Back to Journals » International Journal of Nanomedicine » Volume 19
Opportunities and Challenges for Inhalable Nanomedicine Formulations in Respiratory Diseases: A Review
Authors Feng X, Shi Y, Zhang Y, Lei F, Ren R, Tang X
Received 9 November 2023
Accepted for publication 24 January 2024
Published 17 February 2024 Volume 2024:19 Pages 1509—1538
DOI https://doi.org/10.2147/IJN.S446919
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Yan Shen
Xujun Feng, Yuan Shi, Ye Zhang, Fei Lei, Rong Ren, Xiangdong Tang
Department of Respiratory and Critical Care Medicine, Sleep Medicine Center, Mental Health Center, West China Hospital, Sichuan University, Chengdu, People’s Republic of China
Correspondence: Rong Ren; Xiangdong Tang, Department of Respiratory and Critical Care Medicine, Sleep Medicine Center, Mental Health Center, West China Hospital, Sichuan University, Dian Xin Nan Jie 28#, Chengdu, 610041, People’s Republic of China, Tel +86-28-85422733, Email [email protected]; [email protected]
Abstract: Lungs experience frequent interactions with the external environment and have an abundant supply of blood; therefore, they are susceptible to invasion by pathogenic microorganisms and tumor cells. However, the limited pharmacokinetics of conventional drugs in the lungs poses a clinical challenge. The emergence of different nano-formulations has been facilitated by advancements in nanotechnology. Inhaled nanomedicines exhibit better targeting and prolonged therapeutic effects. Although nano-formulations have great potential, they still present several unknown risks. Herein, we review the (1) physiological anatomy of the lungs and their biological barriers, (2) pharmacokinetics and toxicology of nanomaterial formulations in the lungs; (3) current nanomaterials that can be applied to the respiratory system and related design strategies, and (4) current applications of inhaled nanomaterials in treating respiratory disorders, vaccine design, and imaging detection based on the characteristics of different nanomaterials. Finally, (5) we analyze and summarize the challenges and prospects of nanomaterials for respiratory disease applications. We believe that nanomaterials, particularly inhaled nano-formulations, have excellent prospects for application in respiratory diseases. However, we emphasize that the simultaneous toxic side effects of biological nanomaterials must be considered during the application of these emerging medicines. This study aims to offer comprehensive guidelines and valuable insights for conducting research on nanomaterials in the domain of the respiratory system.
Keywords: nanoparticles, respiratory system, lungs, nanomaterials, nanotechnology
Graphical Abstract:
Introduction
Since frequent interactions occur between the lungs and the surrounding environment, the lungs are vulnerable to exposure to external pollutants and pathogenic microorganisms. At the same time, owing to the rich blood supply in the lungs, exposure to toxic substances or pathogenic microorganisms may lead to systemic diseases. Inhalation therapy involves drug delivery as aerosols, dry powder, or nebulized solution into the respiratory tract, which acts on the mucous membranes of the respiratory tract and alveoli.1 Inhalation therapy is targeted and represents an important methodology for treating respiratory disorders.2
The emergence of nanotechnology has led to the development of diverse nano-formulations, which have opened up new research avenues in the field of biomedicine. Specifically, nanotechnology can be used to create formulations that have great potential for drug delivery to the lungs.3 Nano-formulations are expected to address the issue of inadequate lung targeting observed in traditional treatments; at the same time, they can overcome various barriers to the lungs, enhance drug solubility, and allow the drug to reach the therapeutic concentration, thus producing a better therapeutic effect.4 Currently, many nano-formulations are being used to treat lung diseases.5 Although nano-formulations have great potential, they still pose many unknown risks. For instance, a large body of evidence suggests that some nanoparticles (NPs) can accumulate in the lungs after inhalation and induce lung nodules or even tumors. Therefore, inhalation formulations prepared based on nanotechnology need to be evaluated for potential toxicity.6,7 An in-depth investigation of nanopreparations at the toxicological level can help to further develop nanomedicines with better bioactivity and biosafety.8
As nanoparticles are expected to perform a critical function in the diagnosis and management of respiratory diseases, it is of great significance to explore their bioactivity and toxicological properties at the nanoscale. Therefore, the current investigation aimed to evaluate the therapeutic and diagnostic value of various nanoparticles/formulations in the respiratory system at the nano-level, as well as to evaluate their possible toxic consequences. Throughout the present research, we first describe the anatomical and biological barriers to the respiratory tract and lungs. Subsequently, the pharmacokinetics and toxicology of nanoparticles during administration to the respiratory system are discussed, and various types of nanoparticles, including natural and synthetic nanoscale particles, are categorized and summarized to analyze their biological, physicochemical, and toxicological properties. Finally, we emphasize the application of nanotechnology within the management, diagnosis, and suppression of respiratory disorders. Overall, the current study offers a comprehensive and systematic examination of the role of nanoparticles within the context of respiratory medicine, providing insight into the advancement of nanotechnology in respiratory medicine.
Anatomy and Biological Barriers of the Lung
The lungs play important roles in gas exchange, circulation, and immunity. After inhalation through the nose or oropharynx, the gas substances traverse the trachea and arrive at the lungs. The trachea penetrates downward into the lungs and continuously undergoes bifurcation, resulting in a reduction in the diameter of the airway and a thinning of the epithelial barrier. Pseudostratified columnar epithelial cells transform initially into single columnar epithelial cells, then into cuboidal epithelial cells, and eventually into the very thin respiratory epithelium existent in every alveolus.9 The primary components of the alveoli comprise type I and type II alveolar epithelial cells (pneumocytes), together with fine capillaries. The alveolar surface is predominantly occupied by type I alveolar epithelial cells (AEC I), which account for approximately 96% of the alveolar surface and are characterized by small and extremely thin nuclei as well as the shortest diffusion distance from pulmonary capillary blood.10 Conversely, cuboidal type II alveolar epithelial cells (AEC II), comprising a mere 4% of the alveolar surface area, secrete pulmonary surfactant (PS), which reduces surface tension within the alveoli.11 The epithelial barrier consists of a layer of lysate-like mucus and a gel called mucociliary escalator (MCE), which mediates the movement of drugs across the upper airways. Lung surfactants is the initial line of defense toward inhaled particulate matter and infections, effectively mitigating potential damage. Additionally, they decrease alveolar surface tension and preserve the lungs’ physiological respiratory function.12 The primary physiological role of lung surfactants is to uphold a diminished surface tension at the interface between air and liquid, thereby preventing the collapse of the lungs.13 Prior research has indicated that nanoparticles produced by industrial pollution can damage epithelial cells of the lung, leading to their death and impaired function. The different physicochemical characteristics of nanoparticles (ie, surface charge, particle size, and hydrophobicity) affect their interactions with lung surfactants. These interactions have the potential to disrupt the normal physiological function of lung surfactants or modify the behavior and outcomes of NPs.14 Zhao et al12 evaluated the interactions of PS with silica nanoparticles and polycyclic aromatic hydrocarbons (PAHs) and found that silica substantially changed the physical behavior and foaming capacity of PS. A possible reason for this is the competitive adsorption of PAHs with the PS components on silica. Because of the robust solubilization and adsorption suppression of PAHs by PS, when the NP + PAH complex enters the respiratory tract, the PAHs can be easily separated from silica, which may result in severe lung health consequences, as well as the toxicity of the NPs themselves. Xu et al14 showed that the strength of the interaction of liposomal polymer nanoparticles (LPNs) with the PS component is strongly related to the mobility and surface charge of LPNs. LPNs with higher mobility have stronger interactions with PS, which allows them to accumulate within the PS, leading to a prolonged LPN retention time. However, LPNs with lower mobility are more easily consumed through macrophages and thus are favorable for treating inflammatory lung disorders.15 Alveolar epithelial cells (type II pneumocytes) possess a robust antioxidant capacity and can produce surfactant proteins A and D. These proteins perform an essential function in the modulation of lung inflammation and surfactant lipids that inhibit lymphocyte proliferation. Alterations in the balance of surfactant lipids and proteins that exist on the pulmonary surface may serve as significant regulators of the immunological state within the lungs. The respiratory system encompasses several types of epithelial cells, including plasma cells found in the conducting airways, Clara cells located in the small airways, and alveolar type II epithelial cells situated on the alveolar surface. These cells serve as the primary cytokine-secreting cells within the lungs, producing various peptides and protein antibiotics.
In addition, other types of epithelial cells, including cuprocytes, are found in adult lungs. Cuprocytes are airway epithelial cells, and their primary role is to eliminate pollutants and microorganisms from the lung airways. The extracellular matrix produced by lung fibroblasts maintains the structural integrity of the alveoli. In addition to these cells, several lung immune cells are present. In the lower respiratory tract, alveolar macrophages (AMs) serve as an immune barrier by phagocytosing particles accumulated in the alveolar area; they also perform a critical function in lung infections and disorders.16 AMs effectively remove large peptides or proteinaceous drugs and help clear microorganisms and inhaled particles. Nanoparticles, at relatively low concentrations, stimulate macrophage phagocytosis, whereas higher concentrations decrease phagocytosis.17 The precise mechanism underlying this inhibitory effect remains unclear; however, it may be mostly attributed to the occupation of cell volume or a reduction in membrane surface area, facilitating the ongoing process of invagination and subsequent consumption through phagocytosis or endocytosis.
Lung histology is frequently altered in disease conditions, ultimately resulting in pulmonary obstruction. The airway epithelium comprises various cell types that demonstrate compromised morphology in conditions such as chronic obstructive pulmonary disease (COPD), cystic fibrosis (CF), asthma, and lung cancer.18 In COPD, fine bronchi are obstructed and disappear, leading to emphysema. Basal cell hyperplasia is considered to be the initial lesion in COPD. Recent research has provided evidence supporting the compromised stemness of basal cells in individuals with COPD. Cuprocyte hyperplasia and chemotaxis and reduced ciliated cell quantities were observed in these patients. The remaining ciliated cells exhibited abnormal ciliary function, restricted beat frequency, and reduced shortening. The presence of epithelial squamous metaplasia is also associated with airway obstruction in COPD.19
Nano-Drug Delivery Systems: Nano-Formulations
Definitions
The term “nano” denotes structures that fall in the dimensional range of 1 to 100 nm. By definition, NPs possess dimensions spanning from 1 to 100 nm in all directions. While nanoparticle formulations may contain elements larger than 100 nm, they must be organized at the nanoscale and demonstrate attributes connected to their nanostructure. Furthermore, the surface roughness of nanoparticles has a certain degree of influence on their aerosol characteristics.20,21 The application of nanoparticle-based drug delivery systems in respiratory diseases has tremendous potential.3 Nanoparticles can improve the stability of a loaded drug and promote its uptake by cells, thereby providing better targeting capabilities. In addition, nano-formulations are expected to be administered via inhalation. Compared to oral and intravenous administration, inhalation delivery allows the drug to act directly on the alveolar surface (Figure 1A), avoiding clearance by the circulatory system and first-pass clearance by the liver.22 Distinct forms of nano-formulations have been established based on different materials and preparation processes. The utilization of nanotechnology in the advancement and formulation of nano-drug delivery systems has significant promise in the field of respiratory disorder treatment and diagnosis (Figure 1B).23
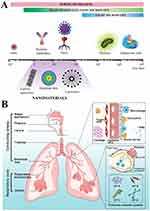 |
Figure 1 Inhalation of nanoparticles into the lungs. (A) Comparison of the relative sizes of nanomaterials with microbiological and other biological entities. Reprinted from Poh TY, Ali N, Mac Aogáin M, et al Inhaled nanomaterials and the respiratory microbiome: clinical, immunological and toxicological perspectives. Particle and fibre toxicology. 2018;15(1):46.22 (B) Diagrammatic illustration of the respiratory tract and physiological factors. Reprinted from Advanced Drug Delivery Reviews, 185, Wang W, Huang Z, Huang Y, et al, Pulmonary delivery nanomedicines towards circumventing physiological barriers: strategies and characterization approaches, 114309, Copyright 2022, with permission from Elsevier.23 |
Polymer Nanoparticles
Polymer nanoparticles are often designed for optimizing drug delivery systems, hence improving the precise delivery of drugs by loading them with various bioactive molecules or drugs. Polymers are categorized into natural (eg, proteins and polysaccharides) and synthetic types (eg, poly [lactic acid] [PLA], poly[lactic acid-co-glycolic acid] [PLGA], poly[caprolactone] [PCL], and poly[ethylene glycol] [PEG]). Polymer nanoparticles often have better biocompatibility and degradability and thus have broader application prospects. Polymer encapsulation is utilized to mitigate the attachment of particles to the mucus network, hence facilitating their enhanced diffusion inside the airway mucus. Fornaguera et al prepared polymeric nanoparticles loaded with dexamethasone using low-energy emulsification for inhalation therapy for lung diseases.24 PLGA was selected because of its biocompatibility and biodegradability. To stabilize the nanoemulsion, polysorbate 80 was added because it is a non-toxic and non-hemolytic surfactant. The polymer nanoparticles developed using the low-energy emulsification method exhibited a particle size of approximately 130 nm and were suitable for inhalation drug delivery. Ziaei et al employed an emulsification solvent evaporation technique to create porous PLGA microspheres loaded with a combination of docetaxel and celecoxib, which is more efficacious and less toxic in treating different lung malignancies.25 Rashid et al prepared PLGA-based rosiglitazone particles, which were optimized to be microporous and spherical in shape, with a release rate of 87.9% ± 6.7% across 24 h.26 In addition, the removal half-life of the rosiglitazone-loaded PLGA particles was 2.5-fold greater than that of the traditional formulation after pulmonary administration. Chitosan is a cationic polymer that exhibits a high affinity for mucin due to electrostatic interactions. The injection of voriconazole in a mouse model demonstrated enhanced pulmonary pharmacokinetics when encapsulated within chitosan-coated PLGA nanoparticles. The formulation resulted in a significant prolongation of the duration needed to achieve peak concentration of uncoated voriconazole particles from 1 to 24 h.27,28 Bandi prepared budesonide-containing PLGA microparticles and successfully maintained budesonide release in vitro for more than 21 days, demonstrating that budesonide-loaded PLGA microparticles may inhibit angiogenesis and tumor development within the lungs by inhibiting VEGF expression and normalizing oxidative stress.29 In addition, the drug levels (intratracheal administration) were higher within the lungs and bronchoalveolar lavage (BAL) throughout a mouse lung tumor model. Yoo et al designed innovative antioxidant polymer nanoparticles to treat inflammatory airway conditions composed of a biodegradable polymeric precursor drug, hydroxybenzyl alcohol (HBA), combined with polyoxalate (HPOX).30 It was shown that intratracheal delivery of HPOX nanoparticles led to a considerable decrease in recruiting inflammatory cells and a suppression of the production of inducible nitric oxide synthase, strong antioxidant activity, hydrolytic degradation of this precursor drug in vivo, and the release of HBA, which has both antioxidant and anti-inflammatory activities. Many inhaled anti-infective drugs formulated in nanoparticle polymers are useful in treating infectious disease pathways. Inhalation of PLGA microspheres containing rifampicin effectively reduced the pulmonary load of Mycobacterium tuberculosis with less toxicity than that of the free drug.
Nanoliposomes
Liposomes are a class of vesicle-like structures with a bilayer phospholipid membrane structure that is amphiphilic in nature and are, therefore, commonly used for drug loading and delivery.31 Liposomal delivery systems provide the capability to encapsulate hydrophilic and lipophilic medications in phospholipid bilayers. Liposomes possess biocompatibility because of their composition resembling that of cell membranes or lung surfactants. Thus, liposomes are less toxic, have the potential to form deep deposits in the lungs, and can load hydrophilic and lipophilic drugs more efficiently.2 Therefore, liposomal drug-carrying systems have important research and treatment applications in the respiratory system. Liposomal drug-carrying systems for the respiratory system underwent extensive investigation. Liposomes were used to encapsulate antibiotics, bronchodilators, hormones, immunosuppressants, peptides, antisense oligonucleotides, and anti-cancer agents for treating a range of respiratory disorders.32 Liposomes have better drug encapsulation and biocompatibility but are often rapidly cleared by macrophages or hepatocytes upon entry into the human body owing to the scavenging effect of the mononuclear phagocytosis system (MPS).33 In vivo experiments in rats performed by Tam et al found that intranasal administration of luciferase-containing mRNA-containing adjuvant lipids significantly increased luminescence expression in the nasal cavity and lungs by a minimum of 10-fold over baseline controls. This was further examined by LoPresti et al,32 who used cationic lipids (DOTAP and ethyl phosphatidylcholine) to assist standard liposomal nanoparticles, which effectively increased their enrichment efficiency in the lungs. Prior investigations have demonstrated that cationic DOTAP liposomes make interactions with heparan sulfate proteoglycans located in the glycocalyx surrounding alveolar endothelial cells to achieve lung-specific delivery through electrostatic interactions (Figure 2A).
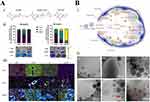 |
Figure 2 (A) Substitution of auxiliary lipids by charged alternatives in lipid nanoparticles enables the specific delivery of mRNA to the spleen and lungs. (i) Structure of three auxiliary lipids. (ii) Helper lipid charge affects the organ location of protein expression after mRNA delivery. (iii) Detection of helper lipid properties affecting the inflammatory state and the cell-specific location of protein expression in organs using firefly luciferase (green), globin-like enzyme (red), and Hoechst (blue) antibody staining.32 Adapted from LoPresti ST, Arral ML, Chaudhary N, Whitehead KA. The replacement of helper lipids with charged alternatives in lipid nanoparticles facilitates targeted mRNA delivery to the spleen and lungs. J Control Release. 2022;345:819–831.32 (B) (i) Schematic demonstration of the fate of nanoparticles inhaled into the alveoli. (ii) Descriptive cryo-TEM images of multilamellar vesicles (MLVs) of PS, LPNs, and LPNs/PS combinations. (a) Non-modified PLGA nanoparticles. (b) non-modified PLGA nanoparticles with MLVs of PS. (c) 15% (w/w) L5N12-modified LPNs. (d) 50% (w/w) L5N12-modified LPNs. (e) 15% (w/w) L5N12-modified LPNs with MLVs of PS. (f) 50% (w/w) L5N12-modified LPNs with MLVs of PS. Red arrows refer to the interaction area between LPNs and PS.14 PS, pulmonary surfactant; LPN, liposomal polymer nanoparticles; Adapted from Xu Y, Parra-Ortiz E, Wan F, et al. Insights into the mechanisms of interaction between inhalable lipid-polymer hybrid nanoparticles and pulmonary surfactant. J Colloid Interface Sci. 2023;633:511–525.14 |
Liposomal drug delivery can be a slow and controlled-release formulation that can effectively reduce pulmonary arterial pressure in patients with pulmonary arterial hypertension. Nahar et al developed magnetic liposomes (average particle size of 164 nm) as carriers for fasudil for the treatment of preferential accumulation of PAHs in the lungs, and 80% of the cumulative drug secretion has been detected for 120 h.34 Fasudil was administered intratracheally in the form of polyethylene glycol (PEG), which is used in treating PAHs across the lungs of individuals diagnosed with pulmonary arterial hypertension. Intratracheal administration of fasudil-containing PEG magnetic liposomes resulted in a 27-fold and 14-fold prolongation of the area under the curve and the half-life, respectively, contrasted with pure fasudil administration. Rashid et al35 employed a combination of fasudil and DETA NONOate (diethylenetriamine dioxide dinitrogen) in CAR-modified liposomes for treating pulmonary arterial hypertension. The outcomes revealed that CAR-modified liposomes were more effective than the pure drug combination, and they reduced the muscularization, amount of collagen accumulation, and thickness of the medial arterial wall to a greater degree.
The interaction between LPNs and lung surface-active substances determines the fate of inhaled LPNs; however, the mechanism underlying this interaction is unclear. Xu et al evaluated the mechanism of interaction among the inhalable lipid polymer-hybridized nanoparticles and PS.14 They found that the physicochemical characteristics of LPNs, such as membrane fluidity, hydrophobicity, and surface charge, had a significant effect on the interaction of nanoparticles with PS, and in particular, the strength of the interaction was strongly associated with the fluidity and the LPN surface charge. Furthermore, the pathological microenvironment (pH 5.4 and ionic strength 150 mM, representing the in vivo inflammatory state) mediated the combining of LPNs to PS (Figure 2B). Lipid nanoparticles targeted overexpressed luteinizing hormone-releasing hormone (LHRH) receptors in non-small cell lung cancer by synthesized LHRH-related peptide ligands. Garbuzenko et al encapsulated libraries of paclitaxel (TAX) and siRNAs using ligand-anchored nanoparticles to inhibit four important forms of epidermal growth factor receptor-tyrosine kinase (EGFR-Tyrosine) signaling.36 The results showed that following inhalation into a mouse model of lung cancer, nanoparticles designed for particular targeting exhibited preferential consumption via cancer cells, resulting in enhanced effectiveness against cancer and fewer adverse effects on the overall system compared with those of non-targeted therapeutic approaches.
Nanostructured lipid carriers (NLCs) and solid lipid nanoparticles (SLNs) represent the two primary categories of lipid matrix nanoparticles that can be efficiently loaded with hydrophilic and lipophilic drugs while retaining liposome biocompatibility. Compared to liposomes, SLNs offer notable benefits in terms of faster production rates and simplified scaling. Nevertheless, SLNs demonstrate a limited capacity for drug encapsulation efficacy and leakage.37 NLCs represent enhanced versions of SLNs that effectively overcome the aforementioned restrictions. NLC formulations are modified at ambient temperature by replacing solid lipids with liquid lipids while maintaining similar physical properties. The modified NLC formulation encapsulated more drugs and minimized leakage during storage.38 Neither SLNs nor NLCs have been extensively studied for inhalation administration. Some studies have shown the promise of controlled drug release following pulmonary administration. For example, Khan et al prepared an NLC using a combination of several solid and liquid lipids,39 which had a 91% encapsulation efficacy of beclomethasone dipropionate after the existence of a liquid lipid component within the formulation. After nebulizing this steroid-containing NLC, a greater number of particles accumulated within the next-generation impactor of the air-jet nebulizer group than in the vibrating-mesh and ultrasonic nebulizer groups.
Dendritic Macromolecules
Dendritic macromolecules are three-dimensional structures consisting of multibranched polymers with diameters of approximately 4–20 nm, and their surfaces can be functionalized with various groups.40 This enhances the versatility and biocompatibility of the dendrimers. Dendritic macromolecules exhibit a significantly smaller size than the majority of nanoparticles and liposomes, hence enhancing their efficacy in facilitating mesenchymal diffusion, uptake, and tumor invasion.41 Additionally, these compounds have the potential to undergo modifications as a result of electrostatic interactions with other charged compounds. Dendritic macromolecules have the capacity to transport drugs of different solubilities. Halevas et al used 2.2-bis(hydroxymethyl)propionic acid (bis-MPA) hyperbranched dendritic nanoscaffolds as nanocarriers for the inhalation delivery of raltegravir.42 Ridecivir has demonstrated efficacy as an antiviral medication in clinical settings, specifically in the management of viral pneumonia. The team prepared a nanoformulation that overcame the low water solubility of raltegravir, while the 80–230 nm size allowed it to be used for inhalation drug delivery. Dendritic macromolecule-based delivery systems can carry drugs to certain malignant sites in the lungs under regulated conditions. Kaminskas et al found that peg-polylysine (PEG-polylysine) dendritic macromolecules coupled with adriamycin can serve as inhalable chemotherapeutic nanomedicines that could enhance drug exposure in resident lung cancer patients.43
Inorganic Nanoparticles
Inorganic nanocarriers, including metal nanoparticles, silica nanoparticles, and carbon nanoparticles, etc., offer numerous beneficial features. For example, these materials possess notable biocompatibility, stability, and resistance to microbial degradation. Additionally, they demonstrate remarkable transport efficiency and possess magnetic characteristics. Iron oxide nanoparticles have been used in the diagnosis of respiratory disorders by various imaging methods, such as positron emission tomography, computed tomography (CT), magnetic resonance imaging (MRI), and gamma scintigraphy. Furthermore, heating with an external magnetic field induces apoptosis in adjacent cells, thereby potentially facilitating the removal of cancerous cells. AuNPs have been employed as nanocarriers in drug delivery systems for the therapeutic management of respiratory disorders. Silver nanoparticles have garnered significant attention in the biomedical domain because of their excellent antimicrobial features and are considered the ultimate line of defense against bacterial resistance and inflammatory responses. However, few investigations have been performed on the biosafety and nano/bio interactions, and unknown toxic side effects limit their development.44 Zamborlin et al44 evaluated the biodistribution and nano/bio interactions of Ag nanomaterials to investigate their biosafety in respiratory disease applications. The results showed that the biodistribution of the nanomaterials was heavily influenced by the design and the chemical properties of the metal. After 24 h of inhalation treatment, the lungs of the mice showed silver deposition, but no inflammation or tissue damage was observed. AgNAs showed good excretion characteristics, with almost negligible accumulation in major organs. However, it should be noted that the authors only evaluated single-dose administration, and further studies on chronic exposure as well as pulmonary endpoints are needed. Tseng et al45 developed biotinylated epidermal growth factor-modified (biotinylated-EGF-modified) cisplatin-based gelatin nanoparticles to particularly target cancer cells that have an upregulation of the epidermal growth factor receptor. Upon inhalation, the site-specific nanoparticle exhibited enhanced release of cisplatin, specifically within the cancerous lung, while demonstrating minimal systemic uptake. Hindi et al prepared a polyphosphate nanoparticle encapsulating a silver-carbene complex with antimicrobial features and nebulized it in mice infected with Pseudomonas aeruginosa.46 The administration of this therapeutic intervention led to a considerable decrease in bacterial load within the lung and spleen, thereby enhancing the overall survival rate of the animals.
Nanogels
The size of hydrogels determines how they are transported and adhere once they enter the vasculature, airway, or gastrointestinal tract. Along with trans-epithelial and local injections (eg, intraperitoneal injections), microgels and nanogels can be administered via other routes. Microgels smaller than 5 μm are utilized for oral or intrapulmonary administration, but they are generally unsuitable for intravascular injection because of their quick circulatory clearance. Nanogels are specifically attractive for the transportation of nucleotide-based drugs such as plasmid DNA for gene therapy. Gene therapy exhibits potential for the therapeutic intervention of cancer, hemophilia, and viral infections. Compared with unencapsulated DNA use, DNA delivery using nanogels improves cellular uptake and extends circulation time. Microgels can be synthesized using microfluidic and micromolding techniques, whereas nanogels can be synthesized via emulsion and nanomolding methods. Javed et al prepared nanogels of diphenhydramine (an antihistamine drug) for nasal mucosal drug delivery and evaluated drug release and penetration efficiency in a New Zealand male white rabbit model.47 The results showed that the benzylamine nanogel had greater penetration and delivery efficiency than direct spray drug delivery. Adhesion polymers, such as hyaluronic acid, decrease cilia clearance and are biocompatible and biodegradable. The polar functional groups of hyaluronic acid (-NH, OH, and COOH) confer this adhesive property by forming hydrogen bonds with negatively charged mucins. HA-coated nanogels increase the lung retention time of salbutamol from 2 to 8 h and reduce systemic exposure in rat models.48
Nanocrystals
Nanocrystals have been a popular topic in nanotechnology research in recent years and are primarily utilized for the transportation of insoluble drugs.49 Nanocrystals are mainly pure drug particles spanning in size from 1–1000 nm, which can enhance adhesion to biofilms because of their large specific surface area, thus improving bioavailability. Nanocrystals possess distinct advantages when employed for pulmonary drug delivery. The utilization of nanocrystal technology has found extensive application within the context of pulmonary inhalation drug delivery systems, particularly for insoluble drugs. This includes the application of nanosuspension-based aerosols, dry powder inhalers, and inhalable nanocomposite particles. Nanocrystals diminish the overuse of harmful non-aqueous solvents and improve the safety of pulmonary drug delivery. C109 exhibits significant inhibitory activity against FtsZ, and Burkholderia cepacia shows strong inhibition. Costabile et al50 prepared nanocrystal-embedded dry powders for inhalation suspensions, including C109 nanocrystals that were subjected to stabilization with D-α-tocopherol polyethylene glycol 1000 succinate (TPGS) encapsulated in hydroxypropyl-β-cyclodextrin (CD). The nanocrystal formulation was not toxic to human bronchial epithelial cells but was active only toward Burkholderia cepacia.50
Exosomes
Exosomes are cell-secreted nanoparticles, which are a subtype of extracellular vesicles that have attracted considerable attention in life sciences in recent years.51 They contain thousands of RNAs, cytokines, and proteins that play an important role in the development and treatment of various diseases. Owing to their double membrane structure, exosomes can be used as unique drug carriers. Popowski et al52 found that lung-derived extracellular vesicles (Lung-Exos) can be used for natural drug delivery to the lungs and administered via nebulization and dry powder inhalation. The Lung-Exos after drug administration was superior to both biological and synthetic nanoparticles in terms of drug distribution and retention. Lung-Exos can still be biologically active when stored at room temperature for 1 month. Han et al53 focused on the therapeutic strategy of delivering small extracellular vesicles, and consequently small RNAs, via nebulization. The results showed that small extracellular vesicles could be delivered to the lungs of mice by a vibrating mesh nebulizer, while the incoming small extracellular vesicles were distributed only in the lungs without escaping to other tissues and organs based on the vivo tracer technique. This study confirms the feasibility of utilizing small extracellular vesicles as inhalation vectors for gene therapy. In addition, exosomes can be engineered to enhance their cellular targeting and therapeutic effects.54 Liu et al55 constructed a biomimetic nanocarrier based on exosomes and enhanced the targeting ability of the nanocarrier by fusing serum exosomes and liposomes while engineering modifications to them. The results showed that the nanocarrier could target macrophages in the lungs, reduce the inflammatory response, and promote the repair of lung injury by remodeling the immune homeostasis.
Virus-Like Particles
Virus-like particles (VLPs) are large particles assembled from one or more structural proteins of multiple viruses.56 Although they have a structure similar to viruses, they do not contain viral nucleic acids and cannot replicate autonomously. VLPs have been mostly studied for vaccine preparation and drug carriers in recent years. During the COVID-19 epidemic, VLPs represented a vaccine platform of great interest. A study by Rothen et al56 showed that a vaccine platform consisting of VLPs of SARS-CoV-2 receptor-binding structural domain (RBD) that was administered intranasally induced protective systemic and locally specific antibody responses in mice. Wang et al57 reported an inhalable exosome-based VLP platform for a new coronavirus vaccine. The vaccine consisted of a recombinant SARS-CoV-2 RBD conjugated to Lung-Exos, which can exert good immunogenicity, and it could also be stored at room temperature for 3 months without inactivation after lyophilization.
Others
Nanoscale materials with attractive research and application prospects for respiratory drug delivery have been favored by researchers, and an increasing number of nanoscale drug-carrying systems have been developed for pulmonary drug delivery. Bilosomes (bile bodies) are vesicular structures designed based on bile salts that have higher stability and drug encapsulation efficiency than traditional liposomes.58 Zakaria et al58 loaded resveratrol using PEGylated bilosomes for improving the bioavailability and solubility and showed that the nanoparticles inhibited the severe acute respiratory syndrome coronavirus type 2 (SARS-CoV-2) Mpro enzyme, a possible drug carrier treating respiratory disorders. Nanosomes are another emerging method of delivering inhalable biologics. Nanosomes refer to small therapeutic proteins (−15 kDa), which are obtained through the extraction of naturally occurring pure heavy-chain antibodies. They exhibited favorable suitability for pulmonary delivery because of their short half-life across the plasma. ALX-0171 is a nanosome designed to specifically target respiratory syncytial virus (RSV)-RSV-specific antigens at sub-nanomolar concentrations. The results demonstrated a significant reduction in both nasal and pulmonary RSV titers when delivered by aerosolization in a rat model.59
Pharmacokinetics and Toxicology of Inhaled Nano-Formulations
Nano-formulations have unique advantages in respiratory drug delivery, and their nanoscale size allows them to be administered by inhalation, deposited in smaller alveoli, and passed through various lung physical barriers; thus, the target drug can enter the alveoli, resulting in better therapeutic effects.60 However, nanopreparations can interact with physiological barriers after inhalation; this can affect their therapeutic efficacy and even result in toxic side effects.61
The chemicophysical characteristics of a drug define its pharmacokinetics, and the type of formulation by which it is administered also affects its pharmacokinetics. Nanomedicines can modulate and enhance the properties of many drugs by reducing the size of the compounds to a degree not possible with conventional formulations, including increasing the solubility of the drugs, protecting them from degradation, enhancing their epithelial uptake, increasing their circulation time, enabling targeted delivery, and enhancing their cellular uptake.62,63
The beneficial effect of inhaled nanoparticle medications mainly relies on the location of nanoparticle deposition in the lungs after drug administration. Inhaled nanoparticles are accumulated in numerous areas of the lungs by three different processes: (1) deposition, (2) inertial impaction, and (3) Brownian diffusion.64,65 Locally acting inhaled drugs remain deep in the lungs for some time after effective drug accumulation. Nevertheless, the residence time of medications accumulated within the lungs is short owing to the self-cleaning mechanism of rapid absorption of the drug via the respiratory epithelium into the circulating airways. The development of inhaled medications with prolonged lung residence necessitates the overcoming of several obstacles in both the respiratory and alveolar areas. These barriers encompass anatomical, physiological, and immunological factors. Anatomical barriers encompass the nasal, tracheobronchial, oropharyngeal, and alveolar barriers. The respiratory tract comprises progressively narrowing airways and branches, with smaller dimensions observed from the upper to lower airways. Overall, larger medication particles tend to be trapped within the oropharyngeal area. The physiological barrier mainly involves mucus and ciliary motilities, whereas the immune barrier mainly involves AMs.66
Pharmacokinetics of Inhaled Nano-Formulations
Physicochemical Properties of Nanopreparations
The mechanism, pattern, and efficacy of particle accumulation within the respiratory tract mainly rely on the thermodynamic or aerodynamic diameters of the inhaled particles. Nanoparticles exhibit a diverse range of sizes and have different properties; therefore, they are efficiently deposited into the respiratory system through the process of diffusion, traversing from the upper airways to the alveoli.67 Because there is no sufficiently strong resistance during inhalation, the aerodynamic diameter is irrelevant and is only related to the thermodynamic diameter. In addition to the above properties, the surface chemistry of nanoparticles affects the biological response of the respiratory epithelium; for instance, the surface charge of nanoparticles affects their final action and modulates specific immune responses to some extent.68 Further, Ruenraroengsak et al69 found that amine-modified polystyrene latex nanoparticles caused human alveolar epithelial-like cells of cell membranes to develop pores, inducing apoptosis and cell death. In contrast, the unmodified and carboxyl-modified nanoparticles did not damage the cell membranes and were less reactive. Arroyabe et al65 investigated the impact of atmospheric nanoparticles on the individual’s health, focusing on the wavelength range of 6–380 nm. Based on the human respiratory model of ICRP 94, the nanoparticles showed an alveolar surface area accumulation platform having a size distribution spanning from 6 to 150 nm. The presence of a negative charge on the nanoparticles can be attributed to the atmospheric nanoparticles primarily accumulated within the alveolar area, where the dominant mechanism of accumulation is Brownian deposition. The surface charge of nanoparticles is a crucial determinant in understanding the Brownian accumulation of the smallest particles within the human respiratory system.
Drug accumulation within the lungs is directly associated with drug absorption. The accumulation of inhaled drug formulations is impacted by various factors, including shape, aerodynamic diameter, particle density, hygroscopicity, and surface characteristics (surface charge, surface morphology, and surface chemistry).70 The aerodynamic diameter of the particles plays a crucial function in the accumulation process. The optimal aerodynamic particle size for lower respiratory tract deposition ranges from 1 to 5 μm. Particles with an aerodynamic diameter < 1 μm are accumulated within the lungs due to Brownian diffusion and are easily exhaled. Particles with an aerodynamic diameter of 5 μm are expelled through the upper airways because of inertial impact. The aerodynamic diameter of a particle is equivalent to the diameter of a sphere having a density of unit (ρ0) with a terminal velocity identical to that of a particle within still air. Thus, this independent factor can be associated with the effects of the particle density and geometric diameter (Figure 3A).71
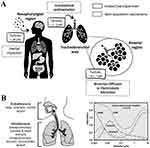 |
Figure 3 Deposition of particulate matter in the respiratory system. (A) Inhaled particles: accumulation mechanisms occurring through the respiratory tract.71 Reprinted from Bessa MJ, Brandão F, Rosário F, et al. Assessing the in vitro toxicity of airborne (nano) particles to the human respiratory system: from basic to advanced models. J Toxicol Environ Health B Crit Rev. 2023;26(2):67–96.71 (B) The respiratory tract of individuals at rest in relation to particle deposition and particle size.72 Reprinted from Geiser M, Kreyling WG. Deposition and biokinetics of inhaled nanoparticles. Part Fibre Toxicol. 2010;7:2.72 |
Mechanisms of Lung Absorption and Clearance
Drug absorption and clearance mechanisms are associated with the innate immune system. The primary innate defensive mechanism of the human respiratory system is mucociliary clearance; it removes foreign bodies from the respiratory tract to the oral cavity for swallowing or coughing. Epithelial and cup cells in the airway region prevent foreign bodies from entering the airways by secreting elastic mucus.73 When the drug cannot pass through the meshwork of elastic mucus, it is encapsulated by sticky mucus, after which the encapsulated material is propelled to the oropharynx for coughing or swallowing through mucus cilia movement.74 Immune clearance is mediated by lung-resident innate immune cells that investigate foreign particles in tissues.75–77 The primary innate immune cells in the lungs are AMs located across the alveolar airways.78 Nanoparticle therapy involves the encounter of AMs after avoiding the physical barrier of the upper conducting airways.76 AMs move across the alveolar surface and recognize and phagocytose dissolved particles.79 Phagocytosis by lung macrophages is mainly related to the phagocytosed particle size, and the ideal particle size range for endocytosis is 1–3 μm; particles within this range are cleared via macrophages.80 There is a positive correlation between molecular weight and residence length in the airway, as well as the duration of contact and consumption via AMs. Phagocytosis by macrophages results in a reduction in the concentration and efficacy of drugs in the lungs. In the majority of instances involving the administration of inhaled LNP used in treating lung disorders, the primary objective is to minimize alveolar macrophage consumption completely.81 Nevertheless, macrophages can serve as cellular targets in specific contexts, such as in the therapeutic intervention against M. tuberculosis.76,82 Hence, the characterization of the disease’s nature plays an important function in elucidating the expected interactions with the physiological barrier of the lungs during drug development. Inhaled insulin requires a 7–8-fold boost in drug concentration to attain comparable impact as subcutaneous insulin.83
Surface-active lung substances are associated with drug absorption and clearance. Studies have shown that certain lipophilic compounds can cross epithelial cells via passive diffusion, whereas hydrophilic compounds are absorbed extracellularly. The greater the lipophilicity, the simpler it is to traverse across the epithelial cells. This may be related to the lipid bilayer of the cells, which has been widely studied for its major role in mammalian respiration. Several studies have shown that hydrophilic nanoparticles tend to migrate rapidly in PS, whereas hydrophobic nanoparticles interact with PS and are retained for longer periods.84
Toxicology of Nanoparticles
The toxicity of nanoparticles pertains to the assessment of the biological behavior of artificially created nanostructures and nanodevices; nanomaterials exhibit distinct optical, chemical, magnetic, and structural features and, therefore, different toxicity characteristics.85 With atmospheric pollution, the air is filled with a wide variety of harmful particles that are mainly composed of nanoparticles that can easily enter the human lungs and cause diseases (Figure 3B);72 the incidence of related diseases is increasing every year.86,87 Inhalation of NPs (including unintentional inhalation) may be a major cause of lung diseases in humans. Inhaled nanoscale particles can cause direct damage to lung tissues and induce lung inflammation; they can also enter the body’s circulation from the lungs, leading to their enrichment in other organs, which can induce several systemic diseases. Although nanoparticles are suitable for drug loading, their prolonged retention in the body after deposition may lead to cellular damage, biological reactions, and adverse effects.1,58 Some studies have established a theoretical model for the deposition of nanoparticles in human alveoli. Because of the greater alveolar wall stiffness of patients with chronic respiratory diseases, which causes the convective effect and rate of deposition and diffusion of nanoparticles to increase, more nanoparticles will be deposited and retained.19,88 When nanoparticles enter the lungs, they may be transferred to other organs by other means, such as the blood, causing damage to other organs.89 The potential toxic effects of nanoparticles within the lungs may depend on the particle surface chemistry and size, and research has indicated that smaller nanoparticles can reduce their toxicity to some extent, cause them to metabolize better in the body, and reduce damage to the body.90,91
The widespread cytotoxicity of nanoparticles is considered the most important factor limiting their use in biomedical applications. Some studies have shown that nano-Ag, TiO2, Al2O3, ZrO2, Fe2O3, Si3N4, chrysotile, BC, and soot from common combustibles are toxic to immortalized human epithelial cells; only the magnitude of toxicity varies, and TiO2 nanoparticles are relatively less toxic to cells.86 Silica nanoparticles are a class of widely used nanoscale particles, and their inhalation can lead to mitochondrial ultrastructural damage and damage to alveolar structures and collagen deposition. Li et al92 thoroughly investigated the toxicological mechanism and found that amorphous silica nanoparticles induced epithelial cell apoptosis and lung injury through reactive oxygen species (ROS)/Ca2+/DRP1-mediated mitochondrial division signaling.
Because of the unique physicochemical properties of nanomaterials, the inhalation of nanoparticles into the lungs induces an inflammatory response. Innate immunity serves as the initial barrier of protection against pathogens, and macrophages perform a crucial function in immunity and in the elimination of NPs; the effect of nanoparticles on the immune system is of great concern.93–95 The inhalation of nanomaterials activates AMs, which aggregate in the area of injury and exhibit different types of cytokines and chemokines that also aggregate macrophages and other immune cells to participate in the immune response. Numerous cytokines, including interleukin-1 and tumor necrosis factor-α, have been identified as capable of initiating the immune response.47,60 These molecules additionally induce activation of epithelial and endothelial cells, leading to the synthesis of pro-inflammatory mediators. Several nanoparticles, including TiO2, carbon black, and cobalt oxide (Co3O4), were established to induce lung damage and inflammation in animal models.10 Silicon dioxide nanoparticles (SiNPs) are extensively employed as drug carriers for enhancing the efficacy of drug delivery and retention. An investigation by Yu et al96 assessed the possible toxicity of SiNPs in the lungs and found a significant increase in inflammation and permeability in rat lungs with elevated concentrations of SiNPs, accompanied by lymphatic endothelial cell injury and increased pulmonary lymphangiogenesis and remodeling. This study provides evidence of SiNP-induced lung injury and illustrates the possible drawbacks of SiNPs as carriers for pulmonary drug delivery.
Metal NPs stimulate free radical generation via the Fenton reaction, causing respiratory damage, inflammation, and oxidative stress.97 In addition, disclosure to Zn, Ti, and Fe NPs elevated ROS levels and apoptosis. Increased oxidative stress results in the induction of the mitogen‑activated protein kinase signaling pathway and enhances the transcriptional levels of essential molecules such as Nrf2 and NF-κB. In turn, the subsequent variables can lead to an increase in the mRNA expression of a range of pro-inflammatory mediators that contributed to the occurrence of several inflammatory disorders.44 In contrast, ROS are widely believed to have a significant impact in regulating the cardiomyocyte microenvironment. NP disclosure can cause cardiotoxicity by increasing ROS production.98 Studies on ischemia-reperfusion injury have shown that elevated ROS concentrations and impaired redox homeostasis are the primary reasons for myocardial injury. The inhalation of NPs can lead to the development of cardiopulmonary disorders in mammalian models. Intratracheal instillation of carbon nanotubes has been reported to cause persistent inflammation in the lungs and cardiovascular system of rats.
Toxicokinetics of Inhaled Nanomaterials
Understanding the toxicokinetics of inhaled nanomaterials is essential for the development of safe nanoinhalation formulations. Various physicochemical properties of nanomaterials not only affect their potential biotoxicity but also their deposition and biodistribution characteristics.99 Relevant physical properties include size, specific surface area, aspect ratio, and surface charge. Several studies have shown that too small a size and higher aspect ratios may lead to stronger cytotoxicity because the cellular substructure may be disrupted, which in turn will lead to an imbalance in redox homeostasis.99,100 The surface charge of nanoparticles has also been correlated with cytotoxicity, with anionic cyanoacrylic acid nanoparticles being more cytotoxic to macrophages than the cationic form.101 Surface modification, in contrast, can mitigate the toxicity of nanomaterials, while also enhancing bioavailability and reducing immunogenicity. PEG modification is one of the more common modifications that can enhance the biosafety and bioavailability of nanoparticles, and is therefore favored by researchers.102
Constructing in vitro lung models for evaluating the pharmacological or toxicological properties of nanomaterials is essential for the development of nanomedicine delivery systems. Waste of resources can be avoided while providing more scientifically accurate data support. Phan et al66 constructed two lung-mimicking models (healthy lung model and diseased lung model) for evaluating new therapeutic regimens or for testing toxicity and damage induced by inhaled agents or pathogens. The two models mimicked the physiological anatomy of the lung, simulated the lung barrier as well as various cellular components, and represented relatively objective in vitro models that are valuable for the study of lung diseases and new drug delivery systems. Meneses et al98 developed a machine-learning (ML) nano-quantitative structure-toxicity relationship (QSTR) model to predict the metal oxide nano-particle-induced potential human lung nanocytotoxicity. The results showed that a decrease in the diameter of nanoparticles can significantly increase their potential ability to enter lung subcellular compartments (eg, mitochondria and nuclei), which in turn leads to potent nanocytotoxicity and epithelial barrier dysfunction.
Nanoparticles for Respiratory Diseases
Poor drug transportation to the lungs when targeting respiratory pathologies is one cause of failure in treating respiratory disorders. Currently, there are three methods for the process of drug transportation to the lungs: endotracheal, inhalation, and intranasal. Nevertheless, the endotracheal pathway of drug delivery is an invasive approach and is, therefore, not feasible in humans. Delivery of genes or oligonucleotides through the intranasal pathway has been examined in rodents; however, the outcomes are hard to extrapolate because the human respiratory system is more complex. The inhalation route is now regarded as the greatest prospective non-invasive technique for drug transportation to the lungs because of its effective and relatively simple setup.3 Aerosol inhalation allows for direct drug transportation to the respiratory system. Nano-delivery systems have the potential to regulate drug release, overcome the lung barrier, particularly in the presence of excessive mucus discharges associated with certain disorders, and enhance drug permeability inside the lungs. This ultimately leads to improved cellular consumption such that therapeutic properties can be achieved with low doses of the drug.103–105 Overall, nano-delivery systems elevate the local drug concentration across the target area, improve therapeutic efficacy, and decrease the overall dose of the administered drug, thereby reducing side effects.
Tumors
The utilization of nanoparticles for the purpose of delivering drug candidates has the potential to enhance bioavailability and overcome natural barriers, such as mucosal barriers. The preferential accumulation of anti-cancer agents loaded in nano-DDS within tumor tissues is primarily facilitated by the enhanced permeability and retention (EPR) features, ie, “passive targeting”, instead of normal tissues (Figure 4A).106–108 EPR is the result of vascular leakage from the tumor caused by inadequate lymphatic drainage of the tumor and the presence of endothelial cell gaps, which provide a possibility for the nanotherapeutic to escape from circulation and be functionally localized across the tumor tissue. Unlike passive targeting, lung-inhaled nanomedicine therapies actively target certain tissues. Active targeting works primarily through receptor-mediated endocytosis and is therefore highly precise relative to EPR effects.107,109 Additionally, inhaled nanoparticles can deliver drugs directly to the lungs, enabling deep lung deposition by regulating the size of the aerosol particles.105,110
 |
Figure 4 Nanoparticles in lung tumors. (A) Smart transformable nanomedicines with a schematic representation for overcoming biological barriers and enhancing anti-tumor efficacy. Reprinted from Biomaterials, 271, Wang Y, Li S, Wang X, et al, Smart transformable nanomedicines for cancer therapy, 120737, Copyright 2021, with permission from Elsevier.108 (B) Synthesis and mechanism of action of SPIO@PSS/CDDP/HSA-MTX NPs.111 Reprinted from Yang SJ, Huang CH, Wang CH, Shieh MJ, Chen KC. The Synergistic Effect of Hyperthermia and Chemotherapy in Magnetite Nanomedicine-Based Lung Cancer Treatment. Int J Nanomedicine. 2020;15:10331–10347.111 Abbreviation: NP, nanoparticle. |
For nanomedicine therapy of respiratory tumors, inhaled active targeting is mainly achieved by targeting tumor cells or the tumor vascular endothelium. Therefore, the key to inhaled nanomedicine therapy for respiratory system tumors lies in the selection of appropriate tumor receptors. The cell membrane of lung cancer cells has a notable upregulation of LHRH receptors relative to that observed in normal cells.113 Taratula et al prepared LHRH-targeted DOX- and PTX-NLCs for pulmonary administration.114 The overall cytotoxicity of the complex was significantly enhanced using siRNAs targeting MRPl and Bcl2 mRNAs as inhibitors of drug resistance in drug-resistant pump and non-pump tumor cells and inhibiting both MRP1 and Bcl2 proteins. The cytotoxic activity of the PTX-siRNA complex was 120 and 16 times greater than that of the free drug and LHRH-NLC-PTX sequentially. Inhalation of LHRH-targeted NLC ensures the preferential aggregation of nanoparticles in tumor cells.114 EGFR is overexpressed in 40–80 NSCLC cells.115 Nanoparticles modified with EGFR can explicitly increase drug uptake by tumor cells. Tseng et al produced Av-bEGF-GNPs by attaching biotinylated epidermal growth factor (bEGF) molecules to affinoprotein (Av)-AV-modified gelatin nanoparticles (GNP).116 Av-bEGF-GNPs exhibited stronger internalization into A549 lung cancer cells (high EGFR expression) than into human lung fibroblasts or squamous lung cancer H520 cells. In vitro assays demonstrated that CIS-bEGF-GNPs had a greater suppressive impact on A549 cells than the unmodified GNP or free CIS. Inhalation nebulization of CIS-bEGF-GNPs targeted mouse lung adenocarcinoma, hence elevating the administered dose and decreasing the nephrotoxicity of CIS. Sadhukha et al developed magnetic nanoparticles for EGFR targeting by inhalation to improve the specificity for lung cancer cells, which resulted in greater drug distribution to lung tumors and attenuated adverse effects.117 Moreover, it has been suggested that thermomagnetic nanomaterials can be used in thermotherapy and chemotherapy of lung tumors to provide a good synergistic treatment effect (Figure 4B).111
Cystic Fibrosis
Nanomedicine technology for the respiratory system is not limited to tumor treatment but has shown good therapeutic effects in research areas targeting lung infections, CF, asthma, and other respiratory systems. CF is a prevalent autosomal recessive disease arising from alterations in both copies of the CF transmembrane conductance regulator protein (CFTR) gene.108,118 Considering CF arises from genetic abnormalities in the CFTR gene, gene therapy is now extensively employed to correct these abnormalities at the cellular level. During this specific technique, the correct copy of CFTR is introduced into the airway epithelial cells that have been impacted by the condition (Figure 5).112 Typically, two major kinds of vectors are utilized for transferring CFTR genes: viral and non-viral. Compared to viral vectors, non-viral vectors, such as nanomaterials, exhibit benefits such as simplicity and cost-effectiveness of production and an extended shelf life. Furthermore, they exhibit a lower immunomodulatory response and have a better tolerance to repeated administration. Non-viral vectors are typically engineered with a positive charge to facilitate electrostatic interactions with therapeutic nucleic acids that have a negative charge. This design aims to enhance the immobilization of particles within the mucus found in CF patients, as this mucus contains abundant amounts of negatively charged macromolecules.119 The initial clinical investigation was the utilization of a non-viral vector based on nanoparticles, specifically employing plasmid DNA containing the CFTR gene, which was contained within polyethylene glycol nanoparticles. Safety and gene transfer efficacy assessments showed that the nanopreparation was a successful gene transfer vector, producing partial potential difference correction without negative outcomes, indicating that this technique could be established in treating patients with CF.120 In addition, the conjugation of dual loading and inhalation nanoparticle approaches has yielded positive outcomes in treating CF. Within a recent investigation, the administration of lumacaftor and ivacaftor, encapsulated in lipid nano-drug carriers, by inhalation, revealed a considerable decrease in the extent of pulmonary tissue injury in mice with CF as in contrast to CF mice that did not receive any treatment.121
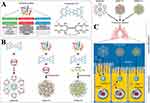 |
Figure 5 Schematic of the multi-modular peptide-based gene transfection platform. (A) Structures of the synthetic multi-modular peptide and poloxamine 704 (T704). (B) Schematic exhibiting nanoparticle formation. (C) A suggested pulmonary delivery mechanism of pDNA-BC, pDNA-TC, and mRNA-TC.112 Reprinted with permission from Guan S, Munder A, Hedtfeld S, et al. Self-assembled peptide-poloxamine nanoparticles enable in vitro and in vivo genome restoration for cystic fibrosis. Nat Nanotechnol. 2019;14(3):287–297. doi:10.1038/s41565-018-0358-x. Copyright 2019 Springer Nature.112 |
Asthma
Asthma is the prevailing chronic inflammatory disorder of the respiratory system, distinguished by intermittent and reversible obstruction of the airways, heightened sensitivity of the bronchial passages, and chronic inflammation inside the airways.122 Unlike inhaled medication, the e-nose is an innovative instrument consisting of sensors that identify specific volatile organic compounds (VOCs) in exhaled breath, enabling the non-invasive detection and identification of lung disorders (Figure 6A).123 According to the activated sputum inflammatory cell counts, there are three inflammatory phenotypes of asthma: eosinophilic, neutrophilic, and oligocytic. Moreover, different types of asthma differ in airway microbiological manifestations and even in their response to corticosteroid therapy. Therefore, the identification of asthma phenotypes holds significant importance in personalizing asthma therapy.124 Recently, there has been a use of electronic nose nanotechnology for the purpose of creating a non-invasive evaluation technique that exhibits a notable level of precision in distinguishing between individuals with eosinophilic, neutrophilic, and oligophilic asthma phenotypes. This development holds significant potential for clinical diagnostic applications.125 In addition, studies have indicated that pharmaceutical agents, such as the mTOR inhibitor rapamycin (Rapa), possess potential significance in the management of airway inflammation linked to pulmonary conditions, which include COPD, asthma, and CF. Craparo et al generated Rapa by nanoprecipitation of amphiphilic polyethylene glycolated-caprolactone/poly (hydroxyethylastenamide)-bearing grafted copolymer (rapamycin)-loaded nanoparticles.126 The nanoparticles that were acquired had a drug loading of 14.4 wt%. These nanoparticles did not exhibit any interaction with mucins. Furthermore, they demonstrated the capability to produce and preserve Rapa from degradation in both simulated lungs and cytosol. The NiM particles exhibited an average diameter of approximately 2 μm, a spherical shape, and showed promising suitability for administration to the respiratory system by a breath-activated dry powder inhaler. Rheology and turbidity investigations revealed that these NiM particles could dissolve in lung-simulating fluids and deliver Rapa-loaded polyethylene glycolized nanoparticles that could diffuse across the mucus layer (Figure 6B and C), thus enabling non-invasive long-term treatment of asthma.126
 |
Figure 6 Nanotechnology applied to the detection and treatment of asthma. (A) The nanomaterial-based selective sensing technique against the nanomaterial-based cross-reactive sensing technique. Used with permission of Future Medicine Ltd, from Nanomaterial-based sensors for detection of disease by volatile organic compounds, Broza YY, Haick H, 8, 5, copyright 2013; permission conveyed through Copyright Clearance Center, Inc.123 (B) The synthetic pathway of PHEA-g-RhB-g-SUCCPCL-g-PEG graft copolymer.126 Copyright 2022, reprinted with permission from Springer.126 (C) Muco-diffusion test of Rapa-loaded nanoPEG by a mucus layer.126 Reprinted from Craparo EF, Drago SE, Quaglia F, Ungaro F, Cavallaro G. Development of a novel rapamycin loaded nano- into micro-formulation for treatment of lung inflammation. Drug Deliv Transl Res. 2022;12(8):1859–1872.126 Abbreviations: Rapa, rapamycin; VOC, volatile organic compound. |
Respiratory Infections
Lung inflammation caused by bacterial infections can often lead to extremely severe clinical symptoms or even death when patients suffer from underlying respiratory diseases.127–130 Therefore, although lung infections are among the most common diseases of the clinical respiratory system, the exploration of nanomaterials for respiratory infections is an area of research that is full of potential and has the fastest clinical application. Nanomedicine-nebulized drug delivery therapy is considered a potential therapeutic alternative to the current oral administration of medications that can maximize drug levels within the lungs while decreasing the risk of systemic toxicity. Costabile et al efficiently encapsulated Ga(III) into hyaluronic acid/chitosan nanoparticles (Ga HA/CS NPs), whose characteristics were adapted to overcome mucus and surrounding bacterial biofilms by rapidly reaching the target lesion.131 Subsequently, for enhancing lung deposition in vivo, Ga HA/CS NPs underwent designing into a mannitol-based NEM (Ga_Man NEM). These powders exhibited the best in vitro aerosol performance and slow-release kinetics in the lung lining fluid. Additionally, good tolerance and antimicrobial features were observed in vitro. Intratracheal blow-in of Ga Man NEM in rats showed significantly improved Ga (III) persistence in the lungs and lower Ga (III) concentrations in the plasma and urine contrasted with GaN solutions. Notably, the constructed formulation considerably alters adverse Ga (III) kinetics, increases Ga (III) levels in the lungs, and prevents Ga (III) deposition in the kidneys.131
Tuberculosis
Technically speaking, tuberculosis is an infectious disease of the respiratory system caused by a specific type of germ. Three FDA-approved drugs (rifampicin, pyrazinamide, and isoniazid) underwent encapsulation in polymeric nano-drug carriers and were ingested weekly by patients experiencing M. tuberculosis infection. The findings of the in vivo investigation revealed that weekly administration had an efficacy similar to that of daily administration. Oral ingestion is the primary pathway of administration for M. tuberculosis infections; however, it has many disadvantages, including quick hepatic first-pass metabolism, reduced intestinal drug absorption, and elevated systemic exposure. Nanoinhalation therapy can effectively address systemic drug exposure and mitigate side effects. In a murine model of M. tuberculosis infection, ethionamide and its enhancers were encapsulated in biodegradable polymer nanoparticles and ingested directly into the lungs. The experimental findings revealed that this nebulized regimen had a much milder drug requirement and systemic organ exposure than oral treatment (Figure 7A).132 Several parameters, such as the deposition effects of inhaled nano-pulmonary drugs, are key indicators of the reliability of a strategy when designing drugs for inhaled nano-drug delivery systems. Huck et al133 investigated the effects of bedaquiline-encapsulated fucosylated and non-fucosylated liposomes on cellular consumption and delivery. Of note, the analysis encompassed crucial factors pertaining to the delivery of substances to the lungs, including the deposition of aerosols and the barriers posed by the non-cellular components of the PS and mucus. Targeting increased liposome consumption in THP-1 cells, peripheral blood monocytes, and lung tissue-derived macrophages. However, aerosol accumulation in the existence of PS masked the effects of active targeting, which altered the antibiotic production depending on the hydrophobicity of the drug, whereas mucus reduced non-targeting mobility to a greater extent than fucosylated liposomes. Spray-dried powder particles of bedaquiline-loaded liposomes showed a high fine particle fraction of > 70% and retained the integrity and targeting function of the liposomes. The antibiotic action was sustained when Mycobacterium abscessus was accumulated as a powdered aerosol over cultivated M. abscessus. The bacterial killing ability was improved when fucosylated variants were administered to THP-1 cells infected with M. abscessus (Figure 7B).133
Apart from acute infections, respiratory diseases are usually characterized by a long illness duration and poor therapeutic effects. The long-term action of inhaled nanomaterial formulations is more suitable for chronic respiratory diseases with longer illness durations. This is because it avoids the problems of repeated drug administration, rapid drug degradation, and systemic toxicity. With ever-changing breakthroughs in nanomaterial research, the therapeutic effects of nanomaterials on respiratory diseases are worthy of more in-depth exploration by researchers and scholars. We believe that research on the application of nanomaterials to the respiratory system is at a stage of vigorous development. However, the toxic outcomes of nanomaterials on the respiratory system, particularly the problem of nanomaterial removal after the disease is cured, may be the biggest difficulty hindering their clinical application. Nevertheless, we acknowledge the excellent progress made in research related to the management of respiratory disorders with nanomaterials and that the clinicalization of nanomaterial drugs for respiratory disorders is an exciting and worthwhile subsequent pursuit.
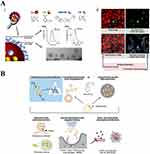 |
Figure 7 Nanoparticles applied to the treatment of lung infections. (A) (i) Chemical structure of PLA NP constituents (nanoemulsion technique) and description of the mean diameter of NPs utilizing three different techniques: PCS (number average data), NTA, and cryo-TEM. (ii) Imaging analysis demonstrated the intracellular anti-tuberculosis activity.132 Reprinted from Costa-Gouveia J, Pancani E, Jouny S, et al. Combination therapy for tuberculosis treatment: pulmonary administration of ethionamide and booster co-loaded nanoparticles. Sci Rep. 2017;7(1):5390.132 (B) Biological barriers within pulmonary drug delivery. Targeted liposomes and liposomal dry powder formulations loaded with bedaquiline (BDQ) and levofloxacin (LVX) sequentially underwent construction to address these barriers.133 Reprinted from Huck BC, Thiyagarajan D, Bali A, et al. Nano-in-Microparticles for Aerosol Delivery of Antibiotic-Loaded, Fucose-Derivatized, and Macrophage-Targeted Liposomes to Combat Mycobacterial Infections: In Vitro Deposition, Pulmonary Barrier Interactions, and Targeted Delivery. Adv Healthc Mater. 2022;11(11):e2102117.133 Abbreviation: NP, nanoparticle. |
Nano Respiratory Vaccines
A variety of vaccines have been used in clinical practice to prevent and treat respiratory diseases. However, the instability and toxicity of traditional vaccines in the human body hinder their utilization.135 Currently, subunit vaccines are considered the most promising therapeutic options because of their ability to effectively minimize side effects.134 However, the challenges of rapid degradation, difficulty in certain targeting, and absence of antigen-presenting cells (APCs) for the cross-presentation of antigens that exist in traditional subunit vaccines are major constraints hindering their further promotion and development. The emergence of nanotechnology has provided an effective solution to the challenges faced in subunit vaccine therapies. This is because nanovaccines composed of antigen-carrying nanoparticles can be preferentially internalized by APCs, thereby achieving rapid antigen presentation.136,137 In addition, delivery systems based on nanomaterials provide good stability features, protect against premature antigen deterioration, and enhance the specificity of antigen transportation to APCs. Moreover, nanoparticles can activate the immune response by inducing cytokine and antibody responses to promote the action of antigens. This unique property extends the possible use of nanoparticles as transportation vehicles for vaccines and adjuvants that can enhance the immunogenicity of vaccines.138,139
The design strategy of traditional tumor vaccines is to activate immune responses that are specific to the tumor by introducing tumor antigens into APCs found in lymph nodes (LNs) and to inhibit tumor growth, recurrence, and metastasis by inducing the body to strengthen long-term immune memory behavior and anti-tumor immunity (Figure 8A).140–142 Nanovaccine technology, however, enhances the targeting and stability of vaccines and effectively facilitates their clinical application. For example, Maarof et al used carbonate apatite nanoparticles, a biodegradable inorganic natural compound, to encapsulate afatinib, which avoids the problems of artificial polymer nanoparticles that are difficult to degrade with poor biocompatibility; the nanoparticles are able to fight NSCLC more consistently and slowly.143 Overall, nanovaccines enhance the utilization efficiency of cancer vaccines through two main pathways: (1) selective triggering of specific stimuli in the tumor immune microenvironment, which mediates the secretion of nanovaccine-loaded antigens/adjuvants (Figure 8B–D),144–147 and (2) utilizing the targeting properties of endogenous nanocarriers to LNs, with which the vaccine can rapidly enter the LNs and promote immune responses in the body.148,149
 |
Figure 8 Nanovaccines for the prevention and treatment of respiratory tumors. (A) Customizing patient-specific cancer vaccines. From Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359(6382):1355–1360. doi:10.1126/science.aar7112. reprinted with permission from American Association for the Advancement of Science (AAAS).140 (B) Description of a MnOx-OVA/tumor cell fragment. Reprinted with permission from Ding B, Zheng P, Jiang F, et al. MnO(x) nanospikes as nanoadjuvants and immunogenic cell death drugs with enhanced antitumor immunity and antimetastatic effect. Angewan Chem. 2020;59(38):16381–16384. doi:10.1002/anie.202005111. © 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim.146 (C) MnOx nanospikes against tumors. (i) H&E, TUNEL, CD8, and CRT stainings to assess treatment efficacy. (ii) H&E staining of lungs within antimetastatic investigations. The red arrows represent the metastatic nodules. Reproduced with permission from Ding B, Zheng P, Jiang F, et al. MnO(x) nanospikes as nanoadjuvants and immunogenic cell death drugs with enhanced antitumor immunity and antimetastatic effect. Angewan Chem. 2020;59(38):16381–16384. doi:10.1002/anie.202005111. © 2020 Wiley-VCH Verlag GmbH & Co. KGaA, Weinheim146 (D) SVMAV decreased the pulmonary metastasis of melanoma. (i) Representative H&E staining and Ki-67 immunohistochemical staining findings of different groups of lung sections. (ii) Photograph of resected lung tissue on day 37; red arrows indicate metastases.147 Reprinted fromZhang L, Huang J, Chen X, et al. Self-assembly nanovaccine containing TLR7/8 agonist and STAT3 inhibitor enhances tumor immunotherapy by augmenting tumor-specific immune response. J Immunother Cancer. 2021;9(8):e003132.147 |
Owing to the introduction of novel coronaviruses to the global healthcare system in 2019, research on vaccines against respiratory viruses has grown rapidly. Nanoparticles can mimic the structure of viruses at the micro-/nanometer level because they are the same nanometer size as the viruses. Therefore, nanotechnology is believed to enhance the functionality and structure of nanovaccines through various design strategies, thereby replacing traditional vaccines and achieving improved immunization against viral infections (Figure 9A).150,151 Among them, nucleic acid vaccines, which involve the transmission of the genetic code during the in-situ secretion of viral proteins, are prospective alternatives to traditional vaccine methods. Although the clinical safety and translatability of nucleic acid vaccines are major impediments to their practical application, nucleic acid vaccine design strategies are highly appealing due to their advantageous characteristics regarding safety, stability, speed, and scalability.150,152,153 In conjunction with antibody and CD4+ T-cell responses, DNA or acid vaccines have the capacity to stimulate CD8+ cytotoxic T-cell responses, which are pivotal in the process of viral eradication. DNA vaccines are more stable than mRNA vaccines, but mRNA is non-integrated and, hence, not at risk of insertional mutagenesis (Figure 9B and C).154,155 Furthermore, the half-life, stability, and immunogenicity of mRNAs were regulated by alterations. The advent of nanotechnology has enabled the targeted delivery of vaccines to specific cell types and subcellular sites, providing a favorable solution to difficulty in coping with antigen presentation.150,154,156 With breakthroughs in nanotechnology, nucleic acid nanovaccines are a promising vaccine design strategy.
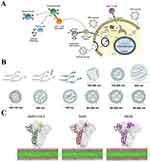 |
Figure 9 Nano virus vaccines in the respiratory system. (A) The antigenic cargo undergoes processing through the APC, and epitopes are introduced via MHC-I and MHC-II, resulting in the synthesis of CD8+ cytotoxic T cells or CD4+ T helper cells needed for antiviral antibody synthesis. Reprinted with permission from Shin MD, Shukla S, Chung YH, et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nature Nanotechnol. 2020;15(8):646–655. doi:10.1038/s41565-020-0737-y. Copyright 2020, Springer Nature.150 (B) Major delivery methods for mRNA vaccines. Copyright 2018, reprinted with permission from Springer Nature.154 (C) Structural modeling of SARS-CoV-2, SARS, and MERS spiny glycoproteins, based on which a targeted DNA nanovaccine was designed (antigen).155 Reprinted from Smith TRF, Patel A, Ramos S, et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat Commun. 2020;11(1):2601.155 Abbreviations: SARS, severe acute respiratory syndrome; CoV, coronavirus; MERS, middle east respiratory syndrome; APC, antigen-presenting cell; MHC, major histocompatibility complex. |
Nano Respiratory Imaging Theranostics
The attainment of precise diagnosis holds utmost importance in enhancing the effectiveness of management and the overall prognosis of respiratory disorders. Tumors and lesions situated in the deep regions of the lungs are often evaluated via the dendritic tracheobronchial tubes, thus opening up a new diagnostic strategy for respiratory diseases via endotracheal delivery. Owing to the special alveolar microenvironment of the lungs and the special anatomical structure of the lungs at all levels, endotracheal delivery of drugs ensures non-invasive and more accurate detection compared with other means of respiratory disease screening.157,158 In contrast, NPs are more specialized for organ-delivered drugs. Nanoparticles loaded with contrast agents can be used to image respiratory foci with high sensitivity at the cellular and molecular levels because of their extremely small particle size.159 Moreover, because nanoparticle size across gas delivery can be tuned in advance, doctors can change the size of nanoparticles to allow them to arrive at the alveolar area of deep lung tissues and improve their distribution and deposition to achieve better imaging effects. In addition, nanoparticles can amplify molecular signals by adding targeted ligands, thereby prolonging their residence time in the lungs, improving imaging results, and protecting secondary organs from immune system attacks.160
MRI is not considered the most preferred test for the respiratory system because of its long detection time and the uncontrollable respiratory motion of the human lungs; however, with the advancement of MRI technology and optimization of imaging sequences, it is possible to obtain more accurate images using nano-sized contrast agents compared to CT, X-ray scanning, and other conventional lung imaging methods. Perfluorocarbon (PFC) is a fluorine-containing compound with excellent oxygen-releasing ability and physicochemical properties that can be combined with conventional MRI contrast agents to enhance the sensitivity of MRI images.161 The present study indicated that PFC nanoparticles (~150 nm in size) administered via an endotracheal tube can diffuse and penetrate deep into lung tumors, produce good fluorescence and 1H- and 19F-MR imaging signals, and persist for a residence time of more than 72 h, making them a highly promising means of lung cancer detection.158 Moreover, because PFC nanoparticles contain a lipid monomolecular layer along with a PFC core, they can bind to various drugs and can be functionalized, further enhancing the detection of PFC nanoparticles in respiratory diseases. PFC nanoparticles can be used as quantitative biomarkers for the molecular imaging of lung cancer by combining them with a number of active targeted agents (Figure 10A).162 Faraj et al used biocompatible antibody-coupled superparamagnetic iron oxide (SPIO) nanoparticles to specifically target and image M1 and M2 macrophage subpopulations in a mouse model of slow-onset pulmonary disease (COPD), which is also applicable to the diagnosis of early pneumonia.163 In addition, Zheng delivered SPIO-NPs into the lungs by nebulized inhalation. This pioneering approach also presented the novel notion of magnetic particle imaging (MPI) as a prospective medical imaging approach. The crucial aspect of this methodology lies in the capacity to accurately identify and monitor aerosols in vivo (eg, assessing the efficacy and uniformity of aerosol delivery and tracking the initial nebulized treatment deposition in vivo), effectively addressing the limitations of SPIO imaging of the lungs because of the difficulty in imaging the lungs with MRI (Figure 10B).164 However, although there is extensive research on SPIO, the imaging technique of SPIO has been observed to result in a decrease in the resolution of soft tissues owing to artifacts produced by superparamagnetism. Moreover, the human body releases iron during apoptosis and lysis, and iron can be phagocytosed by microglia or macrophages, leading to false-positive imaging results.165,166 In particular, different pathological types of lung cancer often have specific molecular targets, such as the EGFR and anaplastic lymphoma kinase (ALK), which are commonly found in non-small cell lung cancer.167 During the combination of molecularly targeted drugs for different lung cancers and PFC nanoparticles, the construction of a targeted MRI detection method for different types of lung cancers to realize the accurate typing and staging of lung cancer has far-reaching research value and may be a popular topic in the future.168
 |
Figure 10 (A) Overlayed 1H (gray) and 19F (red) images of rabbit VX-2 orthotopic lung cancer models. Representative images prior to and at 0.5, 2, 3, 5, and 24 h following intratracheal (i) or intravenous (ii) administration of ανβ3-targeted PFC nanoparticles. Used with permission of Future Medicine Ltd, from (19)F MRI in orthotopic cancer model via intratracheal administration of α(ν)β(3)-targeted perfluorocarbon nanoparticles, Xu X, Zhang R, Liu F, et al, 13, 20, copyright 2018; permission conveyed through Copyright Clearance Center, Inc.162 (B) Magnetic particle imaging (MPI) can evaluate the delivery efficacy of various techniques. (i) Principle of operation of MPI. (ii) MPI evaluation of the delivery efficacy of three distinct techniques.164 Reprinted from Tay ZW, Chandrasekharan P, Zhou XY, Yu E, Zheng B, Conolly S. In vivo tracking and quantification of inhaled aerosol using magnetic particle imaging towards inhaled therapeutic monitoring. Theranostics. 2018;8(13):3676–3687.164 Abbreviation: PFC, perfluorocarbon. |
Compared with MRI nano-imaging technology, optical imaging technology is faster, has higher output, and is cheaper, and it is the mainstream research direction of nano-imaging technology at present. Moreover, the nano-imaging formed using an optical microscope can far exceed the spatial resolution of MRI and other imaging technologies and is difficult to compare with other imaging technologies when observing the single-molecule structure as well as the detailed features of the physiological structure of the cell. Owing to the significant advantages of optical imaging in monitoring and visualization, in optical imaging research related to the lung endotracheal transport strategy, researchers usually focus on developing a design strategy for optical nano-imaging materials and strive to detect lung diseases more accurately and comprehensively. Mizuno et al utilized indocyanine green (ICG) as a fluorescent marker and plasmid DNA pCMV-Luc encoding firefly luciferase as a reporter gene. They used a real-time in vivo imaging system to assess drug transportation to the lungs and gene expression across the lungs by detecting ICG fluorescence and luciferase activity, respectively.169
In addition, the application of nano-detection technology to the respiratory system is not limited to the diagnosis of lung diseases.170 Xu et al used UCNP-PEG-PEI nanoparticles encapsulated with two polymers, PEG and polyethylene imine (PEI), to label human amniotic fluid stem (hAFS) cells in a mouse model of acute lung injury (ALI),171 and they observed the migration of hAFS cells into the lungs by highly sensitive in vivo up-conversion luminescence imaging. The experimental results noted that hAFS cells exhibited significant positive effects on ALI-injured lung tissue repair, including restoration of alveolar capillary membrane integrity, attenuation of trans-epithelial leukocyte and neutrophil migration, and downregulation of pro-inflammatory cytokine and chemokine expression. This technique confirms the potential of optical nano-imaging for guided hAFS cell-based treatment of ALI, thus demonstrating the versatility of optical nano-imaging technology for both detection as well as adjunctive therapy. Similarly, Xie et al prepared a nanomedicine for the treatment of non-small cell lung cancer resistant to tyrosine kinase inhibitors by inhibiting upstream and downstream EGFR signaling pathways.172 This novel nanomedicine was formed based on carbonate bond cross-linking, disulfide bond cross-linking, and encapsulation. The nanomedicine can rapidly accumulate in the in situ NSCLC lesion, release the anticancer drug, and be detected by photoacoustic and fluorescence imaging. Tumor detection and therapeutic effects were achieved simultaneously.
However, studies on the long-term toxicity of nanobiomaterials using both MRI and optical nano-imaging techniques are lacking. Just as diagnostic imaging technologies, including X-ray scanning, CT, and MRI, are constantly advancing and updating, in addition to more accurate and efficient diagnostic capabilities, the harm caused to patients during the diagnostic process should be explored. Nanodiagnostics can only be popularized by constantly balancing the tradeoffs between detection accuracy and side effects. In addition, nano-imaging technology that combines both detection and treatment is an exciting research direction. With the pursuit of multifunctionalization of nanomedicines, exploring improvement strategies to enable nanomedicines to simultaneously perform multiple medical functions, such as therapy, diagnosis, and monitoring, will have more far-reaching clinical significance.
Conclusions and Outlook
With the rapid advancement in nanomaterials and nanomedicine technologies, the utilization of nanomaterials in treating clinical disorders has become highly desirable. Recently, numerous nanomedicine studies have reported on the treatment of multiple clinical disorders. This is mainly because, unlike conventional drug formulations, nanoparticle drugs overcome the pharmacokinetic limitations of conventional drug formulations and are no longer hampered by limited dispersion and rapid degradation of drug action; instead, they can act on the lesion in a sustained manner. For respiratory diseases, inhalation drug delivery of nanomaterials is considered one of the most promising therapeutic approaches, as it can directly pass through the alveolar membrane, act on the lesion, and reasonably avoid the clearance effect of blood circulation and the first-pass clearance effects of the liver, realizing continuous treatment of the lesion. However, because of the very small size of the nanomaterial, it can easily enter the cell through endocytosis. Moreover, inhaled nanomaterials lack normal clearance by the body and are difficult to degrade in the long term, inevitably leading to a build-up effect and biotoxicity. At the same time, nanomaterial biotoxicity is easy to ignore in the current quest for nanomedicine biomaterial. While most nanomaterial formulations exhibit excellent therapeutic effects, most emerging nanomaterials have unknown long-term effects owing to drug deposition and toxic side effects. From an immunological perspective, the human immune system is also subject to the impact of nanomedicines. For example, the scavenging effect of macrophages will lead to the accumulation of nanomedicines in the liver, spleen, and other immune organs after long-term circulation in the body, which may exacerbate organ toxicity and alter the therapeutic effect of nanomedicines on the original lesions. Moreover, as an exogenous drug, nanomedicine will inevitably induce an immune rejection reaction in the human body, which will cause a variety of immune symptoms, although nanomaterials can be modified to allow immune escape. The effectiveness and longevity of such immune escape modifications represent a current issue in nano-immunology and should be further discussed.
How to correctly balance the therapeutic and diagnostic effects of nanomaterials with their long-term toxicological effects in clinical applications is an area worth exploring. To better deal with the pros and cons of nanomaterial formulations, we believe that the core aspect of this task is to dissect the results of more detailed nanotoxicology experiments when designing biomedical nanomaterials. At the cellular level, the uptake and translocation process, as well as the toxic effects of nanomaterials, can be monitored in more detail using mass spectrometry imaging techniques, single-cell techniques, and other methods. Similarly, at the organ level, in vivo quantification as well as in vivo imaging techniques, should be used to monitor the uptake, distribution, metabolism, and excretion of nanomaterials. In particular, for nanobiomaterials applied to the respiratory system, their toxic and deposition effects in non-respiratory organs should not be neglected during the experimental design. Moreover, for nanomaterials that are difficult to metabolize and excrete, it is imperative to monitor their possible long-term effects on experimental models during the experimental design process. Certainly, the possible imaging signal attenuation of the nanomaterials themselves and differences in metabolism of nanomedicines due to individual differences will affect the results of the nanotracking imaging technique. Thus, these issues should be explored in depth. Moreover, potential ethical issues, expenses, and poor patient compliance will also be challenges for the clinicalization of this technology. Although the popularization and application of nanomaterials in respiratory diseases remain to be further advanced, many researchers have been promoting the development of nanomedicine technology through various fields of research over the past decades, and the clinical significance of nanomedicine within the respiratory system is worth looking forward to.
Abbreviations
ALK, Anaplastic lymphoma kinase; ALI, Acute lung injury; AM, Alveolar macrophages; APC, Antigen-presenting cells; CF, Cystic fibrosis; CFTR, CF transmembrane conductance regulator; COPD, Chronic obstructive pulmonary disease; CT, Computed tomography; EGFR, Epidermal growth factor receptor-tyrosine; EPR, Enhanced permeability and retention; hAFS, Human amniotic fluid stem; LHRH, Luteinizing hormone-releasing hormone; LMNP, Lignin micro-/nanoparticles; LN, Lymph nodes; LPN, Liposomal polymer nanoparticles; MPI, Magnetic particle imaging; MRI, Magnetic resonance imaging; NLC, Nanostructured lipid carriers; PAH, Polycyclic aromatic hydrocarbons; PEI, Polyethylene imine; PS, Pulmonary surfactant; ROS, Reactive oxygen species; RSV, Respiratory syncytial virus; SLN, Solid lipid nanoparticles; VARID, Viral Associated Respiratory Infectious Diseases; VOC, Volatile organic compounds.
Consent for Publication
All authors have read and approved the manuscript for submission.
Author Contributions
All authors made a significant contribution to the work reported, whether that is in the conception, study design, execution, acquisition of data, analysis and interpretation, or in all these areas; took part in drafting or writing, revising or critically reviewing the article; gave final approval of the version to be published; have agreed on the journal to which the article has been submitted; and agree to be accountable for the contents of this article.
Funding
This work was supported by the Ministry of Science and Technology of the People’s Republic of China (STI2030-Major Projects2021ZD0201900) and was supported by National Natural Science Foundation of China (82170100, 82120108002).
Disclosure
The authors declare no competing interests.
References
1. Hayes AJ, Bakand S. Toxicological perspectives of inhaled therapeutics and nanoparticles. Expert Opin Drug Metab Toxicol. 2014;10(7):933–947. doi:10.1517/17425255.2014.916276
2. Hye T, Moinuddin SM, Sarkar T, Nguyen T, Saha D, Ahsan F. An evolving perspective on novel modified release drug delivery systems for inhalational therapy. Expert Opin Drug Delivery. 2023;20(3):335–348. doi:10.1080/17425247.2023.2175814
3. Forest V, Pourchez J. Nano-delivery to the lung - by inhalation or other routes and why nano when micro is largely sufficient? Adv Drug Deliv Rev. 2022;183:114173.
4. Zhang YB, Xu D, Bai L, Zhou YM, Zhang H, Cui YL. A review of non-invasive drug delivery through respiratory routes. Pharmaceutics. 2022;14:9. doi:10.3390/pharmaceutics14091974
5. Kamat S, Kumari M, Jayabaskaran C. Nano-engineered tools in the diagnosis, therapeutics, prevention, and mitigation of SARS-CoV-2. J Cont Rel. 2021;338:813–836. doi:10.1016/j.jconrel.2021.08.046
6. Xi J, Longest PW, Martonen TB. Effects of the laryngeal jet on nano- and microparticle transport and deposition in an approximate model of the upper tracheobronchial airways. J Appl Physiol. 2008;104(6):1761–1777. doi:10.1152/japplphysiol.01233.2007
7. Sreedharan S, Zouganelis G, Drake SJ, Tripathi G, Kermanizadeh A. Nanomaterial-induced toxicity in pathophysiological models representative of individuals with pre-existing medical conditions. J Toxicol Env Heal B. 2023;26(1):1–27. doi:10.1080/10937404.2022.2153456
8. Bock S, Rades T, Rantanen J, Scherließ R. Additive manufacturing in respiratory sciences - Current applications and future prospects. Adv. Drug Delivery Rev. 2022;186:114341. doi:10.1016/j.addr.2022.114341
9. Mrsny RJ. Lessons from nature: ”Pathogen-Mimetic” systems for mucosal nano-medicines. Adv Drug Deliv Rev. 2009;61(2):172–192.
10. Nho R. Pathological effects of nano-sized particles on the respiratory system. Nanomedicine. 2020;29:102242. doi:10.1016/j.nano.2020.102242
11. Xing Y, Lu P, Xue Z, et al. Nano-strategies for improving the bioavailability of inhaled pharmaceutical formulations. Mini Rev Med Chem. 2020;20(13):1258–1271. doi:10.2174/1389557520666200509235945
12. Zhao Q, Li Y, Chai X, et al. Interaction of pulmonary surfactant with silica and polycyclic aromatic hydrocarbons: implications for respiratory health. Chemosphere. 2019;222:603–610. doi:10.1016/j.chemosphere.2019.02.002
13. Seyfoori A, Shokrollahi Barough M, Mokarram P, et al. Emerging advances of nanotechnology in drug and vaccine delivery against viral associated respiratory infectious diseases (VARID). Int J Mol Sci. 2021;22:13. doi:10.3390/ijms22136937
14. Xu Y, Parra-Ortiz E, Wan F, et al. Insights into the mechanisms of interaction between inhalable lipid-polymer hybrid nanoparticles and pulmonary surfactant. J Colloid Interface Sci. 2023;633:511–525. doi:10.1016/j.jcis.2022.11.059
15. Zhao J, Su J, Qin L, Zhang X, Mao S. Exploring the influence of inhaled liposome membrane fluidity on its interaction with pulmonary physiological barriers. Biomater Sci. 2020;8(23):6786–6797. doi:10.1039/D0BM01529F
16. Ali ME, McConville JT, Lamprecht A. Pulmonary delivery of anti-inflammatory agents. Expert Opin Drug Deliv. 2015;12(6):929–945. doi:10.1517/17425247.2015.993968
17. Renwick LC, Donaldson K, Clouter A. Impairment of alveolar macrophage phagocytosis by ultrafine particles. Toxicol Appl Pharmacol. 2001;172(2):119–127. doi:10.1006/taap.2001.9128
18. Zhang Y, Wong CYJ, Gholizadeh H, et al. Microfluidics assembly of inhalable liposomal ciprofloxacin characterised by an innovative in vitro pulmonary model. Int J Pharm. 2023;635:122667. doi:10.1016/j.ijpharm.2023.122667
19. Gradon L, Orlicki D, Podgorski A. Deposition and retention of ultrafine aerosol particles in the human respiratory system. Normal and pathological cases. Int J Occup Saf Ergon. 2000;6(2):189–207. doi:10.1080/10803548.2000.11076451
20. Stone V, Johnston H, Clift MJ. Air pollution, ultrafine and nanoparticle toxicology: cellular and molecular interactions. IEEE Trans NanoBiosci. 2007;6(4):331–340. doi:10.1109/TNB.2007.909005
21. Kwok PC, Tunsirikongkon A, Glover W, Chan HK. Formation of protein nano-matrix particles with controlled surface architecture for respiratory drug delivery. Pharm Res. 2011;28(4):788–796. doi:10.1007/s11095-010-0332-2
22. Poh TY, Ali N, Mac Aogáin M, et al. Inhaled nanomaterials and the respiratory microbiome: clinical, immunological and toxicological perspectives. Part Fibre Toxicol. 2018;15(1):46.
23. Wang W, Huang Z, Huang Y, et al. Pulmonary delivery nanomedicines towards circumventing physiological barriers: strategies and characterization approaches. Adv. Drug Delivery Rev. 2022;185:114309. doi:10.1016/j.addr.2022.114309
24. Fornaguera C, Llinas M, Solans C, Caldero G. Design and in vitro evaluation of biocompatible dexamethasone-loaded nanoparticle dispersions, obtained from nano-emulsions, for inhalatory therapy. Colloids Surf B Biointerfaces. 2015;125:58–64. doi:10.1016/j.colsurfb.2014.11.006
25. Ziaei E, Emami J, Rezazadeh M, Kazemi M. Pulmonary delivery of docetaxel and celecoxib by PLGA porous microparticles for their synergistic effects against lung cancer. Anticancer Agents Med Chem. 2022;22(5):951–967. doi:10.2174/1871520621666210811111152
26. Rashid J, Alobaida A, Al-Hilal TA, et al. Repurposing rosiglitazone, a PPAR-gamma agonist and oral antidiabetic, as an inhaled formulation, for the treatment of PAH. J Control Release. 2018;280:113–123.
27. Rasul RM, Tamilarasi Muniandy M, Zakaria Z, et al. A review on chitosan and its development as pulmonary particulate anti-infective and anti-cancer drug carriers. Carbohydr Polym. 2020;250:116800.
28. Paul P, Sengupta S, Mukherjee B, Shaw TK, Gaonkar RH, Debnath MC. Chitosan-coated nanoparticles enhanced lung pharmacokinetic profile of voriconazole upon pulmonary delivery in mice. Nanomedicine (Lond). 2018;13(5):501–520. doi:10.2217/nnm-2017-0291
29. Bandi N, Ayalasomayajula SP, Dhanda DS, Iwakawa J, Cheng PW, Kompella UB. Intratracheal budesonide-poly(lactide-co-glycolide) microparticles reduce oxidative stress, VEGF expression, and vascular leakage in a benzo(a)pyrene-fed mouse model. J Pharm Pharmacol. 2005;57(7):851–860. doi:10.1211/0022357056334
30. Yoo D, Guk K, Kim H, Khang G, Wu D, Lee D. Antioxidant polymeric nanoparticles as novel therapeutics for airway inflammatory diseases. Int J Pharm. 2013;450(1–2):87–94. doi:10.1016/j.ijpharm.2013.04.028
31. Satta S, Shahabipour F, Gao W, et al. Engineering viral genomics and nano-liposomes in microfluidic platforms for patient-specific analysis of SARS-CoV-2 variants. Theranostics. 2022;12(10):4779–4790. doi:10.7150/thno.72339
32. LoPresti ST, Arral ML, Chaudhary N, Whitehead KA. The replacement of helper lipids with charged alternatives in lipid nanoparticles facilitates targeted mRNA delivery to the spleen and lungs. J Cont Rel. 2022;345:819–831. doi:10.1016/j.jconrel.2022.03.046
33. Weber S, Zimmer A, Pardeike J. Solid Lipid Nanoparticles (SLN) and Nanostructured Lipid Carriers (NLC) for pulmonary application: a review of the state of the art. Eur J Pharm Biopharm. 2014;86(1):7–22. doi:10.1016/j.ejpb.2013.08.013
34. Nahar K, Absar S, Patel B, Ahsan F. Starch-coated magnetic liposomes as an inhalable carrier for accumulation of fasudil in the pulmonary vasculature. Int J Pharm. 2014;464(1–2):185–195. doi:10.1016/j.ijpharm.2014.01.007
35. Rashid J, Nahar K, Raut S, Keshavarz A, Ahsan F. Fasudil and DETA NONOate, loaded in a peptide-modified liposomal carrier, Slow PAH progression upon pulmonary delivery. Mol Pharm. 2018;15(5):1755–1765. doi:10.1021/acs.molpharmaceut.7b01003
36. Garbuzenko OB, Kuzmov A, Taratula O, Pine SR, Minko T. Strategy to enhance lung cancer treatment by five essential elements: inhalation delivery, nanotechnology, tumor-receptor targeting, chemo- and gene therapy. Theranostics. 2019;9(26):8362–8376. doi:10.7150/thno.39816
37. Jiang Z, Ma Y, Guo X, et al. Sustainable production of lignin micro-/nano-particles (LMNPs) from biomass: influence of the type of biomass on their self-assembly capability and physicochemical properties. J Hazard Mater. 2021;403:123701. doi:10.1016/j.jhazmat.2020.123701
38. Sakellari GI, Zafeiri I, Batchelor H, Spyropoulos F. Formulation design, production and characterisation of solid lipid nanoparticles (SLN) and nanostructured lipid carriers (NLC) for the encapsulation of a model hydrophobic active. Food Hydrocoll Health. 2021;2021:1.
39. Khan I, Hussein S, Houacine C, et al. Fabrication, characterization and optimization of nanostructured lipid carrier formulations using Beclomethasone dipropionate for pulmonary drug delivery via medical nebulizers. Int J Pharm. 2021;598:120376. doi:10.1016/j.ijpharm.2021.120376
40. Prasanna P, Rathee S, Upadhyay A, Sulakshana S. Nanotherapeutics in the treatment of acute respiratory distress syndrome. Life Sci. 2021;276:119428. doi:10.1016/j.lfs.2021.119428
41. Li H, Sun J, Zhu H, et al. Recent advances in development of dendritic polymer-based nanomedicines for cancer diagnosis. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2021;13(2):e1670. doi:10.1002/wnan.1670
42. Halevas E, Mavroidi B, Kokotidou C, et al. Remdesivir-loaded bis-MPA hyperbranched dendritic nanocarriers for pulmonary delivery. J Drug Deliv Sci Technol. 2022;75:103625. doi:10.1016/j.jddst.2022.103625
43. Kaminskas LM, McLeod VM, Ryan GM, et al. Pulmonary administration of a doxorubicin-conjugated dendrimer enhances drug exposure to lung metastases and improves cancer therapy. J Control Release. 2014;183:18–26. doi:10.1016/j.jconrel.2014.03.012
44. Zamborlin A, Ermini ML, Summa M, et al. The fate of intranasally instilled silver nanoarchitectures. Nano Lett. 2022;22(13):5269–5276. doi:10.1021/acs.nanolett.2c01180
45. Tseng CL, Su WY, Yen KC, Yang KC, Lin FH. The use of biotinylated-EGF-modified gelatin nanoparticle carrier to enhance cisplatin accumulation in cancerous lungs via inhalation. Biomaterials. 2009;30(20):3476–3485. doi:10.1016/j.biomaterials.2009.03.010
46. Hindi KM, Ditto AJ, Panzner MJ, et al. The antimicrobial efficacy of sustained release silver-carbene complex-loaded L-tyrosine polyphosphate nanoparticles: characterization, in vitro and in vivo studies. Biomaterials. 2009;30(22):3771–3779. doi:10.1016/j.biomaterials.2009.03.044
47. Javed H, Shah SNH, Iqbal FM, Javed N, Saeed B. A hematological and histopathological study on diphenhydramine nasal nano-gel and nano-emulgel for the management of allergic rhinitis in animal model. AAPS Pharm Sci Tech. 2023;24(2):55. doi:10.1208/s12249-023-02515-w
48. Li Y, Han M, Liu T, Cun D, Fang L, Yang M. Inhaled hyaluronic acid microparticles extended pulmonary retention and suppressed systemic exposure of a short-acting bronchodilator. Carbohydr Polym. 2017;172:197–204. doi:10.1016/j.carbpol.2017.05.020
49. Yue P, Zhou W, Huang G, et al. Nanocrystals based pulmonary inhalation delivery system: advance and challenge. Drug Deliv. 2022;29(1):637–651. doi:10.1080/10717544.2022.2039809
50. Costabile G, Provenzano R, Azzalin A, et al. PEGylated mucus-penetrating nanocrystals for lung delivery of a new FtsZ inhibitor against Burkholderia cenocepacia infection. Nanomedicine. 2020;23:102113. doi:10.1016/j.nano.2019.102113
51. Ju Y, Hu Y, Yang P, Xie X, Fang B. Extracellular vesicle-loaded hydrogels for tissue repair and regeneration. Mater Today Bio. 2023;2023:18.
52. Popowski KD, Moatti A, Scull G, et al. Inhalable dry powder mRNA vaccines based on extracellular vesicles. Matter. 2022;5(9):2960–2974. doi:10.1016/j.matt.2022.06.012
53. Han Y, Zhu Y, Youngblood HA, et al. Nebulization of extracellular vesicles: a promising small RNA delivery approach for lung diseases. J Control Release. 2022;352:556–569. doi:10.1016/j.jconrel.2022.10.052
54. Zhai Z, Cui T, Chen J, Mao X, Zhang T. Advancements in engineered mesenchymal stem cell exosomes for chronic lung disease treatment. J Transl Med. 2023;21(1). doi:10.1186/s12967-023-04729-9
55. Liu C, Xi L, Liu Y, et al. An inhalable hybrid biomimetic nanoplatform for sequential drug release and remodeling lung immune homeostasis in acute lung injury treatment. ACS Nano. 2023;17(12):11626–11644. doi:10.1021/acsnano.3c02075
56. Rothen DA, Krenger PS, Nonic A, et al. Intranasal administration of a virus like particles‐based vaccine induces neutralizing antibodies against SARS‐CoV‐2 and variants of concern. Allergy. 2022;77(8):2446–2458. doi:10.1111/all.15311
57. Wang Z, Popowski KD, Zhu D, et al. Exosomes decorated with a recombinant SARS-CoV-2 receptor-binding domain as an inhalable COVID-19 vaccine. Nat Biomed Eng. 2022;6(7):791–805. doi:10.1038/s41551-022-00902-5
58. Zakaria MY, El-Halim SM A, Beshay BY, Zaki I, Abourehab MAS. ‘Poly phenolic phytoceutical loaded nano-bilosomes for enhanced caco-2 cell permeability and SARS-CoV 2 antiviral activity’: in-vitro and insilico studies. Drug Deliv. 2023;30(1):2162157. doi:10.1080/10717544.2022.2162157
59. Detalle L, Stohr T, Palomo C, et al. Generation and characterization of ALX-0171, a potent novel therapeutic nanobody for the treatment of respiratory syncytial virus infection. Antimicrob Agents Chemother. 2016;60(1):6–13. doi:10.1128/AAC.01802-15
60. Liu Q, Guan J, Song R, Zhang X, Mao S. Physicochemical properties of nanoparticles affecting their fate and the physiological function of pulmonary surfactants. Acta Biomater. 2022;140:76–87. doi:10.1016/j.actbio.2021.11.034
61. Raine RI. Technology in respiratory medicine. Cont Med Educ. 2003;16(4):200.
62. Barthold S, Kunschke N, Murgia X, Loretz B, Carvalho-Wodarz CS, Lehr CM. Overview of inhaled nanopharmaceuticals. J Aerosol Med Pulm Drug Deliv. 2023;36(3):144–151. doi:10.1089/jamp.2023.29089.sb
63. Chen C, Zhou C, Zhang W, et al. Effect and mechanism of PINK1/parkin-mediated mitochondrial autophagy in rat lung injury induced by nano lanthanum oxide. Nanomaterials. 2022;12:15.
64. Basu S, Kabi P, Chaudhuri S, Saha A. Insights on drying and precipitation dynamics of respiratory droplets from the perspective of COVID-19. Phys Fluids. 2020;32(12):123317. doi:10.1063/5.0037360
65. Fdez-Arroyabe P, Salcines C, Kassomenos P, Santurtún A, Petäjä T. Electric charge of atmospheric nanoparticles and its potential implications with human health. Sci Total Environ. 2022;808:152106. doi:10.1016/j.scitotenv.2021.152106
66. Phan TH, Shi H, Denes CE, et al. Advanced pathophysiology mimicking lung models for accelerated drug discovery. Biomater Res. 2023;27(1):35. doi:10.1186/s40824-023-00366-x
67. Wang H, Wu L, Sun X. Intratracheal delivery of nano- and microparticles and hyperpolarized gases: a promising strategy for the imaging and treatment of respiratory disease. Chest. 2020;157(6):1579–1590. doi:10.1016/j.chest.2019.11.036
68. Blank F, Fytianos K, Seydoux E, et al. Interaction of biomedical nanoparticles with the pulmonary immune system. J Nanobiotechnology. 2017;15(1):6. doi:10.1186/s12951-016-0242-5
69. Ruenraroengsak P, Novak P, Berhanu D, et al. Respiratory epithelial cytotoxicity and membrane damage (holes) caused by amine-modified nanoparticles. Nanotoxicology. 2012;6(1):94–108. doi:10.3109/17435390.2011.558643
70. Doryab A, Taskin MB, Stahlhut P, et al. A bioinspired in vitro lung model to study particokinetics of nano-/microparticles under cyclic stretch and air-liquid interface conditions. Front Bioeng Biotechnol. 2021;9:616830. doi:10.3389/fbioe.2021.616830
71. Bessa MJ, Brandão F, Rosário F, et al. Assessing the in vitro toxicity of airborne (nano)particles to the human respiratory system: from basic to advanced models. J Toxicol Env Heal B. 2023;26(2):67–96. doi:10.1080/10937404.2023.2166638
72. Geiser M, Kreyling WG. Deposition and biokinetics of inhaled nanoparticles. Part Fibre Toxicol. 2010;7:2. doi:10.1186/1743-8977-7-2
73. Kurbatova P, Bessonov N, Volpert V, et al. Model of mucociliary clearance in cystic fibrosis lungs. J Theor Biol. 2015;372:81–88. doi:10.1016/j.jtbi.2015.02.023
74. Bustamante-Marin XM, Ostrowski LE. Cilia and mucociliary clearance. Cold Spring Harbor Perspect. Biol. 2017;9(4):a028241. doi:10.1101/cshperspect.a028241
75. Newman SP. Drug delivery to the lungs: challenges and opportunities. Therap Deliv. 2017;8(8):647–661. doi:10.4155/tde-2017-0037
76. Anderson CF, Grimmett ME, Domalewski CJ, Cui H. Inhalable nanotherapeutics to improve treatment efficacy for common lung diseases. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2020;12:1.
77. Kiyono H, Azegami T. The mucosal immune system: from dentistry to vaccine development. Proc Jpn Acad Ser B Phys Biol Sci. 2015;91(8):423–439. doi:10.2183/pjab.91.423
78. Ardain A, Marakalala MJ, Leslie A. Tissue-resident innate immunity in the lung. Immunology. 2020;159(3):245–256. doi:10.1111/imm.13143
79. Gosens I, Cassee FR, Zanella M, et al. Organ burden and pulmonary toxicity of nano-sized copper (II) oxide particles after short-term inhalation exposure. Nanotoxicology. 2016;10(8):1084–1095. doi:10.3109/17435390.2016.1172678
80. Patton JS, Brain JD, Davies LA, et al. The particle has landed--characterizing the fate of inhaled pharmaceuticals. J Aero Med Pulm Drug Deliv. 2010;23:S71–87.
81. Mühlfeld C, Rothen-Rutishauser B, Blank F, Vanhecke D, Ochs M, Gehr P. Interactions of nanoparticles with pulmonary structures and cellular responses. Am J Physiol Lung Cell Mol Physiol. 2008;294(5):L817–829. doi:10.1152/ajplung.00442.2007
82. Truzzi E, Nascimento TL, Iannuccelli V, et al. In vivo biodistribution of respirable solid lipid nanoparticles surface-decorated with a mannose-based surfactant: a promising tool for pulmonary tuberculosis treatment? Nanomaterials. 2020;10:3.
83. Shah RM, Rajasekaran D, Ludford-Menting M, Eldridge DS, Palombo EA, Harding IH. Transport of stearic acid-based solid lipid nanoparticles (SLNs) into human epithelial cells. Colloids Surf B. 2016;140:204–212. doi:10.1016/j.colsurfb.2015.12.029
84. Guagliardo R, Pérez-Gil J, De Smedt S, Raemdonck K. Pulmonary surfactant and drug delivery: focusing on the role of surfactant proteins. J Cont Rel. 2018;291:116–126. doi:10.1016/j.jconrel.2018.10.012
85. Vega-Villa KR, Takemoto JK, Yáñez JA, Remsberg CM, Forrest ML, Davies NM. Clinical toxicities of nanocarrier systems. Adv Drug Deliv Rev. 2008;60(8):929–938. doi:10.1016/j.addr.2007.11.007
86. Soto KF, Murr LE, Garza KM. Cytotoxic responses and potential respiratory health effects of carbon and carbonaceous nanoparticulates in the Paso del Norte airshed environment. Int J Environ Res Public Health. 2008;5(1):12–25. doi:10.3390/ijerph5010012
87. Cena LG, Chisholm WP, Keane MJ, Chen BT. A field study on the respiratory deposition of the nano-sized fraction of mild and stainless steel welding fume metals. J Occup Environ Hyg. 2015;12(10):721–728. doi:10.1080/15459624.2015.1043055
88. Bierkandt FS, Leibrock L, Wagener S, Laux P, Luch A. The impact of nanomaterial characteristics on inhalation toxicity. Toxicol Res (Camb). 2018;7(3):321–346.
89. Song S, Ding L, Liu G, et al. The protective effects of baicalin for respiratory diseases: an update and future perspectives. Front Pharmacol. 2023;14:1129817.
90. Lee SH, Wang TY, Hong JH, Cheng TJ, Lin CY. NMR-based metabolomics to determine acute inhalation effects of nano- and fine-sized ZnO particles in the rat lung. Nanotoxicology. 2016;10(7):924–934.
91. Dobson J. Toxicological aspects and applications of nanoparticles in paediatric respiratory disease. Paediatr Respir Rev. 2007;8(1):62–66.
92. Li Y, Zhu Y, Zhao B, et al. Amorphous silica nanoparticles caused lung injury through the induction of epithelial apoptosis via ROS/Ca(2+)/DRP1-mediated mitochondrial fission signaling. Nanotoxicology. 2022;16:6–8.
93. Sayers BC, Germolec DR, Walker NJ, et al. Respiratory toxicity and immunotoxicity evaluations of microparticle and nanoparticle C60 fullerene aggregates in mice and rats following nose-only inhalation for 13 weeks. Nanotoxicology. 2016;10(10):1458–1468.
94. Sun D, Zhang G, Xie M, et al. Softness enhanced macrophage-mediated therapy of inhaled apoptotic-cell-inspired nanosystems for acute lung injury. J Nanobiotechnology. 2023;21(1):172.
95. Wei T, Tang M. Biological effects of airborne fine particulate matter (PM(2.5)) exposure on pulmonary immune system. Environ Toxicol Pharmacol. 2018;60:195–201.
96. Yu Y, Pan Y, Chang B, Zhao X, Qu K, Song Y. Silica nanoparticles induce pulmonary damage in rats via VEGFC/D-VEGFR3 signaling-mediated lymphangiogenesis and remodeling. Toxicology. 2023;493:153552.
97. Lee SH, Tang CH, Lin WY, et al. LC-MS-based lipidomics to examine acute rat pulmonary responses after nano- and fine-sized ZnO particle inhalation exposure. Nanotoxicology. 2018;12(5):439–452.
98. Meneses J, González-Durruthy M, Fernandez-de-Gortari E, Toropova AP, Toropov AA, Alfaro-Moreno E. A Nano-QSTR model to predict nano-cytotoxicity: an approach using human lung cells data. Part Fibre Toxicol. 2023;20(1):21.
99. Yusuf A, Almotairy ARZ, Henidi H, Alshehri OY, Aldughaim MS. Nanoparticles as drug delivery systems: a review of the implication of nanoparticles’ physicochemical properties on responses in biological systems. Polymers. 2023;15:7.
100. Huang X, Li C, Wei T, et al. Oropharyngeal aspirated Ag/TiO2 nanohybrids: transformation, distribution and toxicity. Science of the Total Environment. 2024;2024:908.
101. Tomita Y, Rikimaru-Kaneko A, Hashiguchi K, Shirotake S. Effect of anionic and cationic n-butylcyanoacrylate nanoparticles on NO and cytokine production in Raw264.7 cells. Immuno and Immunotoxicology. 2011;33(4):730–737. doi:10.3109/08923973.2011.565345
102. Xu Y, Zheng Y, Ding X, et al. PEGylated pH-responsive peptide-mRNA nano self-assemblies enhance the pulmonary delivery efficiency and safety of aerosolized mRNA. Drug Delivery. 2023;30(1):2219870. doi:10.1080/10717544.2023.2219870
103. Doroudian M, ON A, Mac Loughlin R, Prina-Mello A, Volkov Y, Donnelly SC. Nanotechnology in pulmonary medicine. Curr Opin Pharmacol. 2021;56:85–92. doi:10.1016/j.coph.2020.11.002
104. Omlor AJ, Nguyen J, Bals R, Dinh QT. Nanotechnology in respiratory medicine. Respir Res. 2015;16(1):64. doi:10.1186/s12931-015-0223-5
105. Doroudian M, MacLoughlin R, Poynton F, Prina-Mello A, Donnelly SC. Nanotechnology based therapeutics for lung disease. Thorax. 2019;74(10):965–976. doi:10.1136/thoraxjnl-2019-213037
106. Shim MK, Song SK, Jeon SI, Hwang KY, Kim K. Nano-sized drug delivery systems to potentiate the immune checkpoint blockade therapy. Expert Opin Drug Deliv. 2022;19(6):641–652. doi:10.1080/17425247.2022.2081683
107. Lee HY, Mohammed KA, Nasreen N. Nanoparticle-based targeted gene therapy for lung cancer. Am J Can Res. 2016;6(5):1118–1134.
108. Wang Y, Li S, Wang X, et al. Smart transformable nanomedicines for cancer therapy. Biomaterials. 2021;271:120737. doi:10.1016/j.biomaterials.2021.120737
109. Danhier F. To exploit the tumor microenvironment: since the EPR effect fails in the clinic, what is the future of nanomedicine? J Control Release. 2016;244(Pt A):108–121. doi:10.1016/j.jconrel.2016.11.015
110. Alshammari MK, Almomen EY, Alshahrani KF, et al. Nano-enabled strategies for the treatment of lung cancer: potential bottlenecks and future perspectives. Biomedicines. 2023;11(2). doi:10.3390/biomedicines11020473
111. Yang SJ, Huang CH, Wang CH, Shieh MJ, Chen KC. The synergistic effect of hyperthermia and chemotherapy in magnetite nanomedicine-based lung cancer treatment. Int j Nanomed. 2020;15:10331–10347. doi:10.2147/IJN.S281029
112. Guan S, Munder A, Hedtfeld S, et al. Self-assembled peptide-poloxamine nanoparticles enable in vitro and in vivo genome restoration for cystic fibrosis. Nat Nanotechnol. 2019;14(3):287–297. doi:10.1038/s41565-018-0358-x
113. Kuzmov A, Minko T. Nanotechnology approaches for inhalation treatment of lung diseases. J Control Release. 2015;219:500–518. doi:10.1016/j.jconrel.2015.07.024
114. Taratula O, Kuzmov A, Shah M, Garbuzenko OB, Minko T. Nanostructured lipid carriers as multifunctional nanomedicine platform for pulmonary co-delivery of anticancer drugs and siRNA. J Control Release. 2013;171(3):349–357. doi:10.1016/j.jconrel.2013.04.018
115. Tian X, Gu T, Lee MH, Dong Z. Challenge and countermeasures for EGFR targeted therapy in non-small cell lung cancer. Biochim Biophys Acta Rev Cancer. 2022;1877(1):188645. doi:10.1016/j.bbcan.2021.188645
116. Tseng CL, Wu SY, Wang WH, et al. Targeting efficiency and biodistribution of biotinylated-EGF-conjugated gelatin nanoparticles administered via aerosol delivery in nude mice with lung cancer. Biomaterials. 2008;29(20):3014–3022. doi:10.1016/j.biomaterials.2008.03.033
117. Sadhukha T, Wiedmann TS, Panyam J. Inhalable magnetic nanoparticles for targeted hyperthermia in lung cancer therapy. Biomaterials. 2013;34(21):5163–5171. doi:10.1016/j.biomaterials.2013.03.061
118. Griesenbach U, Pytel KM, Alton EW. Cystic fibrosis gene therapy in the UK and elsewhere. Hum Gene Ther. 2015;26(5):266–275. doi:10.1089/hum.2015.027
119. Suk JS, Kim AJ, Trehan K, et al. Lung gene therapy with highly compacted DNA nanoparticles that overcome the mucus barrier. J Control Release. 2014;178:8–17. doi:10.1016/j.jconrel.2014.01.007
120. Konstan MW, Davis PB, Wagener JS, et al. Compacted DNA nanoparticles administered to the nasal mucosa of cystic fibrosis subjects are safe and demonstrate partial to complete cystic fibrosis transmembrane regulator reconstitution. Hum Gene Ther. 2004;15(12):1255–1269. doi:10.1089/hum.2004.15.1255
121. Garbuzenko OB, Kbah N, Kuzmov A, Pogrebnyak N, Pozharov V, Minko T. Inhalation treatment of cystic fibrosis with lumacaftor and ivacaftor co-delivered by nanostructured lipid carriers. J Control Release. 2019;296:225–231.
122. Holgate ST, Wenzel S, Postma DS, Weiss ST, Renz H, Sly PD. Asthma. Nat Rev Dis Primers. 2015;1(1):15025.
123. Broza YY, Haick H. Nanomaterial-based sensors for detection of disease by volatile organic compounds. Nanomed. 2013;8(5):785–806. doi:10.2217/nnm.13.64
124. Taylor SL, Leong LEX, Choo JM, et al. Inflammatory phenotypes in patients with severe asthma are associated with distinct airway microbiology. J Allergy Clin Immunol. 2018;141(1):94–103.e115. doi:10.1016/j.jaci.2017.03.044
125. Plaza V, Crespo A, Giner J, et al. Inflammatory asthma phenotype discrimination using an electronic nose breath analyzer. J Investig Allergol Clin Immunol. 2015;25(6):431–437.
126. Craparo EF, Drago SE, Quaglia F, Ungaro F, Cavallaro G. Development of a novel rapamycin loaded nano- into micro-formulation for treatment of lung inflammation. Drug Delivery Transl Res. 2022;12(8):1859–1872. doi:10.1007/s13346-021-01102-5
127. Xu J, Xiao N, Zhou D, Xie L. Disease tolerance: a protective mechanism of lung infections. Front Cell Infect Microbiol. 2023;13:1037850. doi:10.3389/fcimb.2023.1037850
128. Pettigrew MM, Tanner W, Harris AD. The lung microbiome and pneumonia. J Infect Dis. 2021;223(12 Suppl 2):S241–s245. doi:10.1093/infdis/jiaa702
129. Zhang H, Zhang Y, Wu J, et al. Risks and features of secondary infections in severe and critical ill COVID-19 patients. Emerg Microbes Infect. 2020;9(1):1958–1964.
130. Vij N, Chandramani-Shivalingappa P, Van Westphal C, Hole R, Bodas M. Cigarette smoke-induced autophagy impairment accelerates lung aging, COPD-emphysema exacerbations and pathogenesis. Am J Physiol Cell Physiol. 2018;314(1):C73–C87. doi:10.1152/ajpcell.00110.2016
131. Costabile G, Mitidieri E, Visaggio D, et al. Boosting lung accumulation of gallium with inhalable nano-embedded microparticles for the treatment of bacterial pneumonia. Int J Pharm. 2022;629:122400. doi:10.1016/j.ijpharm.2022.122400
132. Costa-Gouveia J, Pancani E, Jouny S, et al. Combination therapy for tuberculosis treatment: pulmonary administration of ethionamide and booster co-loaded nanoparticles. Sci Rep. 2017;7(1):5390. doi:10.1038/s41598-017-05453-3
133. Huck BC, Thiyagarajan D, Bali A, et al. Nano-in-microparticles for aerosol delivery of antibiotic-loaded, fucose-derivatized, and macrophage-targeted liposomes to combat mycobacterial infections: in vitro deposition, pulmonary barrier interactions, and targeted delivery. Adv. Healthcare Mater. 2022;11(11):e2102117. doi:10.1002/adhm.202102117
134. Tsoras AN, Champion JA. Protein and Peptide Biomaterials for Engineered Subunit Vaccines and Immunotherapeutic Applications. Annu Rev Chem Biomol. 2019;10:337–359. doi:10.1146/annurev-chembioeng-060718-030347
135. Loo CY, Lee WH, Zhou QT. Recent advances in inhaled nanoformulations of vaccines and therapeutics targeting respiratory viral infections. Pharm Res. 2023;40(5):1015–1036.
136. Zhong W, Zhang X, Zeng Y, Lin D, Wu J. Recent applications and strategies in nanotechnology for lung diseases. Nano Res. 2021;14(7):2067–2089.
137. Liu H, Moynihan KD, Zheng Y, et al. Structure-based programming of lymph-node targeting in molecular vaccines. Nature. 2014;507(7493):519–522. doi:10.1038/nature12978
138. Pati R, Shevtsov M, Sonawane A. Nanoparticle vaccines against infectious diseases. Front Immunol. 2018;9:2224.
139. Al-Halifa S, Gauthier L, Arpin D, Bourgault S, Archambault D. Nanoparticle-based vaccines against respiratory viruses. Front Immunol. 2019;10:22. doi:10.3389/fimmu.2019.00022
140. Sahin U, Türeci Ö. Personalized vaccines for cancer immunotherapy. Science. 2018;359(6382):1355–1360. doi:10.1126/science.aar7112
141. Vermaelen K. Vaccine strategies to improve anti-cancer cellular immune responses. Front Immunol. 2019;10:8. doi:10.3389/fimmu.2019.00008
142. Freeman-Keller M, Goldman J, Gray J. Vaccine immunotherapy in lung cancer: clinical experience and future directions. Pharmacol Ther. 2015;153:1–9. doi:10.1016/j.pharmthera.2015.05.004
143. Maarof NNN, Abdulmalek E, Fakurazi S, Rahman MBA. Biodegradable carbonate apatite nanoparticle as a delivery system to promote afatinib delivery for non-small cell lung cancer treatment. Pharmaceutics. 2022;14(6):1230. doi:10.3390/pharmaceutics14061230
144. Saleh T, Shojaosadati SA. Multifunctional nanoparticles for cancer immunotherapy. Hum Vaccin Immunother. 2016;12(7):1863–1875. doi:10.1080/21645515.2016.1147635
145. Knight FC, Gilchuk P, Kumar A, et al. Mucosal Immunization with a pH-responsive nanoparticle vaccine induces protective CD8(+) lung-resident memory T cells. ACS Nano. 2019;13(10):10939–10960. doi:10.1021/acsnano.9b00326
146. Ding B, Zheng P, Jiang F, et al. MnO(x) nanospikes as nanoadjuvants and immunogenic cell death drugs with enhanced antitumor immunity and antimetastatic effect. Angewan Chem. 2020;59(38):16381–16384. doi:10.1002/anie.202005111
147. Zhang L, Huang J, Chen X, et al. Self-assembly nanovaccine containing TLR7/8 agonist and STAT3 inhibitor enhances tumor immunotherapy by augmenting tumor-specific immune response. Journal for Immunotherapy of Cancer. 2021;9(8):e003132. doi:10.1136/jitc-2021-003132
148. An M, Liu H. Dissolving microneedle arrays for transdermal delivery of amphiphilic vaccines. Small. 2017;13(26). doi:10.1002/smll.201700164
149. Wang S, Gao J, Wang Z. Outer membrane vesicles for vaccination and targeted drug delivery. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2019;11(2):e1523. doi:10.1002/wnan.1523
150. Shin MD, Shukla S, Chung YH, et al. COVID-19 vaccine development and a potential nanomaterial path forward. Nature Nanotechnol. 2020;15(8):646–655. doi:10.1038/s41565-020-0737-y
151. Hussain A, Yang H, Zhang M, et al. mRNA vaccines for COVID-19 and diverse diseases. J Control Release. 2022;345:314–333.
152. Tenchov R, Bird R, Curtze AE, Zhou Q. Lipid nanoparticles─from liposomes to mRNA vaccine delivery, a landscape of research diversity and advancement. ACS Nano. 2021;15(11):16982–17015. doi:10.1021/acsnano.1c04996
153. Yin Y, Su W, Zhang J, et al. Separable microneedle patch to protect and deliver DNA nanovaccines against COVID-19. ACS Nano. 2021;15(9):14347–14359. doi:10.1021/acsnano.1c03252
154. Pardi N, Hogan MJ, Porter FW, Weissman D. mRNA vaccines - a new era in vaccinology. Nat Rev Drug Discov. 2018;17(4):261–279. doi:10.1038/nrd.2017.243
155. Smith TRF, Patel A, Ramos S, et al. Immunogenicity of a DNA vaccine candidate for COVID-19. Nat Commun. 2020;11(1):2601. doi:10.1038/s41467-020-16505-0
156. Taina-González L, de la Fuente M. The potential of nanomedicine to unlock the limitless applications of mRNA. Pharmaceutics. 2022;14(2):460. doi:10.3390/pharmaceutics14020460
157. Siva S, Hardcastle N, Kron T, et al. Ventilation/perfusion positron emission tomography--based assessment of radiation injury to lung. Int J Radiat Oncol Biol Phys. 2015;93(2):408–417. doi:10.1016/j.ijrobp.2015.06.005
158. Wu L, Wen X, Wang X, et al. Local intratracheal delivery of perfluorocarbon nanoparticles to lung cancer demonstrated with magnetic resonance multimodal imaging. Theranostics. 2018;8(2):563–574. doi:10.7150/thno.21466
159. Chow EK, Ho D. Cancer nanomedicine: from drug delivery to imaging. Sci Transl Med. 2013;5(216):216rv214. doi:10.1126/scitranslmed.3005872
160. Aghebati-Maleki A, Dolati S, Ahmadi M, et al. Nanoparticles and cancer therapy: perspectives for application of nanoparticles in the treatment of cancers. J Cell Physiol. 2020;235(3):1962–1972. doi:10.1002/jcp.29126
161. Han Z, Tu X, Qiao L, et al. Phototherapy and multimodal imaging of cancers based on perfluorocarbon nanomaterials. J Mater Chem B. 2021;9(34):6751–6769. doi:10.1039/D1TB00554E
162. Xu X, Zhang R, Liu F, et al. (19)F MRI in orthotopic cancer model via intratracheal administration of α(ν)β(3)-targeted perfluorocarbon nanoparticles. Nanomed. 2018;13(20):2551–2562. doi:10.2217/nnm-2018-0051
163. Al Faraj A, Shaik AS, Afzal S, Al Sayed B, Halwani R. MR imaging and targeting of a specific alveolar macrophage subpopulation in LPS-induced COPD animal model using antibody-conjugated magnetic nanoparticles. Int J Nanomed. 2014;9:1491–1503. doi:10.2147/IJN.S59394
164. Tay ZW, Chandrasekharan P, Zhou XY, Yu E, Zheng B, Conolly S. In vivo tracking and quantification of inhaled aerosol using magnetic particle imaging towards inhaled therapeutic monitoring. Theranostics. 2018;8(13):3676–3687. doi:10.7150/thno.26608
165. Schmieder AH, Caruthers SD, Keupp J, Wickline SA, Lanza GM. Recent Advances in (19) fluorine magnetic resonance imaging with perfluorocarbon emulsions. Engineering (Beijing). 2015;1(4):475–489. doi:10.15302/J-ENG-2015103
166. Himmelreich U, Weber R, Ramos-Cabrer P, et al. Improved stem cell MR detectability in animal models by modification of the inhalation gas. Mol Imaging. 2005;4(2):104–109. doi:10.1162/15353500200504196
167. Rebuzzi SE, Zullo L, Rossi G, et al. Novel emerging molecular targets in non-small cell lung cancer. Int J Mol Sci. 2021;22(5). doi:10.3390/ijms22052625
168. Wu L, Liu F, Liu S, Xu X, Liu Z, Sun X. Perfluorocarbons-Based (19)F magnetic resonance imaging in biomedicine. Int J Nanomed. 2020;15:7377–7395. doi:10.2147/IJN.S255084
169. Mizuno T, Mohri K, Nasu S, Danjo K, Okamoto H. Dual imaging of pulmonary delivery and gene expression of dry powder inhalant by fluorescence and bioluminescence. J Control Release. 2009;134(2):149–154. doi:10.1016/j.jconrel.2008.11.018
170. R A, Han Z, Wang T, Zhu M, Zhou M, Sun X. Pulmonary delivery of nano-particles for lung cancer diagnosis and therapy: recent advances and future prospects. Wiley Interdiscip Rev Nanomed Nanobiotechnol. 2023;e1933. doi:10.1002/wnan.1933
171. Xu Y, Xiang J, Zhao H, et al. Human amniotic fluid stem cells labeled with up-conversion nanoparticles for imaging-monitored repairing of acute lung injury. Biomaterials. 2016;100:91–100. doi:10.1016/j.biomaterials.2016.05.034
172. Xie X, Zhan C, Wang J, Zeng F, Wu S. An activatable nano-prodrug for treating tyrosine-kinase-inhibitor-resistant non-small cell lung cancer and for optoacoustic and fluorescent imaging. Small. 2020;16(38):e2003451. doi:10.1002/smll.202003451
 © 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2024 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
