Back to Journals » Substance Abuse and Rehabilitation » Volume 7
Opioid-induced constipation: rationale for the role of norbuprenorphine in buprenorphine-treated individuals
Authors Webster L , Camilleri M, Finn A
Received 21 November 2015
Accepted for publication 21 March 2016
Published 14 June 2016 Volume 2016:7 Pages 81—86
DOI https://doi.org/10.2147/SAR.S100998
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Professor Li-Tzy Wu
Video abstract presented Lynn R Webster.
Views: 1774
Lynn R Webster,1 Michael Camilleri,2 Andrew Finn3
1PRA Health Sciences, Salt Lake City, UT, 2Mayo Clinic Rochester, MN, 3BioDelivery Sciences, Inc., Raleigh, NC, USA
Abstract: Buprenorphine and buprenorphine–naloxone fixed combinations are effective for managing patients with opioid dependence, but constipation is one of the most common side effects. Evidence indicates that the rate of constipation is lower when patients are switched from sublingual buprenorphine–naloxone tablets or films to a bilayered bioerodible mucoadhesive buccal film formulation, and while the bilayered buccal film promotes unidirectional drug flow across the buccal mucosa, the mechanism for the reduced constipation is unclear. Pharmacokinetic simulations indicate that chronic dosing of sublingually administered buprenorphine may expose patients to higher concentrations of norbuprenorphine than buprenorphine, while chronic dosing of the buccal formulation results in higher buprenorphine concentrations than norbuprenorphine. Because norbuprenorphine is a potent full agonist at mu-opioid receptors, the differences in norbuprenorphine exposure may explain the observed differences in treatment-emergent constipation between the sublingual formulation and the buccal film formulation of buprenorphine–naloxone. To facilitate the understanding and management of opioid-dependent patients at risk of developing opioid-induced constipation, the clinical profiles of these formulations of buprenorphine and buprenorphine-naloxone are summarized, and the incidence of treatment-emergent constipation in clinical trials is reviewed. These data are used to propose a potential role for exposure to norbuprenorphine, an active metabolite of buprenorphine, in the pathophysiology of opioid-induced constipation.
Keywords: opioid, safety, buccal, sublingual, dependence, maintenance
Introduction
Maintenance treatment of opioid-dependent patients typically involves a combination of psychosocial approaches (eg, counseling, prevention education, and recovery support services) and office-based pharmacological substitution therapy with an oral transmucosal agent. Options include buprenorphine or fixed combinations of buprenorphine and naloxone (BN) that are supplied in three formulations: sublingual tablets or single-layered sublingual films for sublingual or buccal use (sublingual buprenorphine–naloxone [SLBN], Suboxone,® Indivior Inc., Richmond, VA, USA) and bilayered bioerodible mucoadhesive buccal films (buccal buprenorphine–naloxone [BBN], Bunavail,® BioDelivery Sciences International, Inc., Raleigh, NC, USA).1,2 These BN agents have been shown to improve outcomes in opioid-dependent patients,3–5 and while they are generally safe and well tolerated, with predictable side-effect profiles, as with all opioids, constipation is among the most common side effects.5
The mechanisms of opioid-induced constipation (OIC) are complex, involving mu-opioid-mediated effects on the enteric nervous system that result in decreased intestinal fluid secretion and increased fluid absorption, as well as decreased muscle contraction and motility of the small intestine and colon, resulting in increased colonic transit time.6–8 Endogenous opioids, including endorphins, enkephalins, and dynorphins, have been shown to reduce acetylcholine-mediated intestinal motor and secretory activities.9–11 Experimental data also indicate that mu-opioid receptors in the brain may also significantly delay intestinal transit.12,13 Local effects on mu-opioid receptors in the intestine may also impact intestinal functions. Thus, the intraluminal administration of opioid receptor antagonists (eg, naloxone, N-methylnaloxone), which are undetectable in the general circulation due to insufficient absorption from the intestinal lumen, may prevent intravascular morphine from depressing motility.14 These effects, together with the increased resting anal sphincter tone and decreased reflex relaxation of the anal sphincter produced by exogenous opioids,15 result in symptoms of OIC.
The significant clinical consequence of the development of OIC is such that patients may reduce or stop their opioid medication to achieve a positive impact on their quality of life,3,11,16,17 and OIC is one of the most common reasons patients avoid or abandon therapeutic opioid use.18,19 Although clinical experience suggests that most opioid-dependent patients experience mild or moderate symptoms that can be managed with over-the-counter laxatives, the potentially serious impact on quality of life, secondary symptoms, and complications of unmanaged constipation underscore its clinical importance (Table 1).17,20,21
  | Table 1 Secondary symptoms and complications of unmanaged constipation |
Methods
To facilitate the understanding of OIC and management of opioid-dependent patients at risk of developing OIC with the goal of preventing the problem, this paper briefly summarizes the clinical profiles of SLBN and BBN and reviews published evidence of the incidence of treatment-emergent constipation associated with these therapies. Separate searches were performed on PubMed for “sublingual buprenorphine naloxone” and “buccal buprenorphine naloxone”, with the filters set to include only clinical trials. Of the records related to SLBN (N=36) or BBN (N=3), only one clinical study with each formulation reported constipation rates.
Buprenorphine
Initially developed for the treatment of pain, buprenorphine is a semisynthetic partial agonist at mu-opioid receptor and an antagonist at kappa opioid receptor sites.22,23 Buprenorphine is widely used to treat opioid-dependent patients because the reward effects are milder than those of full mu-opioid agonists24, its binding to mu-opioid receptors is not easily displaced by other opioids, and it has a lower risk of abuse and dose-limited effects on respiratory depression.25,26 Oral dosing of buprenorphine is not feasible due to extensive first-pass liver metabolism, which markedly limits its bioavailability. In contrast, oral transmucosal administration is associated with bioavailability up to 50%.27
Buprenorphine has been demonstrated to be safe and effective for use in induction, stabilization, and long-term maintenance of opioid-dependent patients, as measured by reduced consumption of illicit opioids.28 A recent Cochrane review found it to be an effective medication in the maintenance treatment of heroin dependence, retaining people in treatment at any sublingual dose >2 mg and suppressing illicit opioid use when administered at sublingual doses ≥16 mg.28 Compared with methadone, buprenorphine substitution treatment has been shown to decrease hospital admissions, morbidity, and mortality,3,4 with potentially less sedation.29 It has a lower abuse potential, carries less stigma, and allows for greater flexibility in treatment than methadone.30 In the gastrointestinal (GI) tract, buprenorphine inhibits acetylcholine-induced ileal muscle contraction, and subcutaneous injections in mice at doses ranging from 1.0 to 20.0 mg/kg can slow GI transit by <50%.31 About 8% of subjects receiving buprenorphine reported an adverse event of constipation after 4 weeks of sublingual treatment with the medication, significantly more than placebo (~3%, P=0.03, Table 2).5
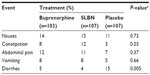  | Table 2 Gastrointestinal adverse events (%) after 4 weeks of treatment with the sublingual tablet formulations of buprenorphine–naloxone (16/4 mg) or buprenorphine (16 mg) |
Buprenorphine combination products
Sublingual buprenorphine–naloxone tablets
SLBN has been approved for the office-based management of patients with opioid dependence since 2002.32 Its efficacy and safety in opioid-dependent patients have been documented in published studies.5,26,30,33 In a controlled clinical trial, it has significantly outperformed placebo for proportion of urine samples that were negative for opiates and the proportion of subjects with opiate craving (P<0.001 for both comparisons).5 Despite an overall rate of adverse events that was roughly similar to placebo, as shown in Table 2, the incidence of constipation in SLBN-treated subjects was four times greater than placebo-treated subjects (P=0.03).5
Buccal buprenorphine–naloxone films
BBN is a novel transmucosal buprenorphine–naloxone combination product formulated as a small, thin, bilayered dissolvable film that adheres to the buccal mucosa and provides approximately twice the bioavailability of buprenorphine compared with SLBN tablets containing double the buprenorphine dose.2,34 Mechanistically, BBN can be distinguished from buccally administered SLBN by the presence of a backing layer, which promotes unidirectional flow across the buccal mucosa; SLBN for buccal administration is a single-layer film without a backing layer.1,2 In an open-label study in 249 adult opioid-dependent subjects stabilized on SLBN tablets or film at a dose of 16 mg/day and switched to a mean dose of 8 mg BBN for 12 weeks, 92% of subjects had urine samples that were negative for nonprescribed opioids. As shown in Figure 1, of the 186 subjects who completed a checklist of common side effects (asking for presence or absence) of BN at baseline and day 84, 41% (76/186) reported constipation at the time of SLBN discontinuation, and before treatment with BBN, and 13% (24/186) reported constipation after 12 weeks of BBN treatment, a risk reduction of 68% (52/76; 95% confidence intervals 60%–77%).34 Approximately 3% (7/249) of subjects on BBN reported treatment-emergent constipation over the course of the 12-week study.34
  | Figure 1 Constipation* at baseline and at week 12 in patients converted from SLBN to BBN (N=186). |
The 2:1 buprenorphine dose conversion ratio from the mean baseline SLBN dose to the mean BBN dose at the end of the study aligns with results from a bioequivalence study in healthy volunteers. In that single-dose, crossover pharmacokinetic study, which compared the rate and extent of BN exposure from 4.2/0.7 mg BBN with 8/2 mg SLBN tablets in 80 healthy adults who had been given naltrexone, buprenorphine exposure from BBN was bioequivalent with SLBN (90% confidence intervals for maximum plasma concentration [Cmax], area under the plasma concentration-time curve from time 0 to the last measurable concentration [AUClast], and AUC extrapolated to infinity [AUCinf] ranged from 88% to 118%), while exposure to naloxone and norbuprenorphine were, respectively, 33% and 40% less than with SLBN (Table 3).35
  | Table 3 Systemic exposure of 4.2/0.7 mg BBN film and 8/2 mg SLBN tablet (N=80) |
Based on the single-dose data from the study comparing SLBN (8 mg) with BBN (4.2 mg) in healthy subjects, plasma buprenorphine and norbuprenorphine concentration exposures (AUC0–24) were modeled for steady-state conditions during daily dosing with SLBN 16 mg or BBN 8 mg. As shown in Table 4, projected norbuprenorphine exposure with SLBN was nearly double that of BBN, while the projected AUC0–24 exposures for buprenorphine were roughly equivalent with both formulations. Importantly, the projected steady-state plasma norbuprenorphine concentrations with SLBN exceeded the steady-state buprenorphine concentrations by ~30%, whereas projected plasma norbuprenorphine concentrations with BBN were nearly 30% lower than buprenorphine. The difference is presumed to be due to higher transmucosal bioavailability and lower amounts of buprenorphine exposed to first-pass liver metabolism.
Potential role of norbuprenorphine in constipation
It is unclear why the rates of constipation decreased in subjects switched from SLBN to BBN. While subcutaneous naloxone (0.8 mg every 6 hours) has been found to accelerate transit in the colon of healthy human volunteers,36 and evidence suggests it can reverse idiopathic chronic constipation, benefit patients with intestinal pseudo-obstruction and constipation-predominant irritable bowel syndrome,37 and improve the symptoms of OIC38,39 when given at high doses orally (eg, 10 mg bid in the constipation-predominant irritable bowel syndrome trial), it seems unlikely to have played a role in the different outcomes with respect to constipation in the study of opioid-dependent subjects stabilized on SLBN and switched to BBN. Not only do both study drugs contain naloxone, but also the rate and extent of naloxone exposure with BBN was one-third lower than with SLBN. Moreover, another orally administered, poorly bioavailable mu-opioid antagonist, naltrexone (50 mg) or subcutaneous methylnaltrexone (0.3 mg/kg), did not accelerate colonic transit in healthy volunteers.40,41 All of these data argue against a role for the naloxone in BBN as the reason for improved bowel function.
Rather, the steady-state plasma concentration projections observed in this study suggest that the lower incidence of constipation with BBN relative to SLBN may be due to reduced exposure of enteric mu-receptors to norbuprenorphine. In randomized studies in opioid-dependent subjects, mean steady-state plasma concentrations of this major peripherally active metabolite of buprenorphine have matched or surpassed the parent compound following sublingual administration.42,43 These data are therefore consistent with the hypothesis that, as a potent full agonist at mu-receptors,44 norbuprenorphine contributes to the overall peripheral pharmacological effects of buprenorphine in the regulation of multiple GI processes in vivo. Its lack of penetration of the blood–brain barrier into the central nervous system suggests little central nervous system contribution of norbuprenorphine to the GI processes.45
Implications for clinical practice
These data indicate that the lower rate of constipation observed with the BBN formulation may be the result of reduced exposure to norbuprenorphine. It therefore appears reasonable to query opioid-dependent patients who are at risk of or report OIC symptoms and possible effects on quality of life in order to enhance adherence to medication regimens. In addition, since there is no significant difference between SLBN tablets and single-layered films with regard to plasma levels of naloxone and norbuprenorphine,46 and treatment-emergent constipation rates with SLBN films across 19 pharmacokinetic studies in healthy volunteers (11%)46 are similar to the clinical study with SLBN tablets (12%),5 if management of OIC is needed for patients who are being treated with SLBN, strategies to minimize exposure to norbuprenorphine may be considered. Evidence suggests that BBN provides adequate symptom control with respect to opioid dependence and is well tolerated, with low reported rates of constipation, good adherence, and favorable patient acceptance.34
Conclusion
Buprenorphine is an effective intervention for the management of opioid dependence, but patients are at risk for a range of GI symptoms, including OIC. Pharmacokinetic simulations indicate that chronic dosing of sublingually administered agents may expose patients to higher concentrations of norbuprenorphine than buprenorphine due to swallowing of unabsorbed buprenorphine, whereas chronic dosing of the bilayered bioerodible mucoadhesive buccal formulation, which provides higher bioavailability and is efficiently absorbed across the buccal mucosa, results in higher buprenorphine concentrations than norbuprenorphine. These data support the hypothesis that exposure to norbuprenorphine, an active metabolite of buprenorphine, plays a role in the pathophysiology of OIC and that differences in norbuprenorphine exposure may explain the observed differences in constipation between SLBN and BBN.
Acknowledgments
The authors wish to thank Sarah DeRossett and Susan Kerls for their assistance in preparing the manuscript. Medical writing services were provided by Christopher Caiazza.
Disclosure
MC is supported by NIH RO1 DK92179. BioDelivery Sciences, Inc., developer of bioerodible mucoadhesive buprenorphine–naloxone buccal film, has provided research support to LRW and MC. AF is a retired employee of BioDelivery Sciences, Inc. The authors report no other conflicts of interests in this work.
References
Suboxone [prescribing information]. Available from: http://www.suboxone.com/content/pdfs/SuboxonePI.pdf. Accessed November 17, 2015. | |
Bunavail [prescribing information]. Available from: http://www.bdsi.com/siteres.aspx?resid=5a738443-a797-41cd-a39a-a8deb2a4a585. Accessed November 17, 2015. | |
Bell J, Trinh L, Butler B, Randall D, Rubin G. Comparing retention in treatment and mortality in people after initial entry to methadone and buprenorphine treatment. Addiction. 2009;104:1193–1200. | |
Jones ES, Moore BA, Sindelar JL, O’Connor PG, Schottenfeld RS, Fiellin DA. Cost analysis of clinic and office-based treatment of opioid dependence:results with methadone and buprenorphine in clinically stable patients. Drug Alcohol Depend. 2009;99:132–140. | |
Fudala PJ, Bridge TP, Herbert S, et al; Buprenorphine/Naloxone Collaborative Study Group. Office-based treatment of opiate addiction with a sublingual tablet formulation of buprenorphine and naloxone. N Engl J Med. 2003;349:949–958. | |
Bagnol D, Mansour A, Akil H, Watson SJ. Cellular localization and distribution of the cloned mu and kappa opioid receptors in rat gastrointestinal tract. Neuroscience. 1997;81:579–591. | |
McKay JS, Linaker BD, Turnberg LA. Influence of opiates on ion transport across rabbit ileal mucosa. Gastroenterology. 1981;80:279–284. | |
Fickel J, Bagnol D, Watson SJ, Akil H. Opioid receptor expression in the rat gastrointestinal tract:a quantitative study with comparison to the brain. Brain Res Mol Brain Res. 1997;46:1–8. | |
Rang HP, Dale MM, Ritter JM. Analgesic drugs. Pharmacology. 1999; 13:579–603. | |
Thomas J. Opioid-induced bowel dysfunction. J Pain Symptom Manage. 2008;35:103–113. | |
Yuan CS, Foss JF, O’Connor M, Moss J, Roizen MF. Gut motility and transit changes in patients receiving long-term methadone maintenance. J Clin Pharmacol. 1998;38:931–935. | |
Thörn SE, Wattwil M, Lindberg G, Säwe J. Systemic and central effects of morphine on gastroduodenal motility. Acta Anaesthesiol Scand. 1996;40:177–186. | |
Manara L, Bianchetti A. The central and peripheral influences of opioids on gastrointestinal propulsion. Annu Rev Pharmacol Toxicol. 1985;25:249–273. | |
Reber P, Brenneisen R, Flogerzi B, Batista C, Netzer P, Scheurer U. Effect of naloxone-3-glucuronide and N-methylnaloxone on the motility of the isolated rat colon after morphine. Dig Dis Sci. 2007;52:502–507. | |
Holzer P. Opioids and opioid receptors in the enteric nervous system:from a problem in opioid analgesia to a possible new prokinetic therapy in humans. Neurosci Lett. 2004;361:192–195. | |
Mancini I, Bruera E. Constipation in advanced cancer patients. Support Care Cancer. 1998;6:356–364. | |
Benyamin R, Trescot AM, Datta S, et al. Opioid complications and side effects. Pain Physician. 2008;11(2 Suppl):S105–S120. | |
Meissner W, Schmidt U, Hartmann M, Kath R, Reinhart K. Oral naloxone reverses opioid-associated constipation. Pain. 2000;84:105–109. | |
Thorpe DM. Management of opioid-induced constipation. Curr Pain Headache Rep. 2001;5:237–240. | |
Kurz A, Sessler DI. Opioid-induced bowel dysfunction:pathophysiology and potential new therapies. Drugs. 2003;63:649–671. | |
Holzer P. Treatment of opioid-induced gut dysfunction. Expert Opin Investig Drugs. 2007;16:181–194. | |
Mello NK, Mendelson J. Behavioral pharmacology of buprenorphine. Drug Alcohol Depend. 1985;14:283–303. | |
Pergolizzi J, Aloisi AM, Dahan A, et al. Current knowledge of buprenorphine and its unique pharmacological profile. Pain Pract. 2010;10:428–450. | |
Lutfy K, Cowan A. Buprenorphine:a unique drug with complex pharmacology. Curr Neuropharmacol. 2004;2:395–402. | |
Mello NK, Walsh S, Preston K, Stitzer M, Cone E, Bigelow G. Clinical pharmacology of buprenorphine:ceiling effects at high doses. Clin Pharmacol Ther. 1994;55:569–580. | |
Strain EC, Preston K, Liebson I, Bigelow G. Buprenorphine effects in methadone-maintained volunteers:effect at two hours after methadone. J Pharmacol Exp Ther. 1995;272:628–638. | |
Yokell MA, Zaller ND, Green TC, Rich JD. Buprenorphine and buprenorphine/naloxone diversion, misuse, and illicit use:an international review. Curr Drug Abuse Rev. 2011;4:28–41. | |
Johnson RE, Jaffe JH, Fudala PJ. A controlled trial of buprenorphine treatment for opioid dependence. JAMA. 1992;267:2750–2755. | |
Mattick RP, Breen C, Kimber J, Davoli M. Buprenorphine maintenance versus placebo or methadone maintenance for opioid dependence. Cochrane Database Syst Rev. 2014;2:CD002207. | |
Mauger S, Fraser R, Gill K. Utilizing buprenorphine-naloxone to treat illicit and prescription-opioid dependence. Neuropsychiatr Dis Treat. 2014;10:587–598. | |
Zhou PL, Li YL, Yan LD, et al. Effect of thienorphine on intestinal transit and isolated guinea-pig ileum contraction. World J Gastroenterol. 2013;19:1444–1450. | |
U.S. Food and Drug Administration. Suboxone Approval History. Available from: http://www.accessdata.fda.gov/drugsatfda_docs/appletter/2002/20732,20733ltr.pdf. | |
Finch JW, Kamien JB, Amass L. Two-year experience with buprenorphine-naloxone (Suboxone) for maintenance treatment of opioid dependence within a private practice setting. J Addict Med. 2007;1:104–110. | |
Sullivan JG, Webster L. Novel buccal film formulation of buprenorphine-naloxone for the maintenance treatment of opioid dependence:a 12-week conversion study. Clin Ther. 2015;37:1064–1075. | |
Vasisht N, Stark J, Bai SA, Finn A. Buprenorphine/naloxone buccal film has a relative buprenorphine bioavailability approximately twice that of buprenorphine/naloxone sublingual tablet. In:2014 ASAM 45th Annual Medical-Scientific Conference, Poster #10, April 10–13, 2014; Orlando, FL. | |
Kaufman PN, Krevsky B, Malmud LS, et al. Role of opiate receptors in the regulation of colonic transit. Gastroenterology. 1988;94:1351–1356. | |
DeHaven-Hudkins DL, DeHaven RN, Little PJ, Techner LM. The involvement of the μ-opioid receptor in gastrointestinal pathophysiology:therapeutic opportunities for antagonism at this receptor. Pharmacol Ther. 2008;117:162–187. | |
DePriest AZ, Miller K. Oxycodone/naloxone:role in chronic pain management, opioid-induced constipation, and abuse deterrence. Pain Ther. 2014;3:1–15. | |
Smith K, Hopp M, Mundin G, et al. Naloxone as part of a prolonged release oxycodone/naloxone combination reduces oxycodone-induced slowing of gastrointestinal transit in healthy volunteers. Expert Opin Investig Drugs. 2011;20:427–439. | |
Foxx-Orenstein AE, Camilleri M, Szarka LA, et al. Does co-administration of a non-selective opiate antagonist enhance acceleration of transit by a 5-HT4 agonist in constipation-predominant irritable bowel syndrome? A randomized controlled trial. Neurogastroenterol Motil. 2007;19:821–830. | |
Wong BS, Rao AS, Camilleri M, et al. The effects of methylnaltrexone alone and in combination with acutely administered codeine on gastrointestinal and colonic transit in health. Aliment Pharmacol Ther. 2010;32:884–893. | |
Kuhlman JJ Jr, Levine B, Johnson RE, Fudala PJ, Cone EJ. Relationship of plasma buprenorphine and norbuprenorphine to withdrawal symptoms during dose induction, maintenance and withdrawal from sublingual buprenorphine. Addiction. 1998;93:549–559. | |
Brown SM, Holtzman M, Kim T, Kharasch ED. Buprenorphine metabolites, buprenorphine-3-glucuronide and norbuprenorphine-3-glucuronide, are biologically active. Anesthesiology. 2011;115:1251–1260. | |
Yassen A, Kan J, Olofsen E, Suidgeest E, Dahan A, Danhof M. Pharmacokinetic-pharmacodynamic modeling of the respiratory depressant effect of norbuprenorphine in rats. J Pharmacol Exp Ther. 2007;321:598–607. | |
Huang P, Kehner GB, Cowan A, Liu-Chen LY. Comparison of pharmacological activities of buprenorphine and norbuprenorphine:norbuprenorphine is a potent opioid agonist. J Pharmacol Exp Ther. 2001;297:688–695. | |
Commonwealth of Australia. Australian Public Assessment Report for Buprenorphine/Naloxone. Available from: https://www.tga.gov.au/auspar/auspar-buprenorphine-naloxone. Accessed March 9, 2016. |
 © 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2016 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.

