R. Radical nephrectomy plus interferon-alfa-based immunotherapy compared with interferon alfa alone in metastatic renal-cell carcinoma: a randomised trial. Lancet. 2001;358(9286):966–970. doi:10.1016/S0140-6736(01)06103-7
7. Flanigan RC, Salmon SE, Blumenstein BA, et al. Nephrectomy followed by interferon alfa-2b compared with interferon alfa-2b alone for metastatic renal-cell cancer. N Engl J Med. 2001;345(23):1655–1659. doi:10.1056/NEJMoa003013
8. Rodriguez-Vida A, Hutson TE, Bellmunt J, Strijbos MH. New treatment options for metastatic renal cell carcinoma. ESMO Open. 2017;2(2):e000185. doi:10.1136/esmoopen-2017-000185
9. Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC. Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol. 1995;13(3):688–696. doi:10.1200/JCO.1995.13.3.688
10. Dutcher JP, Schwartzentruber DJ, Kaufman HL, et al. High dose interleukin-2 (Aldesleukin) - expert consensus on best management practices-2014. J Immunother Cancer. 2014;2(1):26. doi:10.1186/s40425-014-0026-0
11. Motzer RJ, Escudier B, McDermott DF, et al. Nivolumab versus everolimus in advanced renal-cell carcinoma. N Engl J Med. 2015;373(19):1803–1813. doi:10.1056/NEJMoa1510665
12. Hunt JD, van der Hel OL, McMillan GP, Boffetta P, Brennan P. Renal cell carcinoma in relation to cigarette smoking: meta-analysis of 24 studies. Int J Cancer. 2005;114(1):101–108. doi:10.1002/ijc.20618
13. Bjorge T, Tretli S, Engeland A. Relation of height and body mass index to renal cell carcinoma in two million Norwegian men and women. Am J Epidemiol. 2004;160(12):1168–1176. doi:10.1093/aje/kwh345
14. McLaughlin JK, Chow WH, Mandel JS, et al. International renal-cell cancer study. VIII. Role of diuretics, other anti-hypertensive medications and hypertension. Int J Cancer. 1995;63(2):216–221. doi:10.1002/ijc.2910630212
15. Linehan WM, Walther MM, Zbar B. The genetic basis of cancer of the kidney. J Urol. 2003;170(6 Pt 1):2163–2172.
16. Latif F, Tory K, Gnarra J, et al. Identification of the von Hippel-Lindau disease tumor suppressor gene. Science. 1993;260(5112):1317–1320. doi:10.1126/science.8493574
17. Nickerson ML, Jaeger E, Shi Y, et al. Improved identification of von Hippel-Lindau gene alterations in clear cell renal tumors. Clin Cancer Res. 2008;14(15):4726–4734. doi:10.1158/1078-0432.CCR-07-4921
18. Motzer RJ, Bacik J, Murphy BA, Russo P, Mazumdar M. Interferon-alfa as a comparative treatment for clinical trials of new therapies against advanced renal cell carcinoma. J Clin Oncol. 2002;20(1):289–296. doi:10.1200/JCO.20.1.289
19. Mekhail TM, Abou-Jawde RM, Boumerhi G, et al. Validation and extension of the Memorial Sloan-Kettering prognostic factors model for survival in patients with previously untreated metastatic renal cell carcinoma. J Clin Oncol. 2005;23(4):832–841. doi:10.1200/JCO.2005.05.179
20. Heng DY, Xie W, Regan MM, et al. Prognostic factors for overall survival in patients with metastatic renal cell carcinoma treated with vascular endothelial growth factor-targeted agents: results from a large, multicenter study. J Clin Oncol. 2009;27(34):5794–5799. doi:10.1200/JCO.2008.21.4809
21. Heng DY, Xie W, Regan MM, et al. External validation and comparison with other models of the International metastatic renal-cell carcinoma database consortium prognostic model: a population-based study. Lancet Oncol. 2013;14(2):141–148. doi:10.1016/S1470-2045(12)70559-4
22. Motzer RJ, Bacik J, Mazumdar M. Prognostic factors for survival of patients with stage IV renal cell carcinoma: memorial sloan-kettering cancer center experience. Clin Cancer Res. 2004;10(18 Pt 2):6302s–6303s. doi:10.1158/1078-0432.CCR-040031
23. Motzer RJ, Bukowski RM, Figlin RA, et al. Prognostic nomogram for sunitinib in patients with metastatic renal cell carcinoma. Cancer. 2008;113(7):1552–1558. doi:10.1002/cncr.23776
24. Heng DY, Chi KN, Murray N, et al. A population-based study evaluating the impact of sunitinib on overall survival in the treatment of patients with metastatic renal cell cancer. Cancer. 2009;115(4):776–783. doi:10.1002/cncr.24051
25. Otaibi MA, Tanguay S. Locally advanced renal cell carcinoma. Can Urol Assoc J. 2007;1(2 Suppl):S55–61. doi:10.5489/cuaj.68
26. Blute ML, Leibovich BC, Lohse CM, Cheville JC, Zincke H. The Mayo Clinic experience with surgical management, complications and outcome for patients with renal cell carcinoma and venous tumour thrombus. BJU Int. 2004;94(1):33–41. doi:10.1111/j.1464-410X.2004.04897.x
27. Levy DA, Slaton JW, Swanson DA, Dinney CP. Stage specific guidelines for surveillance after radical nephrectomy for local renal cell carcinoma. J Urol. 1998;159(4):1163–1167. doi:10.1016/S0022-5347(01)63541-9
28. Frank I, Blute ML, Leibovich BC, Cheville JC, Lohse CM, Zincke H. Independent validation of the 2002 American Joint Committee on cancer primary tumor classification for renal cell carcinoma using a large, single institution cohort. J Urol. 2005;173(6):1889–1892. doi:10.1097/01.ju.0000158043.94525.d6
29. Méjean A, Ravaud A, Thezenas S, et al. Sunitinib alone or after nephrectomy in metastatic renal-cell carcinoma. N Engl J Med. 2018;379(5):417–427. doi:10.1056/NEJMoa1803675
30. Rini BI, Dorff TB, Elson P, et al. Active surveillance in metastatic renal-cell carcinoma: a prospective, phase 2 trial. Lancet Oncol. 2016;17(9):1317–1324. doi:10.1016/S1470-2045(16)30196-6
31. Nabi S, Kessler ER, Bernard B, Flaig TW, Lam ET. Renal cell carcinoma: a review of biology and pathophysiology. F1000Res. 2018;7:307. doi:10.12688/f1000research.13179.1
32. Kaelin WG
33. Zhou L, Liu XD, Sun M, et al. Targeting MET and AXL overcomes resistance to sunitinib therapy in renal cell carcinoma. Oncogene. 2016;35(21):2687–2697. doi:10.1038/onc.2015.343
34. Yakes FM, Chen J, Tan J, et al. Cabozantinib (XL184), a novel MET and VEGFR2 inhibitor, simultaneously suppresses metastasis, angiogenesis, and tumor growth. Mol Cancer Ther. 2011;10(12):2298–2308. doi:10.1158/1535-7163.MCT-11-0264
35. Motzer RJ, Hutson TE, Tomczak P, et al. Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med. 2007;356(2):115–124. doi:10.1056/NEJMoa065044
36. Sternberg CN, Davis ID, Mardiak J, et al. Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol. 2010;28(6):1061–1068. doi:10.1200/JCO.2009.23.9764
37. Sternberg CN, Hawkins RE, Wagstaff J, et al. A randomised, double-blind phase III study of pazopanib in patients with advanced and/or metastatic renal cell carcinoma: final overall survival results and safety update. Eur J Cancer. 2013;49(6):1287–1296. doi:10.1016/j.ejca.2012.12.010
38. Motzer RJ, Hutson TE, Cella D, et al. Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med. 2013;369(8):722–731. doi:10.1056/NEJMoa1303989
39. Choueiri TK, Halabi S, Sanford BL, et al. Cabozantinib versus sunitinib as initial targeted therapy for patients with metastatic renal cell carcinoma of poor or intermediate risk: the alliance A031203 CABOSUN trial. J Clin Oncol. 2017;35(6):591–597. doi:10.1200/JCO.2016.70.7398
40. Rini BI, Escudier B, Tomczak P, et al. Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet. 2011;378(9807):1931–1939. doi:10.1016/S0140-6736(11)61613-9
41. Motzer RJ, Escudier B, Tomczak P, et al. Axitinib versus sorafenib as second-line treatment for advanced renal cell carcinoma: overall survival analysis and updated results from a randomised phase 3 trial. Lancet Oncol. 2013;14(6):552–562. doi:10.1016/S1470-2045(13)70093-7
42. Laplante M, Sabatini DM. mTOR signaling in growth control and disease. Cell. 2012;149(2):274–293. doi:10.1016/j.cell.2012.03.017
43. Hudes G, Carducci M, Tomczak P, et al. Temsirolimus, interferon alfa, or both for advanced renal-cell carcinoma. N Engl J Med. 2007;356(22):2271–2281. doi:10.1056/NEJMoa066838
44. Niu G, Chen X. Vascular endothelial growth factor as an anti-angiogenic target for cancer therapy. Curr Drug Targets. 2010;11(8):1000–1017. doi:10.2174/138945010791591395
45. Burnet FM. The concept of immunological surveillance. Prog Exp Tumor Res. 1970;13:1–27.
46. Burnet M. Immunological factors in the process of carcinogenesis. Br Med Bull. 1964;20:154–158. doi:10.1093/oxfordjournals.bmb.a070310
47. Dunn GP, Bruce AT, Ikeda H, Old LJ, Schreiber RD. Cancer immunoediting: from immunosurveillance to tumor escape. Nat Immunol. 2002;3(11):991–998. doi:10.1038/ni1102-991
48. Dighe AS, Richards E, Old LJ, Schreiber RD. Enhanced in vivo growth and resistance to rejection of tumor cells expressing dominant negative IFN gamma receptors. Immunity. 1994;1(6):447–456. doi:10.1016/1074-7613(94)90087-6
49. Clynes RA, Towers TL, Presta LG, Ravetch JV. Inhibitory Fc receptors modulate in vivo cytotoxicity against tumor targets. Nat Med. 2000;6(4):443–446. doi:10.1038/74704
50. Chang AJ, Zhao L, Zhu Z, et al. The past, present and future of immunotherapy for metastatic renal cell carcinoma. Anticancer Res. 2019;39(6):2683–2687. doi:10.21873/anticanres.13393
51. Noessner E, Brech D, Mendler AN, Masouris I, Schlenker R, Prinz PU. Intratumoral alterations of dendritic-cell differentiation and CD8(+) T-cell anergy are immune escape mechanisms of clear cell renal cell carcinoma. Oncoimmunology. 2012;1(8):1451–1453. doi:10.4161/onci.21356
52. Alexandrescu DT, Dasanu CA. Kidney cancer therapy: new perspectives and avenues. Expert Opin Pharmacother. 2006;7(18):2481–2493. doi:10.1517/14656566.7.18.2481
53. Yang JC, Childs R. Immunotherapy for renal cell cancer. J Clin Oncol. 2006;24(35):5576–5583. doi:10.1200/JCO.2006.08.3774
54. Motzer RJ, Bukowski RM. Targeted therapy for metastatic renal cell carcinoma. J Clin Oncol. 2006;24(35):5601–5608. doi:10.1200/JCO.2006.08.5415
55. McDermott DF, Cheng SC, Signoretti S, et al. The high-dose aldesleukin “select” trial: a trial to prospectively validate predictive models of response to treatment in patients with metastatic renal cell carcinoma. Clin Cancer Res. 2015;21(3):561–568. doi:10.1158/1078-0432.CCR-14-1520
56. Allard CB, Gelpi-Hammerschmidt F, Harshman LC, et al. Contemporary trends in high-dose interleukin-2 use for metastatic renal cell carcinoma in the United States. Urol Oncol. 2015;33(11):
57. Minasian LM, Motzer RJ, Gluck L, Mazumdar M, Vlamis V, Krown SE. Interferon alfa-2a in advanced renal cell carcinoma: treatment results and survival in 159 patients with long-term follow-up. J Clin Oncol. 1993;11(7):1368–1375. doi:10.1200/JCO.1993.11.7.1368
58. Escudier B, Pluzanska A, Koralewski P, et al. Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet. 2007;370(9605):2103–2111. doi:10.1016/S0140-6736(07)61904-7
59. Pardoll DM. The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer. 2012;12(4):252–264. doi:10.1038/nrc3239
60. Wei SC, Levine JH, Cogdill AP, et al. Distinct cellular mechanisms underlie anti-CTLA-4 and Anti-PD-1 checkpoint blockade. Cell. 2017;170(6):1120–1133.e1117. doi:10.1016/j.cell.2017.07.024
61. Motzer RJ, Tannir NM, McDermott DF, et al. Nivolumab plus ipilimumab versus sunitinib in advanced renal-cell carcinoma. N Engl J Med. 2018;378(14):1277–1290. doi:10.1056/NEJMoa1712126
62. Tannir NM, Frontera OA, Hammers HJ, et al. Thirty-month follow-up of the phase III CheckMate 214 trial of first-line nivolumab + ipilimumab (N+I) or sunitinib (S) in patients (pts) with advanced renal cell carcinoma (aRCC). J Clin Oncol. 2019;37(7_suppl):547. doi:10.1200/JCO.2019.37.7_suppl.547
63. Tannir NM, McDermott DF, Escudier B, et al. Overall survival and independent review of response in CheckMate 214 with 42-month follow-up: first-line nivolumab + ipilimumab (N+I) versus sunitinib (S) in patients (pts) with advanced renal cell carcinoma (aRCC). J Clin Oncol. 2020;38(6_suppl):609. doi:10.1200/JCO.2020.38.6_suppl.609
64. Joseph RW, Millis SZ, Carballido EM, et al. PD-1 and PD-L1 expression in renal cell carcinoma with sarcomatoid differentiation. 2015;3(12):1303–1307.
65. McDermott DF, Choueiri TK, Motzer RJ, et al. CheckMate 214 post-hoc analyses of nivolumab plus ipilimumab or sunitinib in IMDC intermediate/poor-risk patients with previously untreated advanced renal cell carcinoma with sarcomatoid features. J Clin Oncol. 2019;37(15_suppl):4513. doi:10.1200/JCO.2019.37.15_suppl.4513
66. Motzer RJ, Penkov K, Haanen J, et al. Avelumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1103–1115. doi:10.1056/NEJMoa1816047
67. McDermott DF, Atkins MB, Motzer RJ, et al. A phase II study of atezolizumab (atezo) with or without bevacizumab (bev) versus sunitinib (sun) in untreated metastatic renal cell carcinoma (mRCC) patients (pts). J Clin Oncol. 2017;35(6_suppl):431. doi:10.1200/JCO.2017.35.6_suppl.431
68. Rini BI, Powles T, Atkins MB, et al. Atezolizumab plus bevacizumab versus sunitinib in patients with previously untreated metastatic renal cell carcinoma (IMmotion151): a multicentre, open-label, phase 3, randomised controlled trial. Lancet. 2019;393(10189):2404–2415. doi:10.1016/S0140-6736(19)30723-8
69. Motzer RJ, Powles T, Atkins MB, et al. IMmotion151: a randomized Phase III study of atezolizumab plus bevacizumab vs sunitinib in untreated Metastatic Renal Cell Carcinoma (mRCC). J Clin Oncol. 2018;36(6_suppl):578. doi:10.1200/JCO.2018.36.6_suppl.578
70. Rini BI, Motzer RJ, Powles T, et al. Atezolizumab (atezo)+ bevacizumab (bev) versus sunitinib (sun) in pts with untreated metastatic renal cell carcinoma (mRCC) and sarcomatoid (sarc) histology: iMmotion151 subgroup analysis. Journal of Clinical Oncology. 2019;37(15_suppl):4512. doi:10.1200/JCO.2019.37.15_suppl.4512
71. Atkins MB, Plimack ER, Puzanov I, et al. Axitinib in combination with pembrolizumab in patients with advanced renal cell cancer: a non-randomised, open-label, dose-finding, and dose-expansion phase 1b trial. Lancet Oncol. 2018;19(3):405–415. doi:10.1016/S1470-2045(18)30081-0
72. Rini BI, Melichar B, Ueda T, et al. Axitinib with or without dose titration for first-line metastatic renal-cell carcinoma: a randomised double-blind phase 2 trial. Lancet Oncol. 2013;14(12):1233–1242. doi:10.1016/S1470-2045(13)70464-9
73. Hutson TE, Lesovoy V, Al-Shukri S, et al. Axitinib versus sorafenib as first-line therapy in patients with metastatic renal-cell carcinoma: a randomised open-label phase 3 trial. Lancet Oncol. 2013;14(13):1287–1294. doi:10.1016/S1470-2045(13)70465-0
74. McDermott DF, Lee J-L, Szczylik C, et al. Pembrolizumab monotherapy as first-line therapy in advanced clear cell renal cell carcinoma (accRCC): results from cohort A of KEYNOTE-427. J Clin Oncol. 2018;36(15_suppl):4500. doi:10.1200/JCO.2018.36.15_suppl.4500
75. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab (pembro) plus axitinib (axi) versus sunitinib as first-line therapy for metastatic renal cell carcinoma (mRCC): outcomes in the combined IMDC intermediate/poor risk and sarcomatoid subgroups of the phase 3 KEYNOTE-426 study. Am Soc Clin Oncol. 2019;37(15_suppl):4500. doi:10.1200/JCO.2019.37.15_suppl.4500
76. Rini BI, Plimack ER, Stus V, et al. Pembrolizumab plus axitinib versus sunitinib for advanced renal-cell carcinoma. N Engl J Med. 2019;380(12):1116–1127. doi:10.1056/NEJMoa1816714
77. Tierney JF, Stewart LA, Ghersi D, Burdett S, Sydes MR. Practical methods for incorporating summary time-to-event data into meta-analysis. Trials. 2007;8:16. doi:10.1186/1745-6215-8-16
78. Sperling AS, Gibson CJ, Ebert BL. The genetics of myelodysplastic syndrome: from clonal haematopoiesis to secondary leukaemia. Nat Rev Cancer. 2017;17(1):5–19. doi:10.1038/nrc.2016.112
79. Higgins JP, Altman DG, Gotzsche PC, et al. The Cochrane Collaboration’s tool for assessing risk of bias in randomised trials. BMJ. 2011;343:d5928. doi:10.1136/bmj.d5928
80. Bejar R, Lord A, Stevenson K, et al. TET2 mutations predict response to hypomethylating agents in myelodysplastic syndrome patients. Blood. 2014;124(17):2705–2712. doi:10.1182/blood-2014-06-582809
81. Moher D, Liberati A, Tetzlaff J, Altman DG. Preferred reporting items for systematic reviews and meta-analyses: the PRISMA statement. Ann Intern Med. 2009;151(4):264–269, W264. doi:10.7326/0003-4819-151-4-200908180-00135
82. Payne R, Glenn L, Hoen H, et al. Durable responses and reversible toxicity of high-dose interleukin-2 treatment of melanoma and renal cancer in a community hospital biotherapy program. J Immunother Cancer. 2014;2:13. doi:10.1186/2051-1426-2-13
83. Amin MB, Amin MB, Tamboli P, et al. Prognostic impact of histologic subtyping of adult renal epithelial neoplasms: an experience of 405 cases. Am J Surg Pathol. 2002;26(3):281–291. doi:10.1097/00000478-200203000-00001
84. Delahunt B, Eble JN, McCredie MR, Bethwaite PB, Stewart JH, Bilous AM. Morphologic typing of papillary renal cell carcinoma: comparison of growth kinetics and patient survival in 66 cases. Hum Pathol. 2001;32(6):590–595. doi:10.1053/hupa.2001.24984
85. Warren AY, Harrison D. WHO/ISUP classification, grading and pathological staging of renal cell carcinoma: standards and controversies. World J Urol. 2018;36(12):1913–1926. doi:10.1007/s00345-018-2447-8
86. Cancer Genome Atlas Research Network, Linehan WM, Spellman PT, et al. Comprehensive molecular characterization of papillary renal-cell carcinoma. N Engl J Med. 2016;374(2):135–145.
87. Shuch B, Amin A, Armstrong AJ, et al. Understanding pathologic variants of renal cell carcinoma: distilling therapeutic opportunities from biologic complexity. Eur Urol. 2015;67(1):85–97. doi:10.1016/j.eururo.2014.04.029
88. Tan MH, Wong CF, Tan HL, et al. Genomic expression and single-nucleotide polymorphism profiling discriminates chromophobe renal cell carcinoma and oncocytoma. BMC Cancer. 2010;10:196. doi:10.1186/1471-2407-10-196
89. Maher ER. Hereditary renal cell carcinoma syndromes: diagnosis, surveillance and management. World J Urol. 2018;36(12):1891–1898. doi:10.1007/s00345-018-2288-5
90. Srigley JR, Delahunt B. Uncommon and recently described renal carcinomas. Mod Pathol. 2009;22(Suppl 2):S2–S23. doi:10.1038/modpathol.2009.70
91. Priolo C, Khabibullin D, Reznik E, et al. Impairment of gamma-glutamyl transferase 1 activity in the metabolic pathogenesis of chromophobe renal cell carcinoma. Proc Natl Acad Sci U S A. 2018;115(27):. doi:10.1073/pnas.1710849115
92. Gandhi JS, Malik F, Amin MB, Argani P, Bahrami A. MiT family translocation renal cell carcinomas: a 15th anniversary update. Histol Histopathol. 2019;18159.
93. National Comprehensive Cancer Network. Kidney Cancer (Version 2.2020). 2019. Available from: https://www.nccn.org/professionals/physician_gls/PDF/kidney.pdf.
94. Lee C-H, Makker V, Rasco DW, et al. Lenvatinib + pembrolizumab in patients with renal cell carcinoma: updated results. J Clin Oncol. 2018;36(15_suppl):4560. doi:10.1200/JCO.2018.36.15_suppl.4560
95. Chowdhury S, McDermott DF, Voss MH, et al. A phase I/II study to assess the safety and efficacy of pazopanib (PAZ) and pembrolizumab (PEM) in patients (pts) with advanced renal cell carcinoma (aRCC). J Clin Oncol. 2017;35(15_suppl):4506. doi:10.1200/JCO.2017.35.15_suppl.4506
 © 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2020 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
