Back to Journals » Risk Management and Healthcare Policy » Volume 10
Opinions, practice patterns, and perceived barriers to lung cancer screening among attending and resident primary care physicians
Authors Henderson LM, Jones LM , Marsh MW, Brenner AT, Goldstein AO, Benefield TS, Greenwood-Hickman MA , Molina PL , Rivera MP, Reuland DS
Received 3 June 2017
Accepted for publication 9 November 2017
Published 22 January 2018 Volume 2017:10 Pages 189—195
DOI https://doi.org/10.2147/RMHP.S143152
Checked for plagiarism Yes
Review by Single anonymous peer review
Peer reviewer comments 2
Editor who approved publication: Dr Kent Rondeau
Louise M Henderson,1 Laura M Jones,1 Mary W Marsh,1 Alison T Brenner,2,3 Adam O Goldstein,4 Thad S Benefield,1 Mikael Anne Greenwood-Hickman,5 Paul L Molina,1 M Patricia Rivera,2 Daniel S Reuland2,3
1Department of Radiology, The University of North Carolina, Chapel Hill, NC, 2Department of Medicine, 3The University of North Carolina Lineberger Comprehensive Cancer Center, 4Department of Family Medicine, The University of North Carolina, Chapel Hill, NC, 5Kaiser Permanente Washington Health Research Institute, Seattle, WA, USA
Introduction: The US Preventive Services Task Force recommended annual lung cancer screening with low-dose computed tomography (LDCT) for high-risk patients in December 2013. We compared lung cancer screening-related opinions and practices among attending and resident primary care physicians (PCPs).
Methods: In 2015, we conducted a 23-item survey among physicians at a large academic medical center. We surveyed 100 resident PCPs (30% response rate) and 86 attending PCPs (49% response rate) in Family Medicine and Internal Medicine. The questions focused on physicians’ opinions, knowledge of recommendations, self-reported practice patterns, and barriers to lung cancer screening. In 2015 and 2016, we compared responses among attending versus resident PCPs using chi-square/Fisher’s exact tests and 2-samples t-tests.
Results: Compared with resident PCPs, attending PCPs were older (mean age =47 vs 30 years) and more likely to be male (54% vs 37%). Over half of both groups concurred that inconsistent recommendations make deciding whether or not to screen difficult. A substantial proportion in both groups indicated that they were undecided about the benefit of lung cancer screening for patients (43% attending PCPs and 55% resident PCPs). The majority of attending and resident PCPs agreed that barriers to screening included limited time during patient visits (62% and 78%, respectively), cost to patients (74% and 83%, respectively), potential for complications (53% and 70%, respectively), and a high false-positive rate (67% and 73%, respectively).
Conclusion: There was no evidence to suggest that attending and resident PCPs had differing opinions about lung cancer screening. For population-based implementation of lung cancer screening, physicians and trainees will need resources and time to address the benefits and harms with their patients.
Keywords: lung neoplasms, mass screening, physician behavior, surveys, questionnaires, low dose computed tomography, benefits, harms
Introduction
Lung cancer is the leading cause of cancer deaths in the US, resulting in more deaths than breast, colorectal, cervical, and prostate cancers combined.1 Since the signs and symptoms of lung cancer are nonspecific and do not appear until the disease is advanced, the majority of lung cancers are detected at late stages when treatment is less effective and survival is poor.2 While randomized controlled trials assessing early lung cancer detection through screening with conventional radiography failed to find a reduction in lung cancer mortality, the National Lung Screening Trial (NLST) showed a 20% reduction in lung cancer mortality among current and former heavy smokers who were screened with low-dose computed tomography (LDCT) versus chest radiography.3 Despite concerns over the high false-positive rate reported in the NLST and the fact that NLST results may not be generalizable to the US population as a whole, in December 2013, the US Preventive Services Task Force (USPSTF) began recommending annual lung cancer screening with LDCT in adults aged 55–80 years who are current or former (quit within the past 15 years) smokers and have a 30 pack-year smoking history.4 In 2015, the Centers for Medicaid and Medicare Services (CMS) began covering lung cancer screening with LDCT for those meeting the screening criteria; however, the extent to which screening practices will change with the new USPSTF recommendation and CMS coverage is unknown.5 Approximately 8.7 million people in the US meet the NLST criteria of “high risk” (based on age and smoking history) and would qualify for screening under the USPSTF guidelines.6
Although several groups have endorsed screening,4,7–9 there is concern that the risk-to-benefit ratio observed in the NLST will not translate into real-world settings.10,11 This may explain why uptake of lung cancer screening has been relatively slow. According to the 2015 National Health Interview Survey, only 3.9% of eligible smokers reported LDCT screening in the prior month.12 Understanding the opinions and knowledge of published recommendations for lung cancer screening among resident and attending primary care physicians (PCPs) are likely to influence implementation of lung cancer screening in future years. Prior research has shown that physician knowledge of screening guidelines is positively correlated with increased utilization of screening procedures.13
The purpose of this study was to understand and compare perceptions of lung cancer screening among attending and resident PCPs at one US academic medical center. Specifically, we explored the relationship between practitioner type (attending or resident physician) and screening opinions, ordering and referral patterns, and perceived barriers. We hypothesized that attending and resident PCPs would both report following USPSTF recommendations but that resident PCPs would perceive more barriers to screening than attending PCPs since resident PCPs are more likely to see uninsured patients, those with low literacy, and more patients with major psychosocial problems than attending PCPs.14,15
Methods
Survey methodology
We designed, pre-tested, and implemented an online survey to attending physicians and residents at a single large academic medical center using the Tailored Design Method by Dillman et al.16 The survey content was developed through collaboration with a multidisciplinary Advisory Group comprised of a survey methodologist, an epidemiologist and physicians from Internal Medicine, Family Medicine, Thoracic Radiology, Pulmonary Medicine, and Pathology. A total of 23 survey questions were included with a focus on opinions about lung cancer screening, recommendations and guidelines for lung cancer screening, referral patterns and barriers for lung cancer screening, and respondent socio-demographics. Survey questions were comprised of Likert scale items, multiple choice, and short answer. The survey was administered online, via the Qualtrics software.
We pre-tested the survey among five physicians outside of the academic medical center where the survey took place to obtain feedback on the survey flow, length, design, and ease of understanding and responding to the survey questions. Based on responses from the pre-testing, we made slight modifications to clarify the intent of a few questions.
Through the use of online resources (student service coordinators and department websites), we created a list of 186 potential Internal and Family Medicine survey respondents. Of these, 86 (46.2%) were attending physicians and 100 (53.8%) were residents. We collected participant names, email addresses, and campus box numbers and sent a pre-notification postcard in 2015 to each potential participant to introduce the study and survey. One week later, we emailed a survey link to each participant through Qualtrics. At 1, 5, 8, and 9 weeks post survey delivery, we sent reminder emails to the physicians who had not yet responded. In addition, we mailed a reminder postcard 2 weeks after survey deployment. Participation for the study was determined by return of the survey. Those who participated in the study were given the opportunity to enter into a random drawing for an iPad incentive, as offering an incentive has been shown to increase response rates for physicians.17–19 Responses from returned surveys were stored within the Qualtrics system and exported upon survey closure. This study was reviewed and approved by the University of North Carolina at Chapel Hill Institutional Review Board (Study #13-2672).
Statistical analyses
We describe the characteristics and responses of the survey by attending and resident physicians. We compare survey responses between these groups with a 2-sample t-test using the Satterthwaite approximation for unequal variances for continuous outcomes and chi-square or Fisher’s exact tests for categorical outcomes, depending on the expected cell size. All analyses were performed in 2015 and 2016 using SAS v9.4.
Results
Sociodemographic characteristics of the attending and resident PCPs
A total of 72 attending and resident PCPs responded to the survey for an overall response rate of 38.7%. The response rate was significantly higher for attending PCPs (response rate = 42/86 = 48.8%) than for resident PCPs (response rate = 30/100 =30.0%), p-value =0.01. Attending physicians were older with more years in clinical practice and more patients were seen per week in the clinic compared with resident physicians (Table 1).
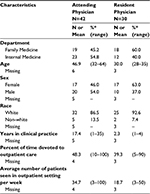  | Table 1 Sociodemographic characteristics of the respondents by provider type Note: *Percentages are among those with non-missing data. |
Physicians were asked their opinions about screening patients for lung cancer (Table 2). When asked whether they were convinced that screening for lung cancer is beneficial for patients, 42.5% of attending and 55.2% of resident physicians were undecided, with a similar proportion agreeing or strongly agreeing with the statement (40% vs 34.5% respectively). In both physician groups, the majority of respondents agreed or strongly agreed that inconsistent recommendations about lung cancer screening make it difficult to decide whether or not to screen (56.1% attending vs 65.5% resident). The majority of attending (60.0%) and resident PCPs (62.1%) agreed or strongly agreed that they had enough knowledge to explain the pros and cons of lung cancer screening to patients. More than half of attending and resident PCPs agreed or strongly agreed (61.6% and 75.9%, respectively) that time restrictions during patient visits result in other problems having higher priority than screening for lung cancer.
  | Table 2 Provider attitudes about lung cancer screening, by provider type Notes: aChi-square test or Fisher’s exact test. *Percentages are among those with non-missing data. |
We asked physicians about the initiation of lung cancer screening discussions, as well as ordering and referral practices during the 12 months prior to the survey to ascertain the extent to which these were occurring (Table 3). While 37.1% of attending physicians reported that patients asked whether they should be screened for lung cancer, only 18.5% of resident physicians reported that patients asked about lung cancer screening. The majority of the attending (66.7%) and resident (57.1%) physicians reported initiating discussion of the risks and benefits of lung cancer screening with patients, with a smaller proportion reporting ordering LDCT screening (46.2% of attending and 34.5% of resident physicians). Approximately 53% of attending and 32% of resident physicians reported discussing results of LDCT lung cancer screening with the patients.
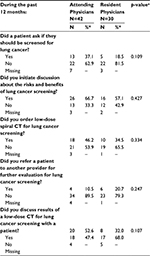  | Table 3 Lung cancer screening discussion, ordering, and referral by provider type Notes: aChi-square test or Fisher’s exact test. *Percentages are among those with non-missing data. |
Physicians were also asked about perceived barriers to recommending lung cancer screening (Table 4). Overall, most attending and resident physicians selected at least one barrier. In both attending and resident groups, cost to the patient was the most cited concern (72.8% vs 83.3%, respectively). Physicians were also concerned with too many false positives, potential for complications, potential for emotional harm, and cost to the health care system. Less than 15% of physicians reported false negatives/missed cancers as a barrier to screening.
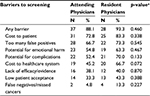  | Table 4 Provider reported barriers to lung cancer screening by provider type Note: aChi-square test or Fisher’s exact test. |
Discussion
We found no evidence to suggest that attending and resident PCPs in one large academic medical center share dissimilar reservations about the benefits, risks, and barriers associated with lung cancer screening with LDCT. Differences between attending and resident PCPs may indeed exist, but were not detectable given our sample size. The survey developed and pilot tested in this study should be conducted in a larger population to allow for more in-depth analyses.
Although resident PCPs typically see patients who are less likely to have private insurance and more likely to have comorbidities than attending physicians,14,15 our survey results indicated no difference in terms of barriers to screening among resident and attending PCPs. Both types of physicians reported being unsure of the benefits of lung cancer screening and cited many concerns about screening. Regardless of resident or attending status, respondents identified inconsistent recommendations, time restrictions during visits, cost to patients, potential complications, and a high false-positive rate as barriers to lung cancer screening. While more than half of physicians initiated discussions about the risks and benefits of lung cancer screening, of these, approximately two-thirds of physicians reported ordering an LDCT scan and 15%–38% reported referring their patient to another provider for further evaluation for screening.
Prior to the USPSTF guidelines being released in December 2013, several studies examined provider practices around lung cancer screening with sputum cytology, chest radiography, or LDCT.13,20–22 These studies found that US PCPs’ beliefs and recommendations regarding lung cancer screening were inconsistent with guidelines20 and that providers frequently ordered screening tests even though they were not recommended by expert groups.21 Another study conducted telephone focus groups with 28 US PCPs and identified factors influencing physicians’ decisions to screen, including perceptions of the test effectiveness, attitudes toward recommended guidelines, practice experience, perceptions of the patient’s lung cancer risk, cost of screening, concerns about litigation, and patient request for screening.22 An additional study was conducted after the results of the NLST were published, during the time when some professional society guidelines endorsed LDCT but prior to the release of updated USPSTF guidelines. This study found that providers ordered radiography for lung cancer screening more often than LDCT (21% vs 12%, respectively).13 Providers in this study also cited that the most common barriers to screening included cost to the patient, false-positive findings, patients’ lack of awareness, incidental findings, and insurance coverage.13 Our finding that over half of respondents agreed that inconsistent recommendations about lung cancer screening make it difficult to decide whether or not to screen is likely reflective of the fact that the American Academy of Family Physicians does not currently endorse lung cancer screening and that 51% of our respondents are from the Family Medicine Department.
Several studies on provider attitudes about lung cancer screening have been conducted since the USPSTF recommendation was published.23–26 Two of these focused on specialist physicians25,26 while the other two focused on PCPs.23,24 Hoffman et al conducted semi-structured interviews with 10 New Mexico PCPs in 2014.19 None of the 10 PCPs had screened with LDCT and viewed the NLST results with skepticism, particularly the high false-positive rate, the high number needed to screen to prevent one lung cancer death, and the low enrollment of minority participants. Potential barriers noted in this study were following up on abnormal tests, concern that New Mexico lacked the infrastructure to support high-quality screening programs as required by guidelines, challenges of screening rural patients in terms of available technology and travel burdens for patients, costs for follow-up testing, overloading a primary care system that is already taxed, and competing demands during the patient visit. In the other study of PCPs, Ersek et al distributed a 32-item questionnaire via email to all active South Carolina Academy of Family Physicians and via paper form to a subset of physicians at a meeting in 2015.23 Among the 101 physicians who responded, the majority reported that screening with LDCT increased the odds of detecting disease at earlier stages and that the benefits outweighed the harms. In the prior 12 months, 47% of PCPs had not referred a patient for LDCT and some PCPs continued to recommend chest radiography for screening. Concerns about screening included unnecessary diagnostic procedures, psychological harms, and exposure to radiation.
The results of our physician survey have some similarities and differences to the Hoffman and Ersek studies. First, the proportion of respondents in our survey who agreed or strongly agreed that lung cancer screening is beneficial for patients was lower than that in the South Carolina group (35%–40% versus 75%). Second, unlike the South Carolina study in which 31% of respondents agreed or strongly agreed that LDCT screening is cost-effective, only 6.9% of residents and 19.5% of attending PCPs in our study perceived lung cancer screening as being cost-effective. Similar to the Hoffman study, cost to patients was the most frequently reported barrier to lung cancer screening among our respondents. While lung cancer screening with LDCT is covered by most of the insurance plans for those patients meeting the high-risk definition, the work-up of pulmonary nodules or incidental findings will likely incur out-of-pocket expenses for the patient. Third, providers in all three studies raised concern about the high false-positive rate. In a retrospective analysis, application of the American College of Radiology Lung-RADS to NLST data suggests that use of Lung-RADS may potentially reduce the false-positive rates,27 which may alleviate some provider concerns about false positives in the future.
Limitations
Since our study was conducted at a single US academic medical center, our results are limited in generalizability to other similar settings. As a safety net system, our institution serves a diverse patient population, and the concerns that physicians raised about screening are likely reflective of the potential burden that would be placed on these patients. By asking physicians about their ordering and referral patterns, we rely on their reporting, and thus, responses are subject to recall bias. In addition, our sample was underpowered to detect small differences between attending and resident physicians. There is also the potential for nonresponse bias given our overall response rate of 38.7%. This nonresponse bias may differentially impact the attending and resident physician groups, as the response rates were significantly different for the two. We are unable to ascertain whether those who did not respond to the survey differed in a systematic way than those who did respond. Where feasible, future studies should address nonresponse bias by collecting information from non-respondents. Where this is impossible, future studies should include auxiliary data on the sampling frame in order to derive nonresponse adjustments.
Conclusion
Findings from our study suggest that attending and resident physicians have similar opinions, ordering and referral patterns, or perceived barriers for lung cancer screening with LDCT. Both attending and resident PCPs reported being undecided on the utility of screening, and while they report having the knowledge to explain the pros and cons of screening, they do not have enough time during the clinical visit to adequately discuss screening. For lung cancer screening to be implemented at the population level, physicians will need resources and time to fully address the benefits and harms of screening with their patients. Furthermore, US attending and resident PCPs may need more evidence on the benefits of lung cancer screening and its impact on patients in real-world settings.
Acknowledgments
The research presented in this paper is that of the authors and does not reflect the official policy of the National Institutes of Health (NIH). This work was supported by a grant from the NIH/ National Cancer Institute (NCI) under R21CA175983.
Article contents have been previously presented as a poster at the February 2016 American Society of Clinical Oncology (ASCO) Quality Care Symposium, entitled “Do Perceived Barriers to Lung Cancer Screening Differ between Attending Physicians and Residents?”
Author contributions
LMH, MWM, TSB, LMJ, DSR, ATB, AOG, MAGH, PLM, and MPR contributed substantially to the study design/implementation, data analysis and interpretation, and the writing of the manuscript.
Disclosure
LMH received funding from the NIH/NCI under R21CA175983. LMJ received funding from the NIH/NCI under R21CA175983. MWM received funding from the NIH/NCI under R21CA175983. ATB received intramural pilot research support from the University of North Carolina (UNC) Lineberger Comprehensive Cancer Center and the North Carolina Translational and Clinical Sciences Institute. TSB received funding from the NIH/NCI under R21CA175983. MAGH received funding from the NIH/NCI under R21CA175983. PLM received funding from the NIH/NCI under R21CA175983. MPR received funding from the NIH/NCI under R21CA175983. DSR received intramural pilot research support from the University of North Carolina (UNC) Lineberger Comprehensive Cancer Center and the North Carolina Translational and Clinical Sciences Institute. AOG reports no conflicts of interest in this work.
References
American Cancer Society. Cancer Facts & Figures 2017. Atlanta, GA: American Cancer Society; 2017. | ||
Howlader N, Noone AM, Krapcho M, et al. SEER Cancer Statistics Review, 1975-2014, National Cancer Institute. Bethesda, MD. Based on November 2016 SEER data submission, posted to the SEER web site, April 2017. Available from: https://seer.cancer.gov/csr/1975_2014/. Accessed October 30, 2017. | ||
Aberle DR, Adams AM, Berg CD, et al. Reduced lung-cancer mortality with low-dose computed tomographic screening. N Engl J Med. 2011;365(5):395–409. | ||
Moyer VA. Screening for lung cancer: U.S. Preventive Services Task Force recommendation statement. Ann Intern Med. 2014;160(5):330–338. | ||
Centers for Medicare & Medicaid Services. Decision memo for screening for lung cancer with low dose computed tomography (LDCT). 2015; Available from: https://www.cms.gov/medicare-coverage-database/details/nca-decision-memo.aspx?NCAId=274. Accessed June 7, 2016. | ||
Doria-Rose VP, White MC, Klabunde CN, et al. Use of lung cancer screening tests in the United States: results from the 2010 National Health Interview Survey. Cancer Epidemiol Biomarkers Prev. 2012;21(7):1049–1059. | ||
Watson KS, Blok AC, Buscemi J, et al. Society of Behavioral Medicine supports implementation of high quality lung cancer screening in high-risk populations. Transl Behav Med. 2016;6(4):669–671. | ||
Smith RA, Andrews K, Brooks D, et al. Cancer screening in the United States, 2016: a review of current American Cancer Society guidelines and current issues in cancer screening. CA Cancer J Clin. 2016;66(2):96–114. | ||
Mazzone P, Powell CA, Arenberg D, et al. Components necessary for high-quality lung cancer screening: American College of Chest Physicians and American Thoracic Society Policy Statement. Chest. 2015;147(2):295–303. | ||
American Academy of Family Physicians. Summary of Recommendations for Clinical Preventive Services. 2017. Available from: www.aafp.org/dam/AAFP/documents/patient_care/clinical_recommendations/cps-recommendations.pdf. Accessed October 30, 2017. | ||
Medicare Evidence Development & Coverage Advisory Committee. Lung cancer screening with low dose computed tomography. April 30, 2014. Available from: https://www.cms.gov/medicare-coverage-database/details/medcac-meeting-details.aspx?MEDCACId=68. Accessed October 30, 2017. | ||
Jemal A, Fedewa SA. Lung cancer screening with low-dose computed tomography in the United States-2010 to 2015. JAMA Oncol. 2017; 3(9):1278–1281. | ||
Lewis JA, Petty WJ, Tooze JA, et al. Low-dose CT lung cancer screening practices and attitudes among primary care providers at an academic medical center. Cancer Epidemiol Biomarkers Prev. 2015;24(4):664–670. | ||
Charlson ME, Karnik J, Wong M, McCulloch CE, Hollenberg JP. Does experience matter? A comparison of the practice of attendings and residents. J Gen Intern Med. 2005;20(6):497–503. | ||
Zallman L, Ma J, Xiao L, Lasser KE. Quality of US primary care delivered by resident and staff physicians. J Gen Inter Med. 2010;25(11):1193–1197. | ||
Dillman DA, Smyth JD, Christian LM. Internet, Mail and Mixed-Mode Surveys: The Tailored Design Method. 3rd ed. Hoboken, NJ: Wiley; 2008. | ||
VanGeest JB, Johnson TP, Welch VL. Methodologies for improving response rates in surveys of physicians: a systematic review. Eval Health Prof. 2007;30(4):303–321. | ||
Klabunde CN, Willis GB, McLeod CC, et al. Improving the quality of surveys of physicians and medical groups: a research agenda. Eval Health Prof. 2012;35(4):477–506. | ||
Thorpe C, Ryan B, McLean SL, et al. How to obtain excellent response rates when surveying physicians. Family practice. 2009;26(1):65–68. | ||
Klabunde CN, Marcus PM, Silvestri GA, et al. U.S. primary care physicians’ lung cancer screening beliefs and recommendations. Am J Prev Med. 2010;39(5):411-–420. | ||
Klabunde CN, Marcus PM, Han PK, et al. Lung cancer screening practices of primary care physicians: results from a national survey. Ann Fam Med. 2012;10(2):102–110. | ||
Henderson S, DeGroff A, Richards TB, et al. A qualitative analysis of lung cancer screening practices by primary care physicians. J Commun Health. 2011;36(6):949–956. | ||
Ersek JL, Eberth JM, McDonnell KK, et al. Knowledge of, attitudes toward, and use of low-dose computed tomography for lung cancer screening among family physicians. Cancer. 2016;122(15):2324–2331. | ||
Hoffman RM, Sussman AL, Getrich CM, et al. Attitudes and beliefs of primary care providers in new mexico about lung cancer screening using low-dose computed tomography. Prev Chronic Dis. 2015;12:E108. | ||
Iaccarino JM, Clark J, Bolton R, et al. A national survey of pulmonologists’ views on low-dose computed tomography screening for lung cancer. Ann Am Thorac Soc. 2015;12(11):1667–1675. | ||
Simmons J, Gould MK, Woloshin S, Schwartz LM, Wiener RS. Attitudes about low-dose computed tomography screening for lung cancer: a survey of American Thoracic Society Clinicians. Am J Respir Crit Care Med. 2015;191(4):483–486. | ||
Pinsky PF, Gierada DS, Black W, et al. Performance of lung-RADS in the National Lung Screening Trial: a retrospective assessment. Ann Intern Med. 2015;162(7):485–491. |
 © 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
© 2018 The Author(s). This work is published and licensed by Dove Medical Press Limited. The full terms of this license are available at https://www.dovepress.com/terms.php and incorporate the Creative Commons Attribution - Non Commercial (unported, v3.0) License.
By accessing the work you hereby accept the Terms. Non-commercial uses of the work are permitted without any further permission from Dove Medical Press Limited, provided the work is properly attributed. For permission for commercial use of this work, please see paragraphs 4.2 and 5 of our Terms.
